Engineering of Multifunctional Nanocomposite Membranes for Wastewater Treatment: Oil/Water Separation and Dye Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.3. Membrane Characterization
2.4. Antibacterial Properties
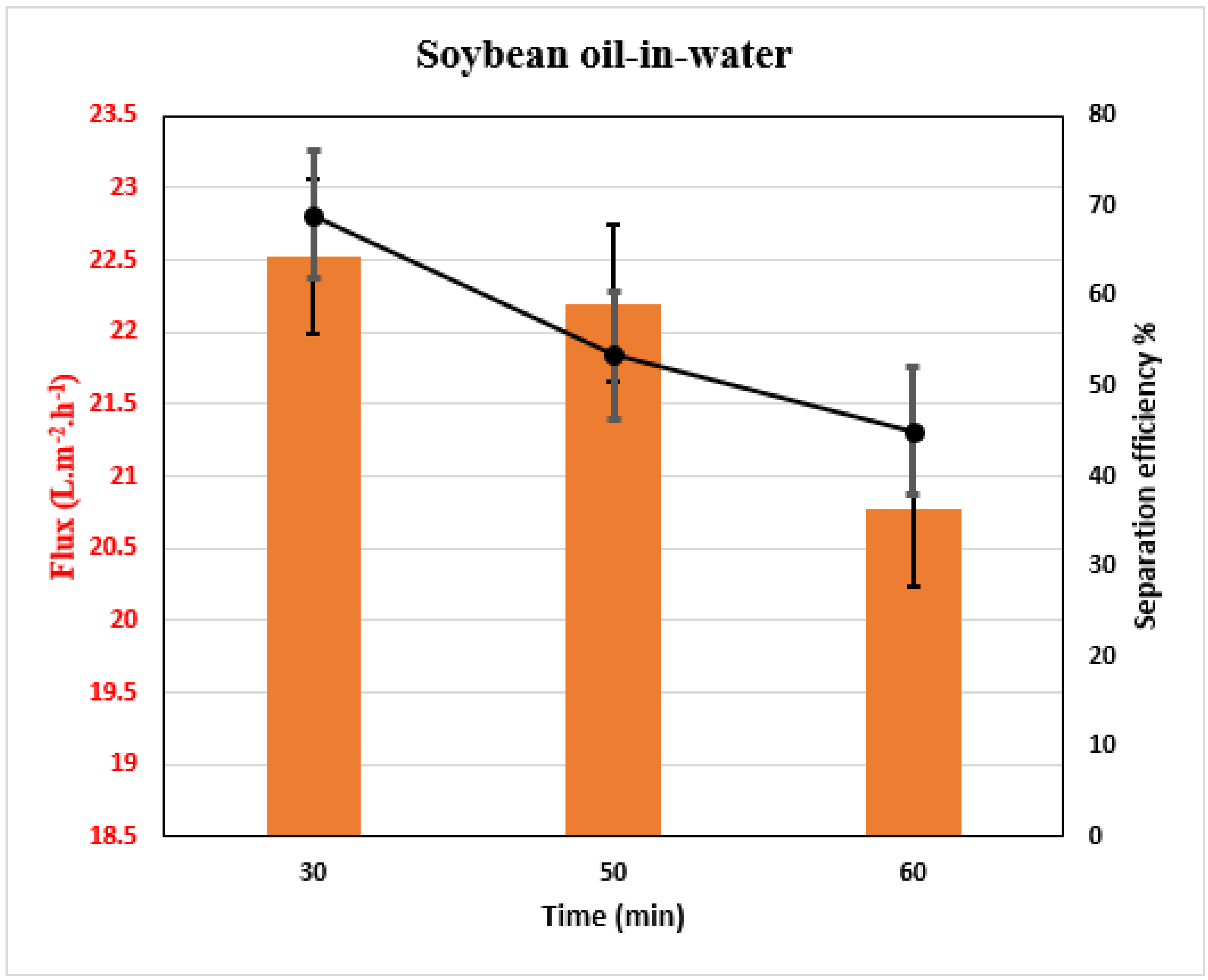
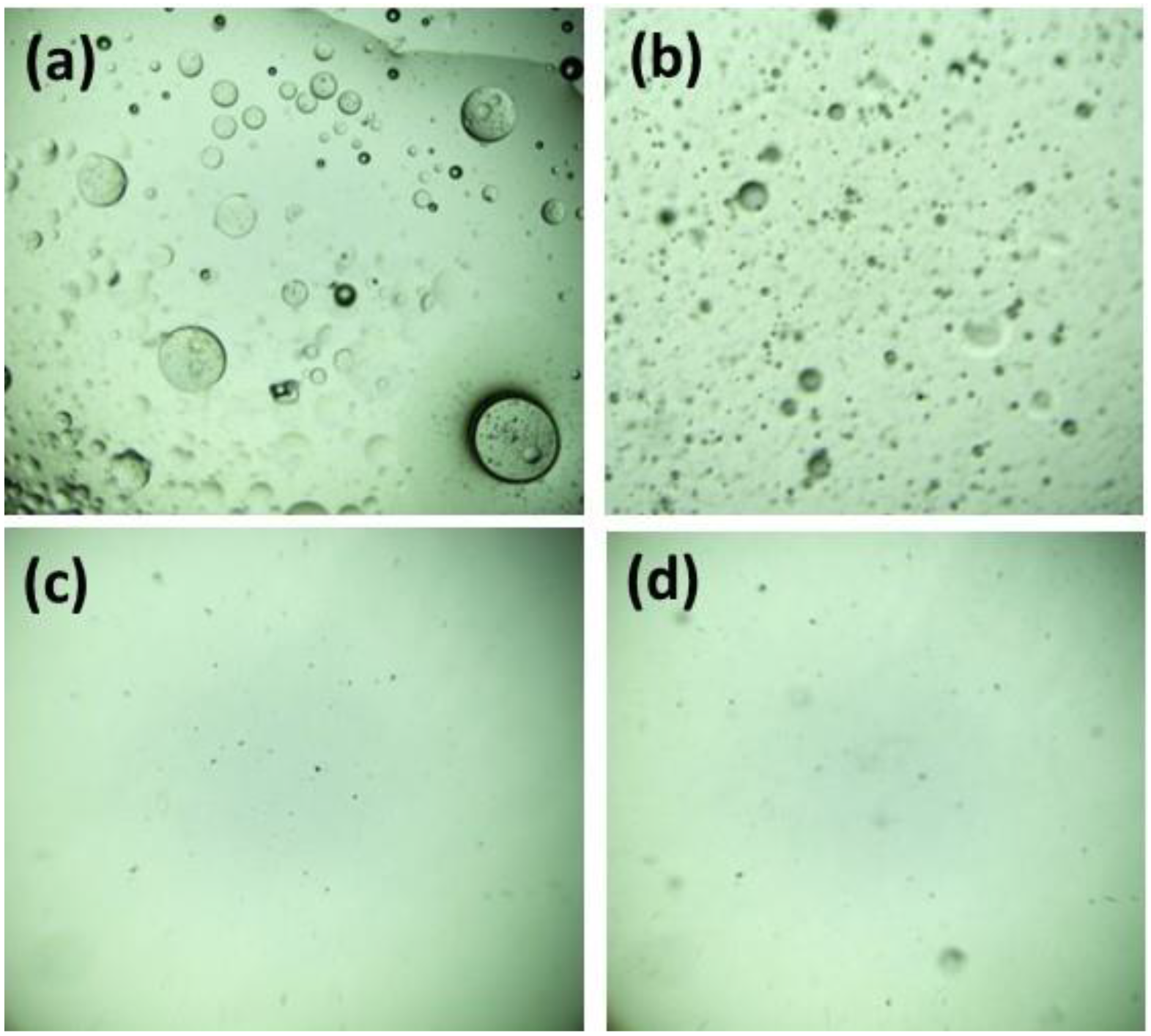
2.5. Measurements of Membrane Performance
3. Results
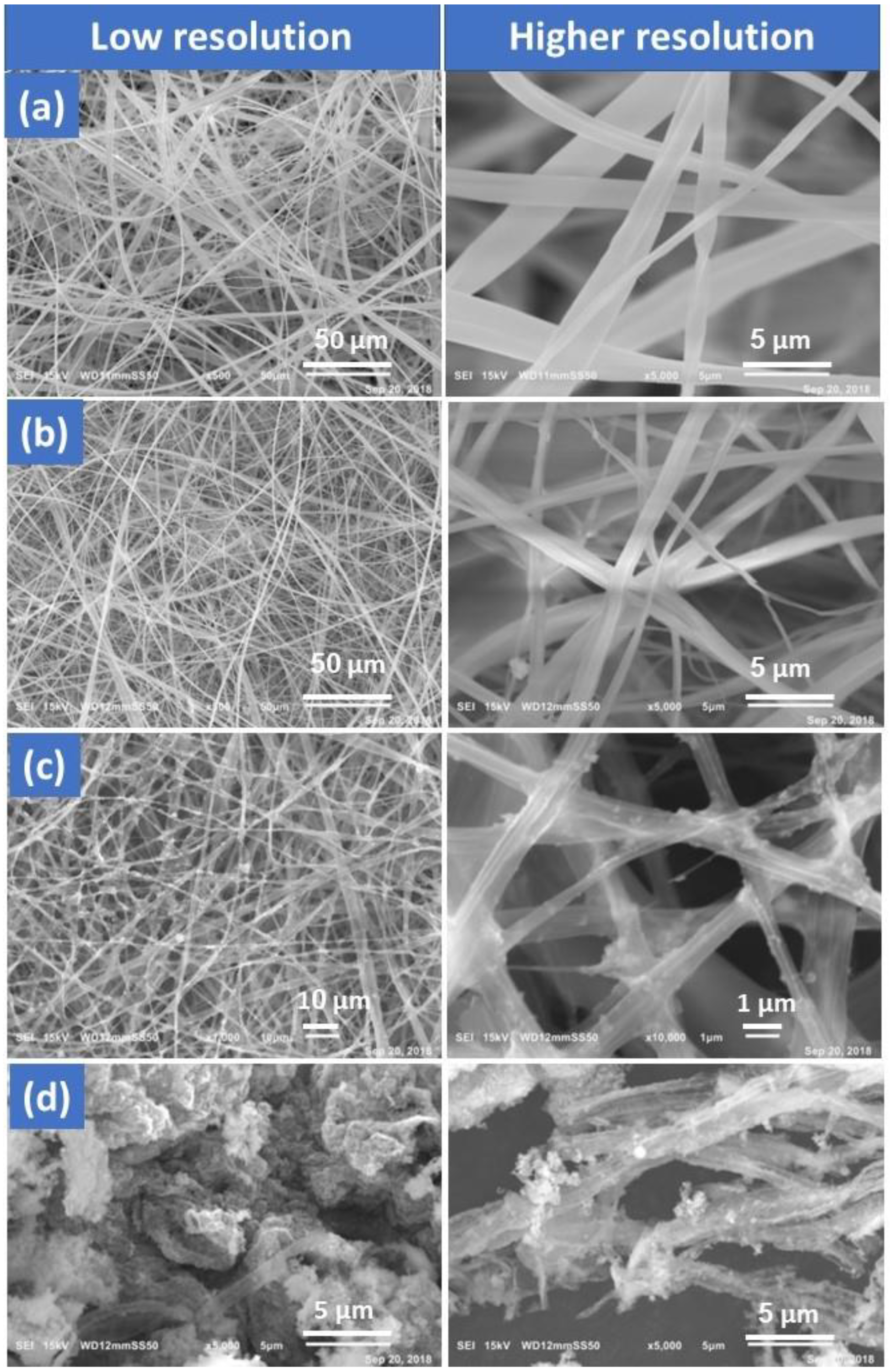
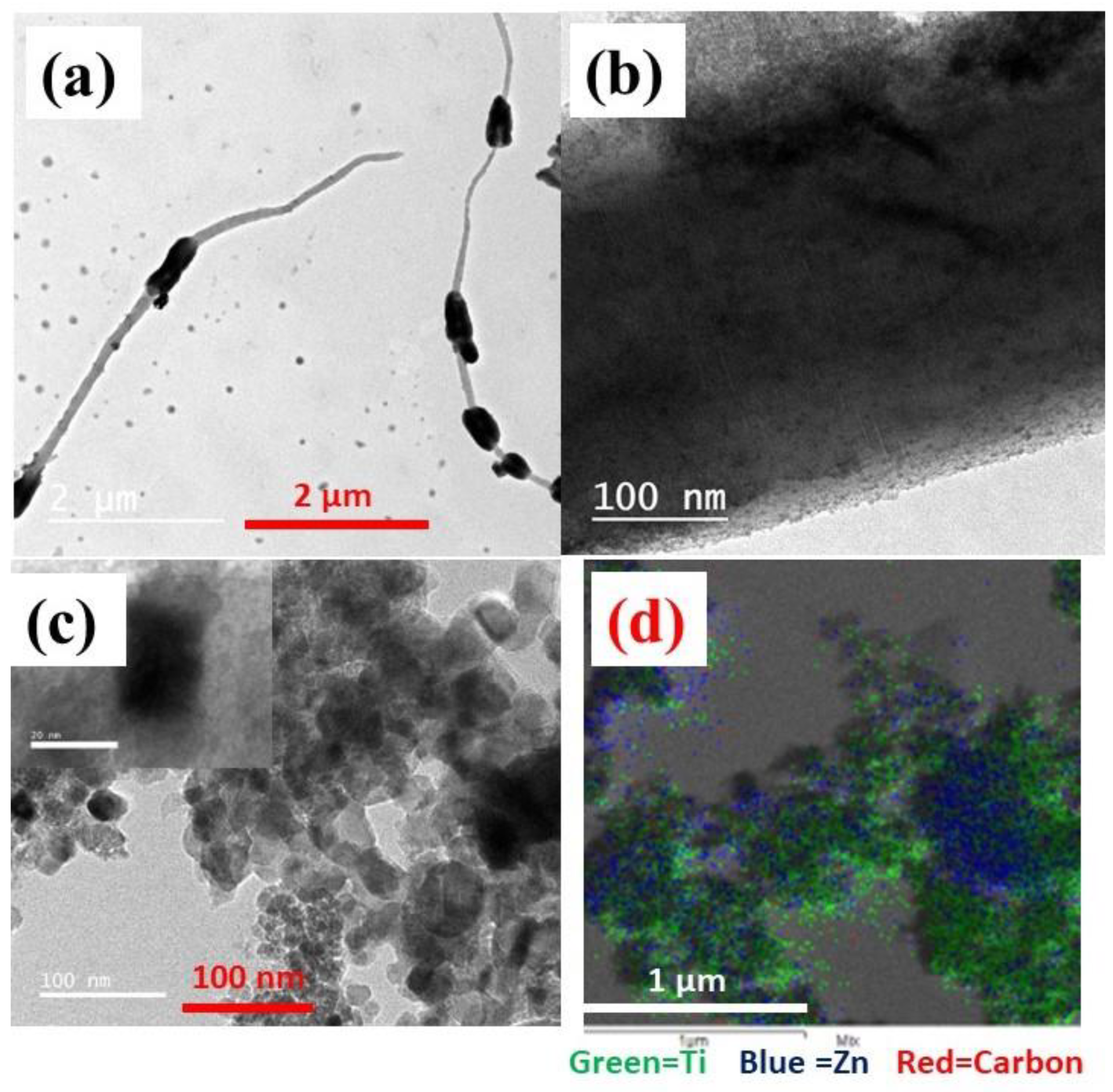
3.1. Morphology and Structure Analysis
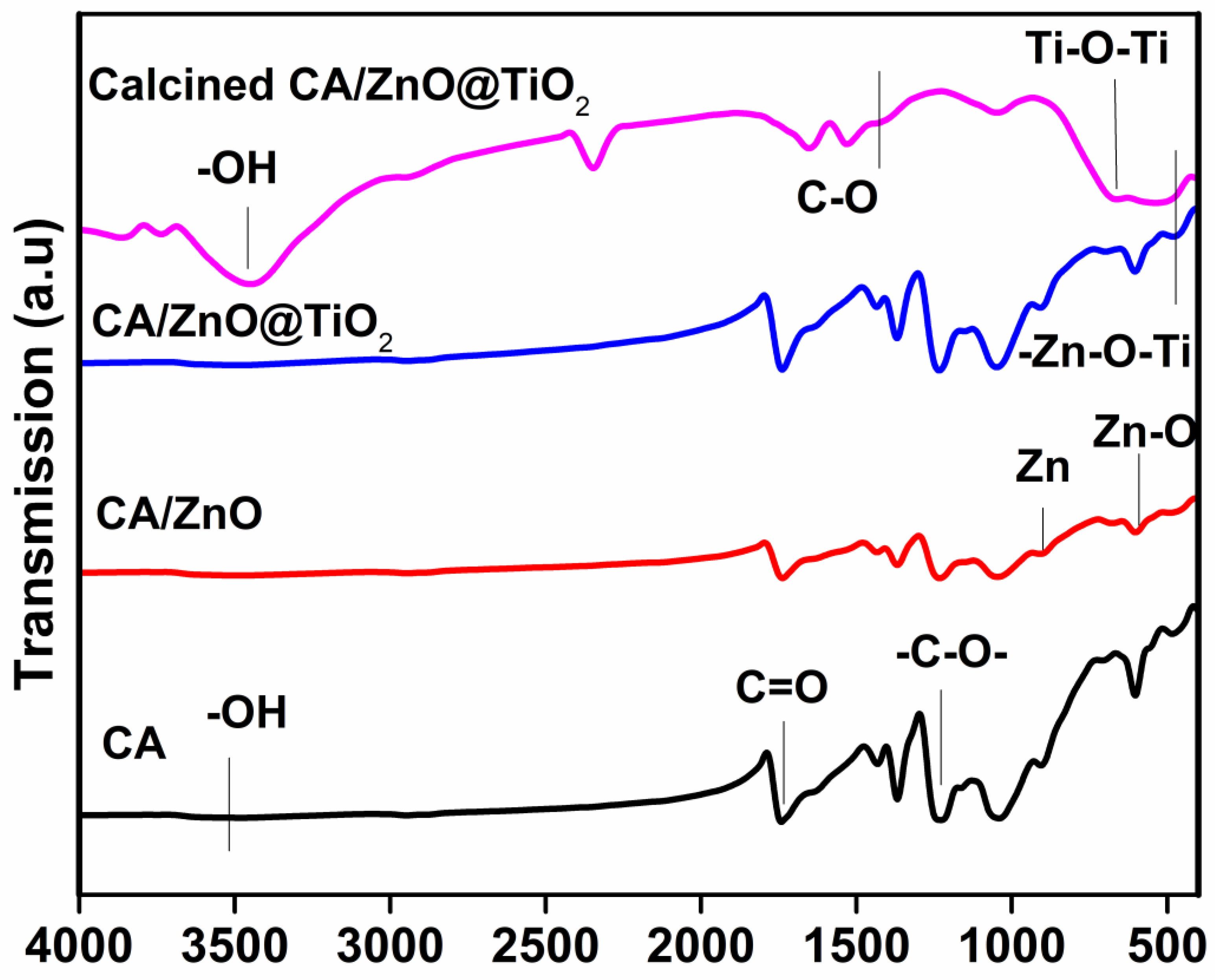
3.2. Chemical Composition (FTIR)
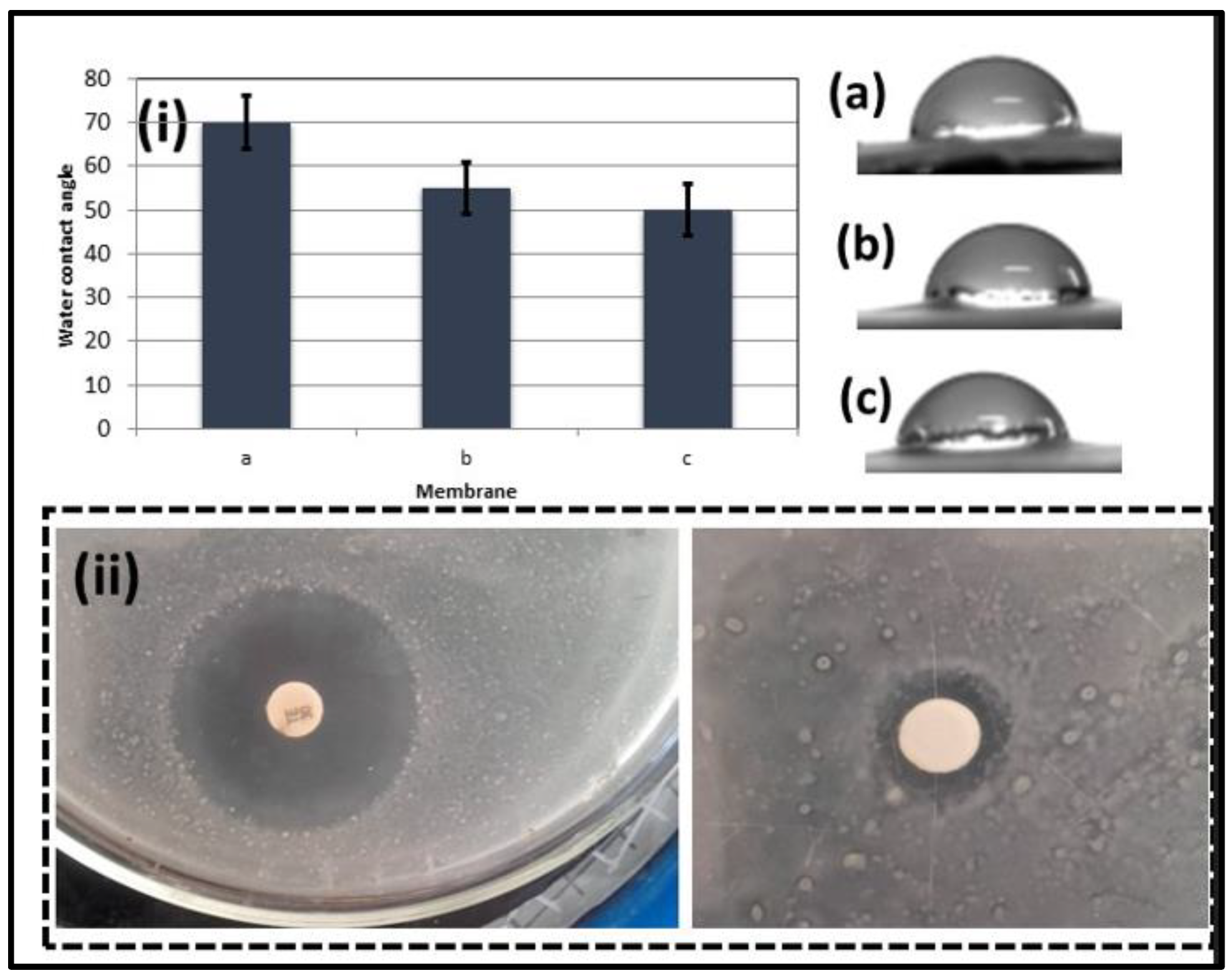
3.3. Wettability and Antibacterial Test
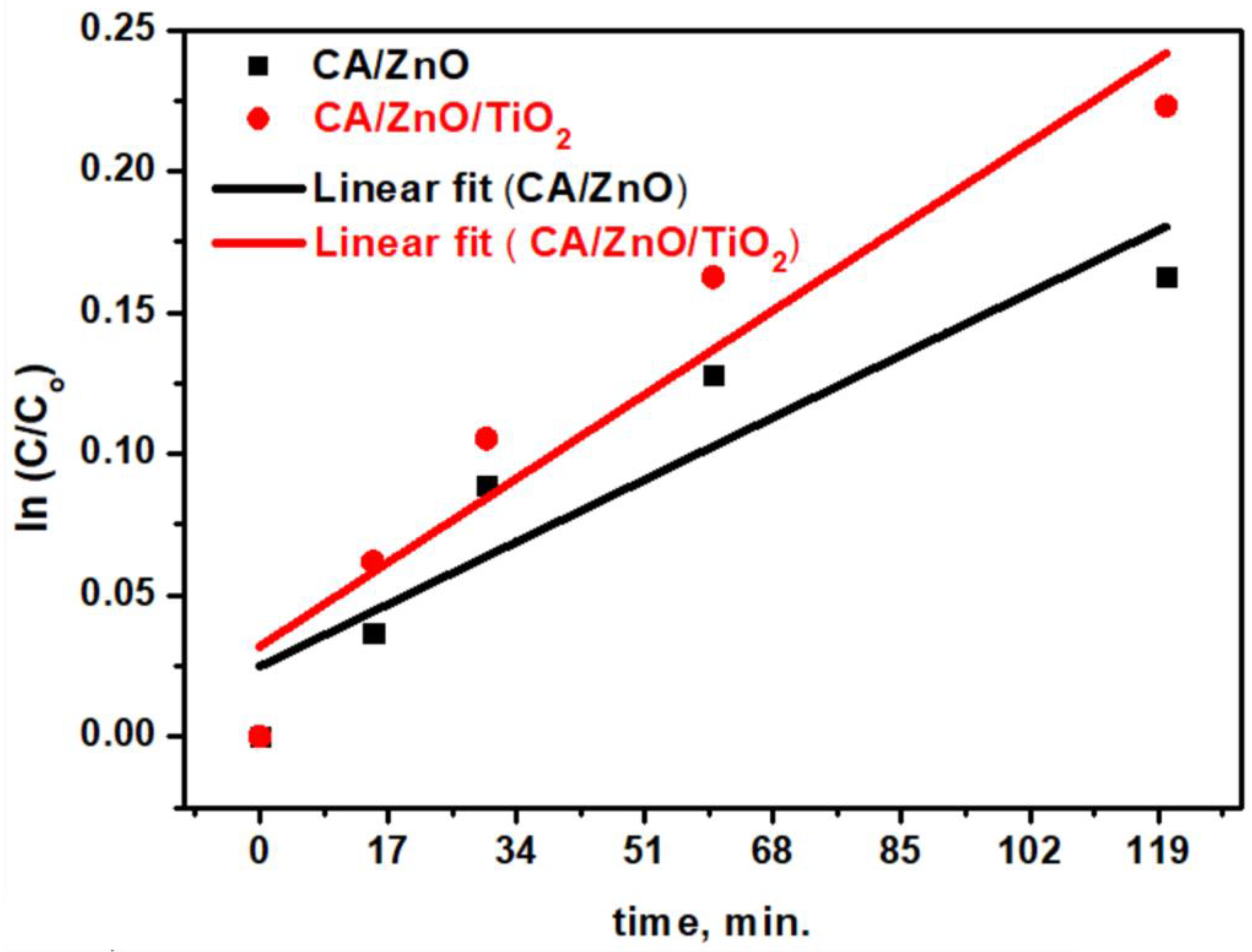
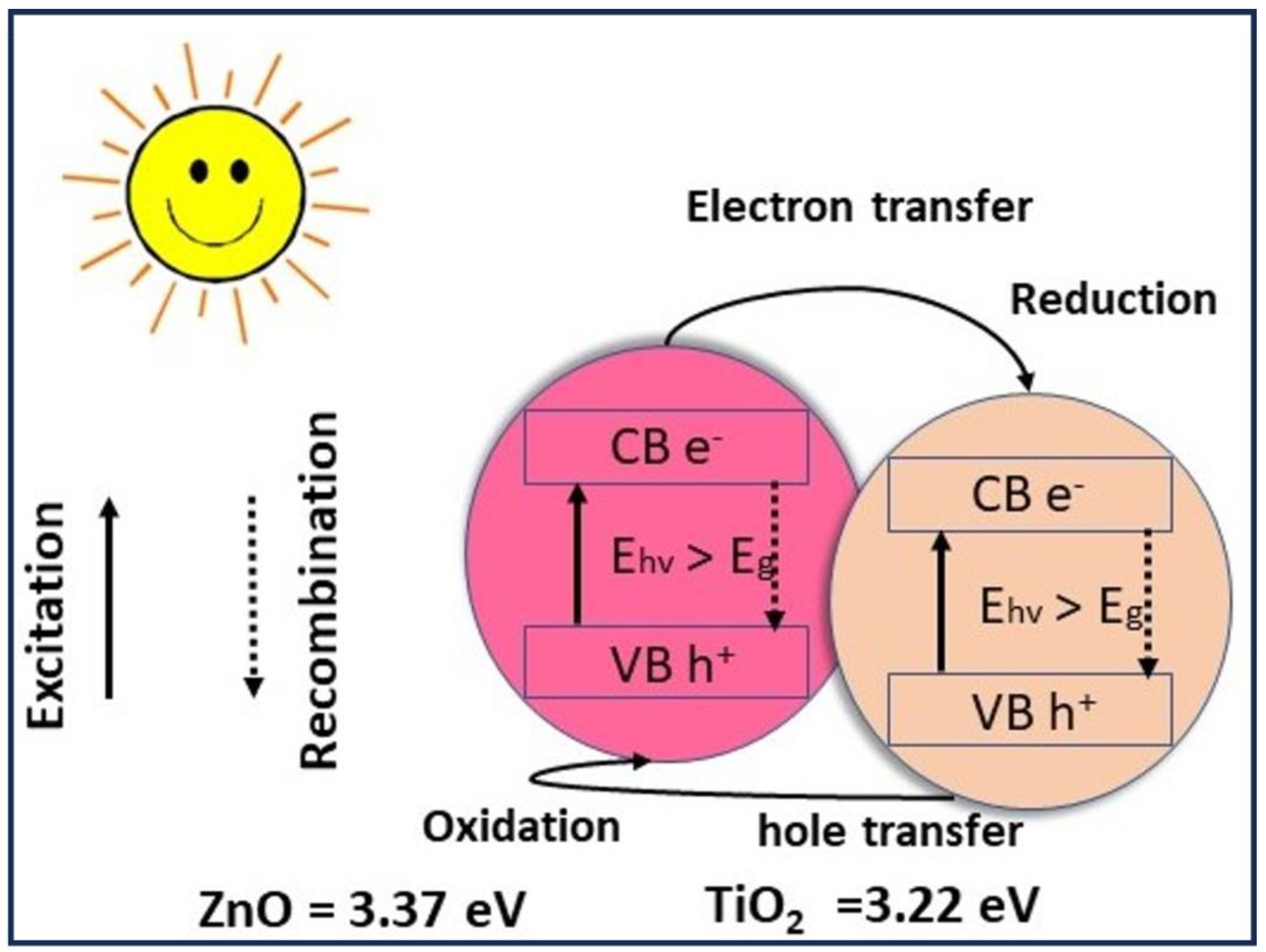
3.4. Membranes’ Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasalingam, S.; Peng, R.; Koodali, R.T. An Insight into the Adsorption and Photocatalytic Degradation of Rhodamine B in Periodic Mesoporous Materials. Appl. Catal. B Environ. 2015, 174–175, 49–59. [Google Scholar] [CrossRef]
- Arslan, I.; Balcioglu, I.A. Degradation of Commercial Reactive Dyestuffs by Heterogenous and Homogenous Advanced Oxidation Processes: A Comparative Study. Dye. Pigment. 1999, 43, 95–108. [Google Scholar] [CrossRef]
- Wu, Y.; Ji, H.; Liu, Q.; Sun, Z.; Li, P.; Ding, P.; Guo, M.; Yi, X.; Xu, W.; Wang, C.C.; et al. Visible Light Photocatalytic Degradation of Sulfanilamide Enhanced by Mo Doping of BiOBr Nanoflowers. J. Hazard. Mater. 2022, 424, 127563. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Khosla, N.; Govindasamy, V.; Saini, A.; Annapurna, K.; Dhakate, S.R. Trimetallic Composite Nanofibers for Antibacterial and Photocatalytic Dye Degradation of Mixed Dye Water. Appl. Nanosci. 2020, 10, 4191–4205. [Google Scholar] [CrossRef]
- Galindo, C.; Jacques, P.; Kalt, A. Photooxidation of the Phenylazonaphthol AO20 on TIO2: Kinetic and Mechanistic Investigations. Chemosphere 2001, 45, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Kaviyarasu, K.; Ayeshamariam, A.; Manikandan, E.; Kennedy, J.; Ladchumananandasivam, R.; Umbelino Gomes, U.; Jayachandran, M.; Maaza, M. Solution Processing of CuSe Quantum Dots: Photocatalytic Activity under RhB for UV and Visible-Light Solar Irradiation. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2016, 210, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhai, S.; Wang, M.; Ji, H.; He, L.; Ye, C.; Wang, C.; Fang, S.; Zhang, H. Photocatalytic Degradation of Rhodamine B by Using a Nanocomposite of Cuprous Oxide, Three-Dimensional Reduced Graphene Oxide, and Nanochitosan Prepared via One-Pot Synthesis. J. Alloys Compd. 2016, 659, 101–111. [Google Scholar] [CrossRef]
- Awasthi, G.P.; Adhikari, S.P.; Ko, S.; Kim, H.J.; Park, C.H.; Kim, C.S. Facile Synthesis of ZnO Flowers Modified Graphene like MoS2 Sheets for Enhanced Visible-Light-Driven Photocatalytic Activity and Antibacterial Properties. J. Alloys Compd. 2016, 682, 208–215. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Gloria, D.C.S.; Brito, C.H.V.; Mendonça, T.A.P.; Brazil, T.R.; Domingues, R.A.; Vieira, N.C.S.; Santos, E.B.; Gonçalves, M. Preparation of TiO2/Activated Carbon Nanomaterials with Enhanced Photocatalytic Activity in Paracetamol Degradation. Mater. Chem. Phys. 2023, 305, 127947. [Google Scholar] [CrossRef]
- Mezher, T.; Fath, H.; Abbas, Z.; Khaled, A. Techno-Economic Assessment and Environmental Impacts of Desalination Technologies. Desalination 2011, 266, 263–273. [Google Scholar] [CrossRef]
- Kundu, S.; Karak, N. Polymeric Photocatalytic Membrane: An Emerging Solution for Environmental Remediation. Chem. Eng. J. 2022, 438, 135575. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.K.; Diniz Da Costa, J.C. Recent Progresses on Fabrication of Photocatalytic Membranes for Water Treatment. Catal. Today 2014, 230, 47–54. [Google Scholar] [CrossRef]
- Mousa, H.M.; Alenezi, J.F.; Mohamed, I.M.A.; Yasin, A.S.; Hashem, A.F.M.; Abdal-hay, A. Synthesis of TiO2@ZnO Heterojunction for Dye Photodegradation and Wastewater Treatment. J. Alloys Compd. 2021, 886, 161169. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Pinjari, D.V.; Sonawane, S.H.; Bhanvase, B.A.; Sheikh, J.; Sillanpää, M. Cellulose-Based Nanomaterials for Water and Wastewater Treatments: A Review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar] [CrossRef]
- Saien, J.; Nejati, H. Enhanced Photocatalytic Degradation of Pollutants in Petroleum Refinery Wastewater under Mild Conditions. J. Hazard. Mater. 2007, 148, 491–495. [Google Scholar] [CrossRef]
- Yu, X.; Ji, Q.; Zhang, J.; Nie, Z.; Yang, H. Photocatalytic Degradation of Diesel Pollutants in Seawater under Visible Light. Reg. Stud. Mar. Sci. 2018, 18, 139–144. [Google Scholar] [CrossRef]
- Bae, K.L.; Kim, J.; Lim, C.K.; Nam, K.M.; Song, H. Colloidal Zinc Oxide-Copper(I) Oxide Nanocatalysts for Selective Aqueous Photocatalytic Carbon Dioxide Conversion into Methane. Nat. Commun. 2017, 8, 1156. [Google Scholar] [CrossRef]
- Revathi, M.; Pricilla Jeyakumari, A.; Saravanan, S.P. Design and Fabrication of ZnO/CdS Heterostructured Nanocomposites for Enhanced Hydrogen Evolution from Solar Water Splitting. Inorg. Chem. Commun. 2021, 134, 20407–20415. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.M.; Abdelrazek Khalil, K.; Yousef, A.; Jeon, K.S.; Kim, H.Y. Encapsulation of CoS Nanoparticles in PAN Electrospun Nanofibers: Effective and Reusable Catalyst for Ammonia Borane Hydrolysis and Dyes Photodegradation. Ceram. Int. 2013, 39, 1469–1476. [Google Scholar] [CrossRef]
- Bickley, R.I.; Slater, M.J.; Wang, W.J. Engineering Development of a Photocatalytic Reactor for Waste Water Treatment. Process Saf. Environ. Prot. 2005, 83, 205–216. [Google Scholar] [CrossRef]
- Madkhali, N.; Prasad, C.; Malkappa, K.; Choi, H.Y.; Govinda, V.; Bahadur, I.; Abumousa, R.A. Recent Update on Photocatalytic Degradation of Pollutants in Waste Water Using TiO2-Based Heterostructured Materials. Results Eng. 2023, 17, 100920. [Google Scholar] [CrossRef]
- Chi, Y.; Yuan, Q.; Li, Y.; Zhao, L.; Li, N.; Li, X.; Yan, W. Magnetically Separable Fe3O4@SiO2@TiO2-Ag Microspheres with Well-Designed Nanostructure and Enhanced Photocatalytic Activity. J. Hazard. Mater. 2013, 262, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Pližingrová, E.; Klementová, M.; Bezdička, P.; Boháček, J.; Barbieriková, Z.; Dvoranová, D.; Mazúr, M.; Krýsa, J.; Šubrt, J.; Brezová, V. 2D-Titanium Dioxide Nanosheets Modified with Nd, Ag and Au: Preparation, Characterization and Photocatalytic Activity. Catal. Today 2017, 281, 165–180. [Google Scholar] [CrossRef]
- Roşu, M.C.; Socaci, C.; Floare-Avram, V.; Borodi, G.; PogǍcean, F.; Coroş, M.; MǍgeruşan, L.; Pruneanu, S. Photocatalytic Performance of Graphene/TiO2-Ag Composites on Amaranth Dye Degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 Nanomaterials for Photocatalytic Degradation of Pollutants: A Review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A. TiO2/RGO Composites: Its Achievement and Factors Involved in Hydrogen Production. Renew. Sustain. Energy Rev. 2017, 76, 1384–1392. [Google Scholar] [CrossRef]
- Yi, H.; Huang, D.; Qin, L.; Zeng, G.; Lai, C.; Cheng, M.; Ye, S.; Song, B.; Ren, X.; Guo, X. Selective Prepared Carbon Nanomaterials for Advanced Photocatalytic Application in Environmental Pollutant Treatment and Hydrogen Production. Appl. Catal. B Environ. 2018, 239, 408–424. [Google Scholar] [CrossRef]
- Noorjahan, M.; Kumari, V.D.; Subrahmanyam, M.; Boule, P. A Novel and Efficient Photocatalyst: TiO2-HZSM-5 Combinate Thin Film. Appl. Catal. B Environ. 2004, 47, 209–213. [Google Scholar] [CrossRef]
- Luque-Morales, P.A.; López-Peraza, A.; Nava-Olivas, O.J.; Amaya-Parra, G.; Báez-López, Y.A.; Orozco-Carmona, V.M.; Garrafa-Gálvez, H.E.; Chinchillas-Chinchillas, M.D.J. Zno Semiconductor Nanoparticles and Their Application in Photocatalytic Degradation of Various Organic Dyes. Materials 2021, 14, 7537. [Google Scholar] [CrossRef]
- Marcì, G.; Augugliaro, V.; López-Muñoz, M.J.; Martín, C.; Palmisano, L.; Rives, V.; Schiavello, M.; Tilley, R.J.D.; Venezia, A.M. Preparation Characterization and Photocatalytic Activity of Polycrystalline ZnO/TiO2 Systems. 2. Surface, Bulk Characterization, and 4-Nitrophenol Photodegradation in Liquid-Solid Regime. J. Phys. Chem. B 2001, 105, 1033–1040. [Google Scholar] [CrossRef]
- Tian, J.; Chen, L.; Yin, Y.; Wang, X.; Dai, J.; Zhu, Z.; Liu, X.; Wu, P. Photocatalyst of TiO2/ZnO Nano Composite Film: Preparation, Characterization, and Photodegradation Activity of Methyl Orange. Surf. Coat. Technol. 2009, 204, 205–214. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Zhang, L.; Kang, P.; Xie, Y.; Lv, Y.; Xu, R.; Zhang, X. Sonocatalytic Degradation of Some Dyestuffs and Comparison of Catalytic Activities of Nano-Sized TiO2, Nano-Sized ZnO and Composite TiO2/ZnO Powders under Ultrasonic Irradiation. Ultrason. Sonochem. 2009, 16, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nascimben Santos, E.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G. Photocatalytic Membrane Filtration and Its Advantages over Conventional Approaches in the Treatment of Oily Wastewater: A Review. Asia-Pac. J. Chem. Eng. 2020, 15, e2533. [Google Scholar] [CrossRef]
- Chinchillas-Chinchillas, M.J.; Orozco-Carmona, V.M.; Alvarado-Beltrán, C.G.; Almaral-Sánchez, J.L.; Sepulveda-Guzman, S.; Jasso-Ramos, L.E.; Castro-Beltrán, A. Synthesis of Recycled Poly(Ethylene Terephthalate)/Polyacrylonitrile/Styrene Composite Nanofibers by Electrospinning and Their Mechanical Properties Evaluation. J. Polym. Environ. 2019, 27, 659–669. [Google Scholar] [CrossRef]
- Vatanpour, V.; Kose-Mutlu, B.; Koyuncu, I. Electrospraying Technique in Fabrication of Separation Membranes: A Review. Desalination 2022, 533, 115765. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Hamdy Makhlouf, A.S.; Khalil, K.A. Novel, Facile, Single-Step Technique of Polymer/TiO2 Nanofiber Composites Membrane for Photodegradation of Methylene Blue. ACS Appl. Mater. Interfaces 2015, 7, 13329–13341. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.M.; Hussein, K.H.; Sayed, M.M.; Abd El-Rahman, M.K.; Woo, H.M. Development and Characterization of Cellulose/Iron Acetate Nanofibers for Bone Tissue Engineering Applications. Polymers 2021, 13, 1339. [Google Scholar] [CrossRef]
- Da Silva-Neto, M.L.; de Oliveira, M.C.A.; Dominguez, C.T.; Lins, R.E.M.; Rakov, N.; de Araújo, C.B.; Menezes, L.D.S.; de Oliveira, H.P.; Gomes, A.S.L. UV Random Laser Emission from Flexible ZnO-Ag-Enriched Electrospun Cellulose Acetate Fiber Matrix. Sci. Rep. 2019, 9, 11765. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Wang, Y.; Wang, Z. ZnO/Graphene-Oxide Nanocomposite with Remarkably Enhanced Visible-Light-Driven Photocatalytic Performance. J. Colloid Interface Sci. 2012, 377, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kuo, D.H.; Lu, D. N-Doped Mesoporous TiO2 Nanoparticles Synthesized by Using Biological Renewable Nanocrystalline Cellulose as Template for the Degradation of Pollutants under Visible and Sun Light. Chem. Eng. J. 2016, 295, 192–200. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Du, X.W.; Fu, Y.S.; Sun, J.; Han, X.; Liu, J. Complete UV Emission of ZnO Nanoparticles in a PMMA Matrix. Semicond. Sci. Technol. 2006, 21, 1202–1206. [Google Scholar] [CrossRef]
- Nair, M.G.; Nirmala, M.; Rekha, K.; Anukaliani, A. Structural, Optical, Photo Catalytic and Antibacterial Activity of ZnO and Co Doped ZnO Nanoparticles. Mater. Lett. 2011, 65, 1797–1800. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, W.; Wu, Z.; Guo, S. Band Structure and Visible Light Photocatalytic Activity of Multi-Type Nitrogen Doped TiO2 Nanoparticles Prepared by Thermal Decomposition. J. Hazard. Mater. 2009, 162, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effects of Surface Functional Groups on Protein Adsorption and Subsequent Cell Adhesion Using Self-Assembled Monolayers. J. Mater. Chem. 2007, 17, 4079–4087. [Google Scholar] [CrossRef]
- Mo, J.; Son, S.H.; Jegal, J.; Kim, J.; Lee, Y.H. Preparation and Characterization of Polyamide Nanofiltration Composite Membranes with TiO2 Layers Chemically Connected to the Membrane Surface. J. Appl. Polym. Sci. 2007, 105, 1267–1274. [Google Scholar] [CrossRef]
- Alhoshan, M.; Alam, J.; Dass, L.A.; Al-Homaidi, N. Fabrication of Polysulfone/ZnO Membrane: Influence of ZnO Nanoparticles on Membrane Characteristics. Adv. Polym. Technol. 2013, 32, 21369. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, Y.; Xiang, Y.; Zhu, J. Construction of Urchin-Like ZnO/TiO2 Direct Z-Scheme System to Improve Charge Separation. ChemistrySelect 2019, 4, 12963–12970. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpai, S.K. Preparation, Characterization and Antibacterial Applications of ZnO-Nanoparticles Coated Polyethylene Films for Food Packaging. Colloids Surf. B Biointerfaces 2012, 90, 16–20. [Google Scholar] [CrossRef]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured Materials for Photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous Photodegradation of Industrial Dyes: An Insight to Different Mechanisms and Rate Affecting Parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Al Kausor, M.; Gupta, S.S.; Chakrabortty, D. Ag3PO4-Based Nanocomposites and Their Applications in Photodegradation of Toxic Organic Dye Contaminated Wastewater: Review on Material Design to Performance Enhancement. J. Saudi Chem. Soc. 2020, 24, 20–41. [Google Scholar] [CrossRef]
- Mandal, B.; Panda, J.; Paul, P.K.; Sarkar, R.; Tudu, B. MnFe2O4 Decorated Reduced Graphene Oxide Heterostructures: Nanophotocatalyst for Methylene Blue Dye Degradation. Vacuum 2020, 173, 109150. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.H.; Park, J.W.; Yip, A.C.K. Photocatalysts for Degradation of Dyes in Industrial Effluents: Opportunities and Challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Park, S.Y. TiO2/Amidoxime-Modified Polyacrylonitrile Nanofibers and Its Application for the Photodegradation of Methyl Blue in Aqueous Medium. Desalin. Water Treat. 2015, 54, 3146–3151. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Y.; Men, L.; Wang, X.; He, D.; Chai, Y.; Zhao, B.; Ghoshroy, S.; Tang, Q. A Highly Efficient TiO2@ZnO N-p-n Heterojunction Nanorod Photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Zhao, C.; Yang, F. Improvement of Antifouling Performances for Modified PVDF Ultrafiltration Membrane with Hydrophilic Cellulose Nanocrystal. Appl. Surf. Sci. 2018, 440, 1091–1100. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, H.M.; Sayed, M.M.; Mohamed, I.M.A.; El-sadek, M.S.A.; Nasr, E.A.; Mohamed, M.A.; Taha, M. Engineering of Multifunctional Nanocomposite Membranes for Wastewater Treatment: Oil/Water Separation and Dye Degradation. Membranes 2023, 13, 810. https://doi.org/10.3390/membranes13100810
Mousa HM, Sayed MM, Mohamed IMA, El-sadek MSA, Nasr EA, Mohamed MA, Taha M. Engineering of Multifunctional Nanocomposite Membranes for Wastewater Treatment: Oil/Water Separation and Dye Degradation. Membranes. 2023; 13(10):810. https://doi.org/10.3390/membranes13100810
Chicago/Turabian StyleMousa, Hamouda M, Mostafa M. Sayed, Ibrahim M. A. Mohamed, M. S. Abd El-sadek, Emad Abouel Nasr, Mohamed A. Mohamed, and Mohamed Taha. 2023. "Engineering of Multifunctional Nanocomposite Membranes for Wastewater Treatment: Oil/Water Separation and Dye Degradation" Membranes 13, no. 10: 810. https://doi.org/10.3390/membranes13100810
APA StyleMousa, H. M., Sayed, M. M., Mohamed, I. M. A., El-sadek, M. S. A., Nasr, E. A., Mohamed, M. A., & Taha, M. (2023). Engineering of Multifunctional Nanocomposite Membranes for Wastewater Treatment: Oil/Water Separation and Dye Degradation. Membranes, 13(10), 810. https://doi.org/10.3390/membranes13100810







