Study of the Efficiency of a Polycation Using the Diafiltration Technique in the Removal of the Antibiotic Oxytetracycline Used in Aquaculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
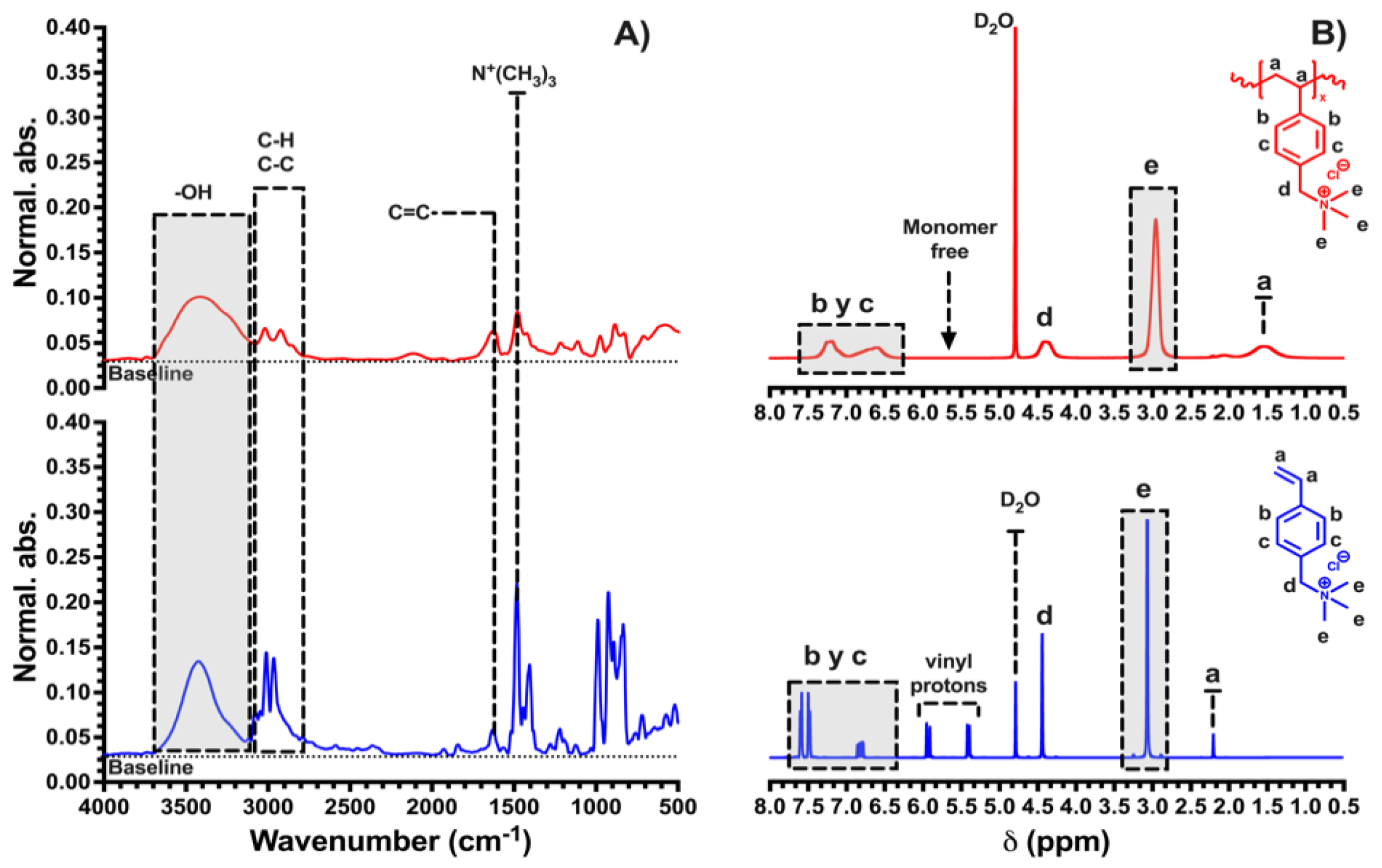
2.2. Synthesis of Polycation—Homopolymer Polyelectrolyte (HPE)
2.3. Characterization
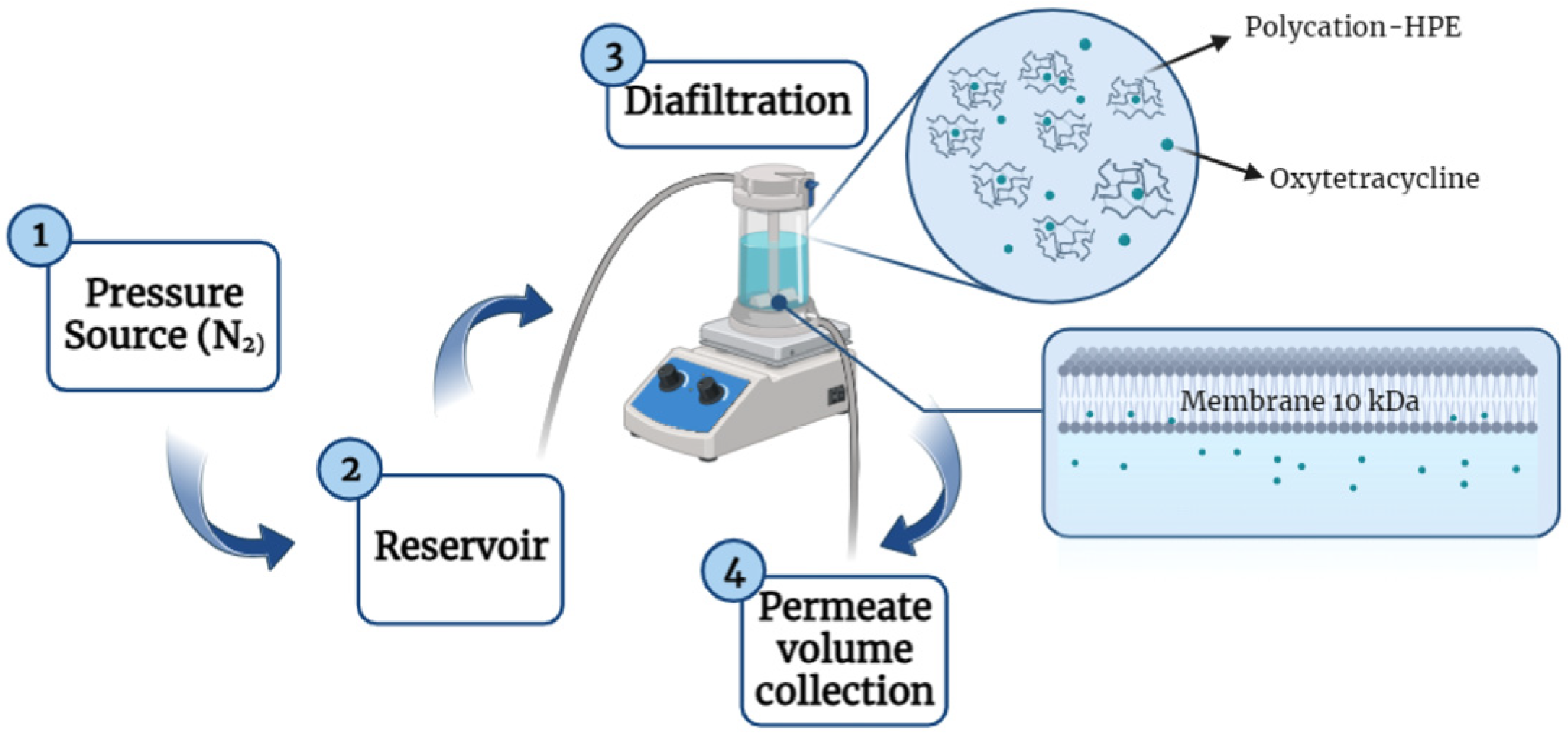
2.4. OXT Removal Using Diafiltration Technique
2.4.1. Washing Method
2.4.2. Enrichment Method
2.5. OXT Removal Using Diafiltration Technique
3. Results and Discussion
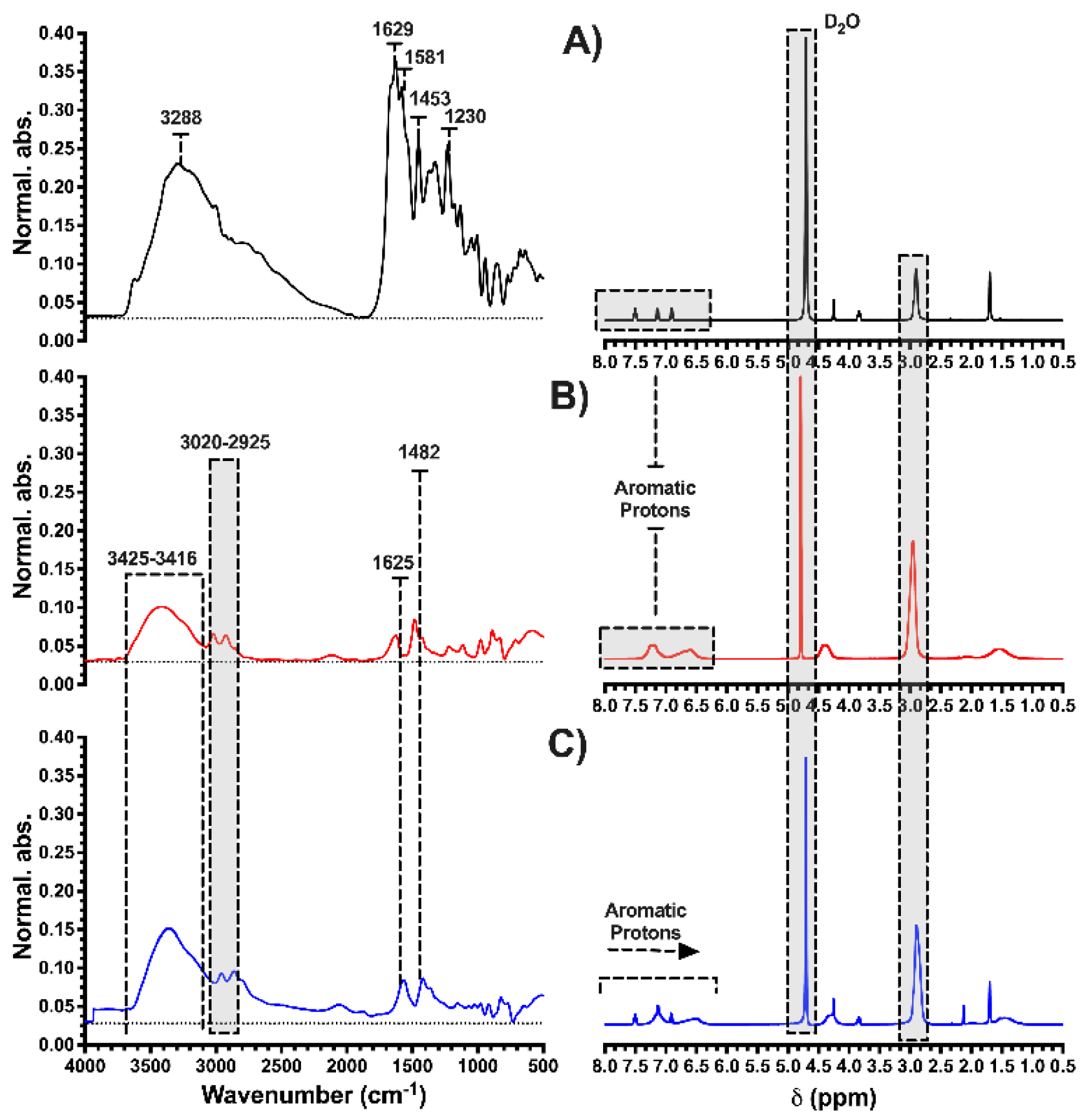
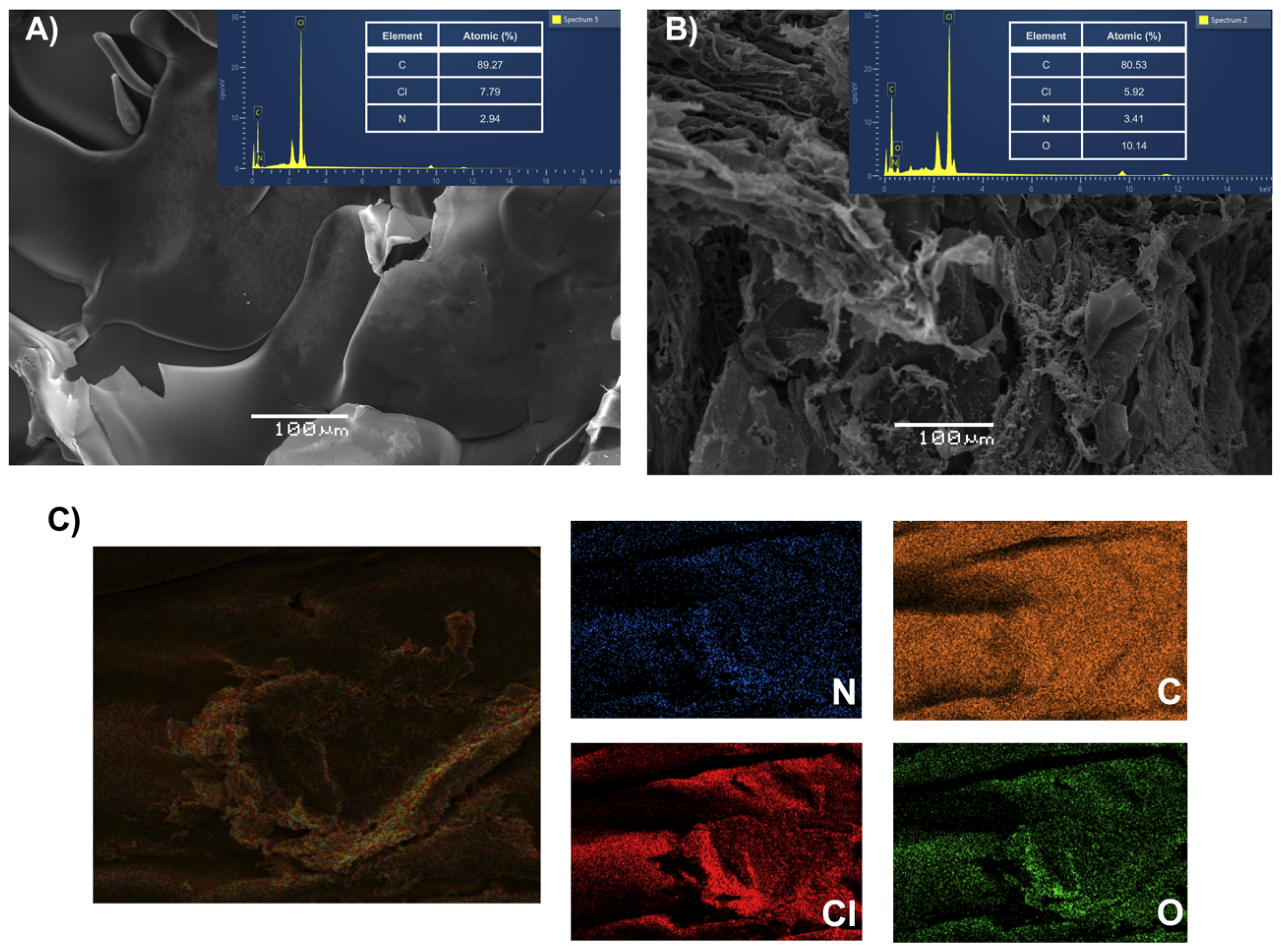
3.1. Characterization of HPE
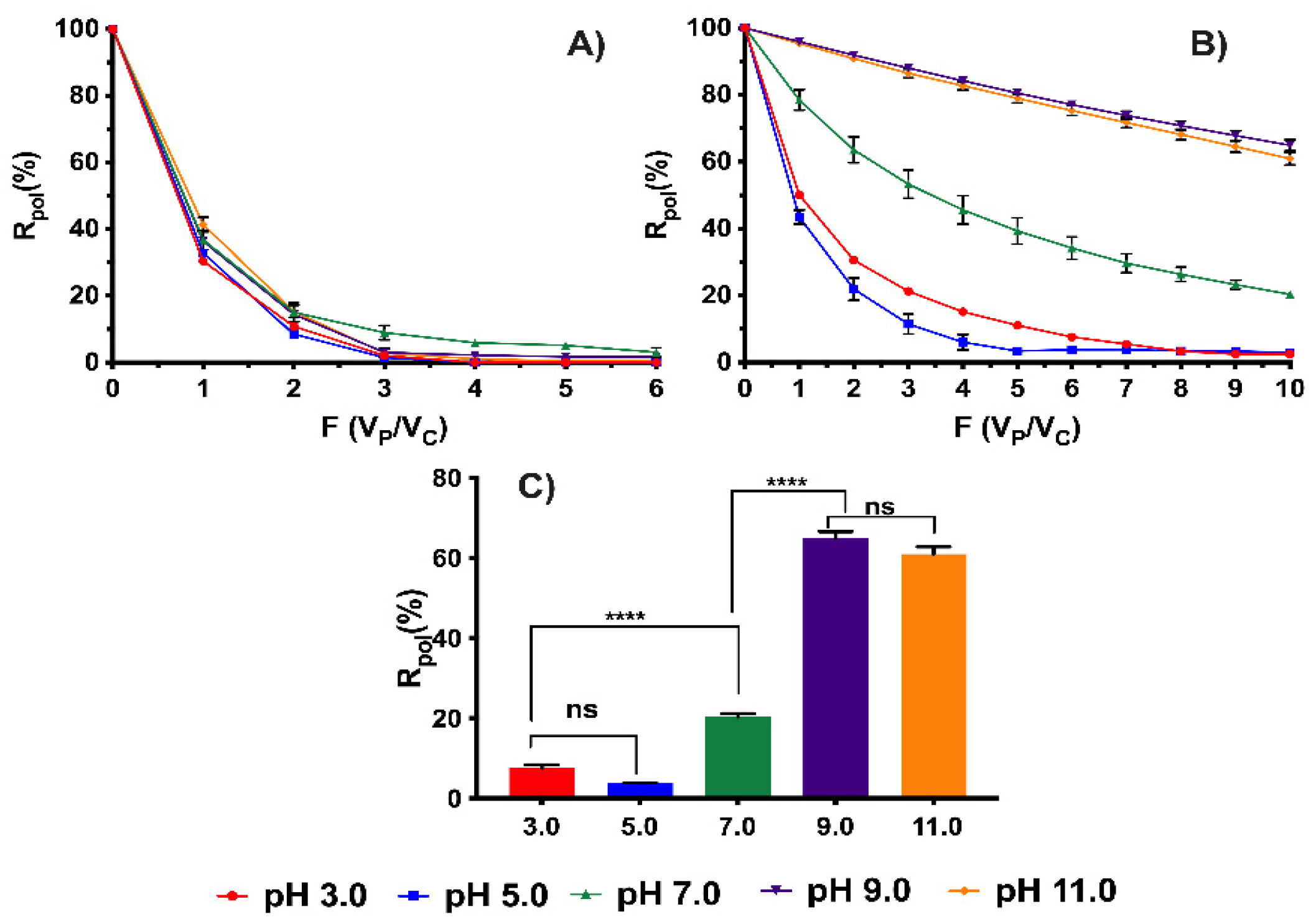
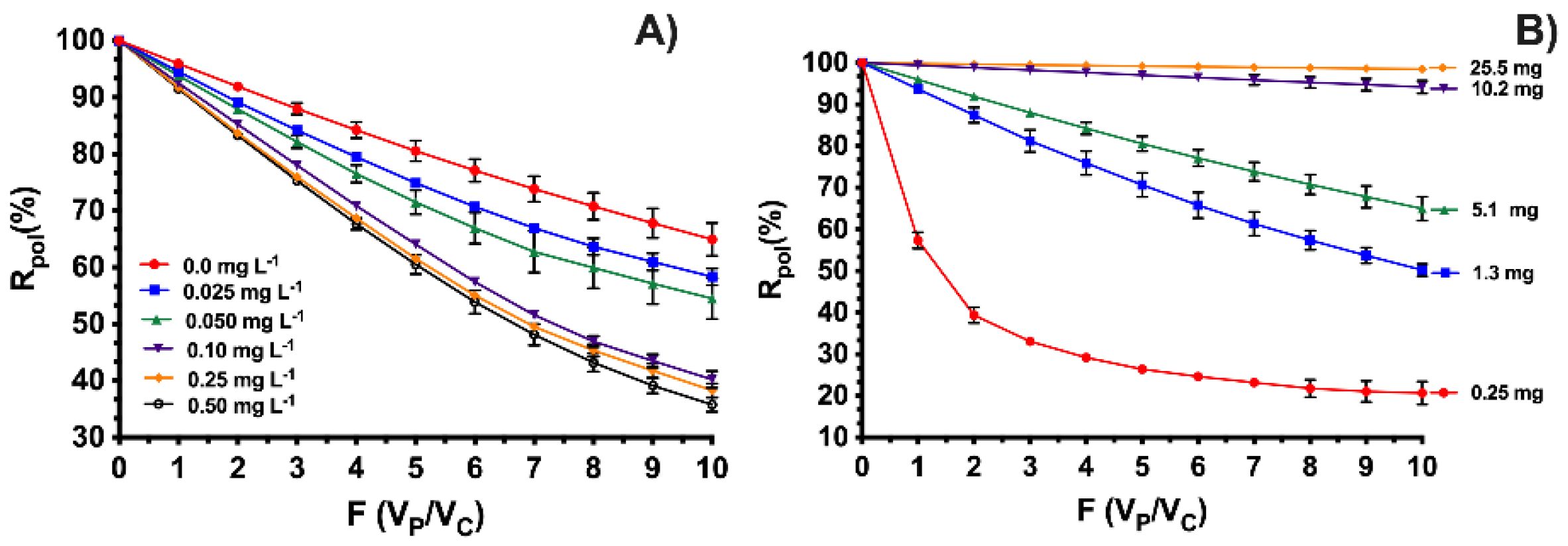
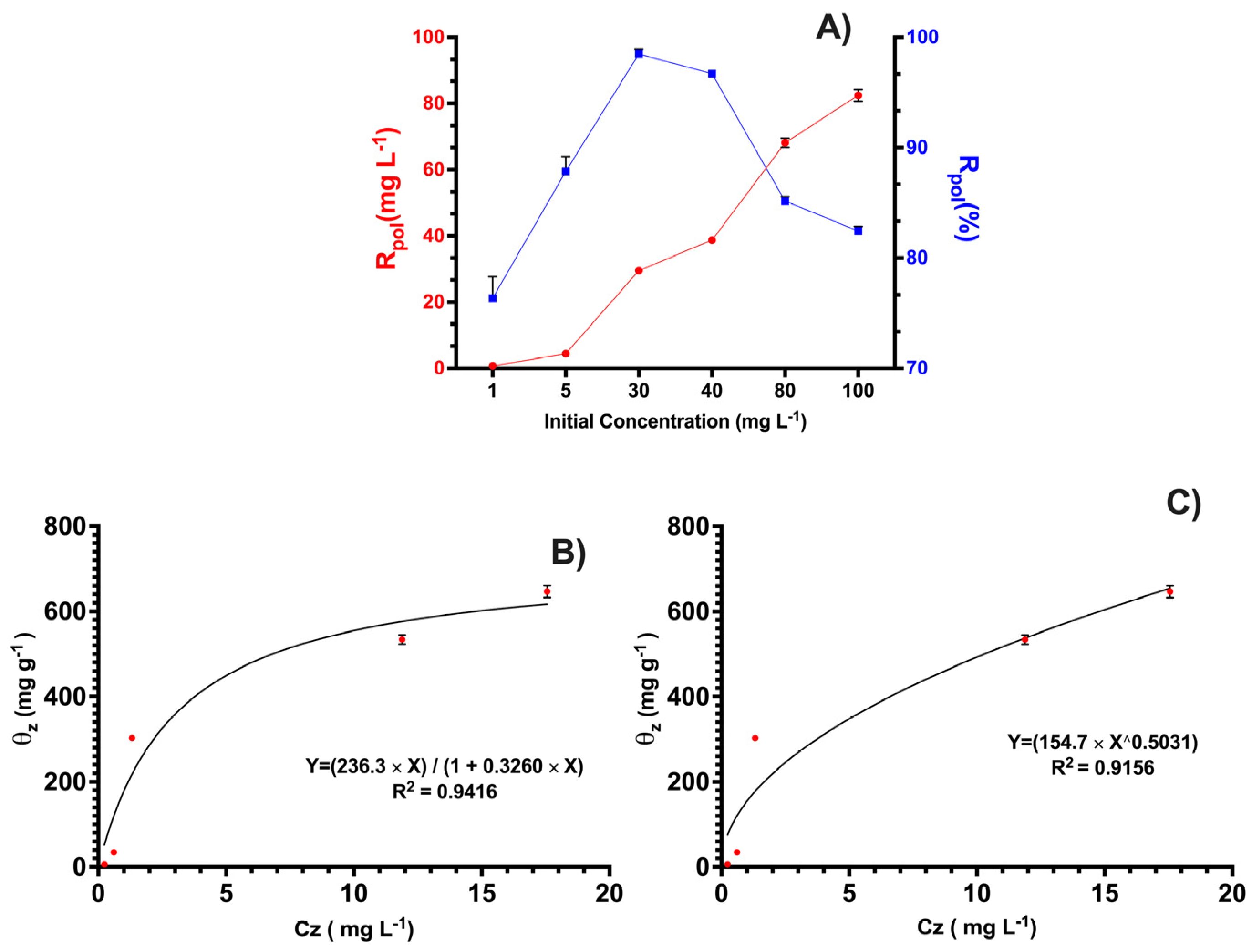
3.2. Removal Studies
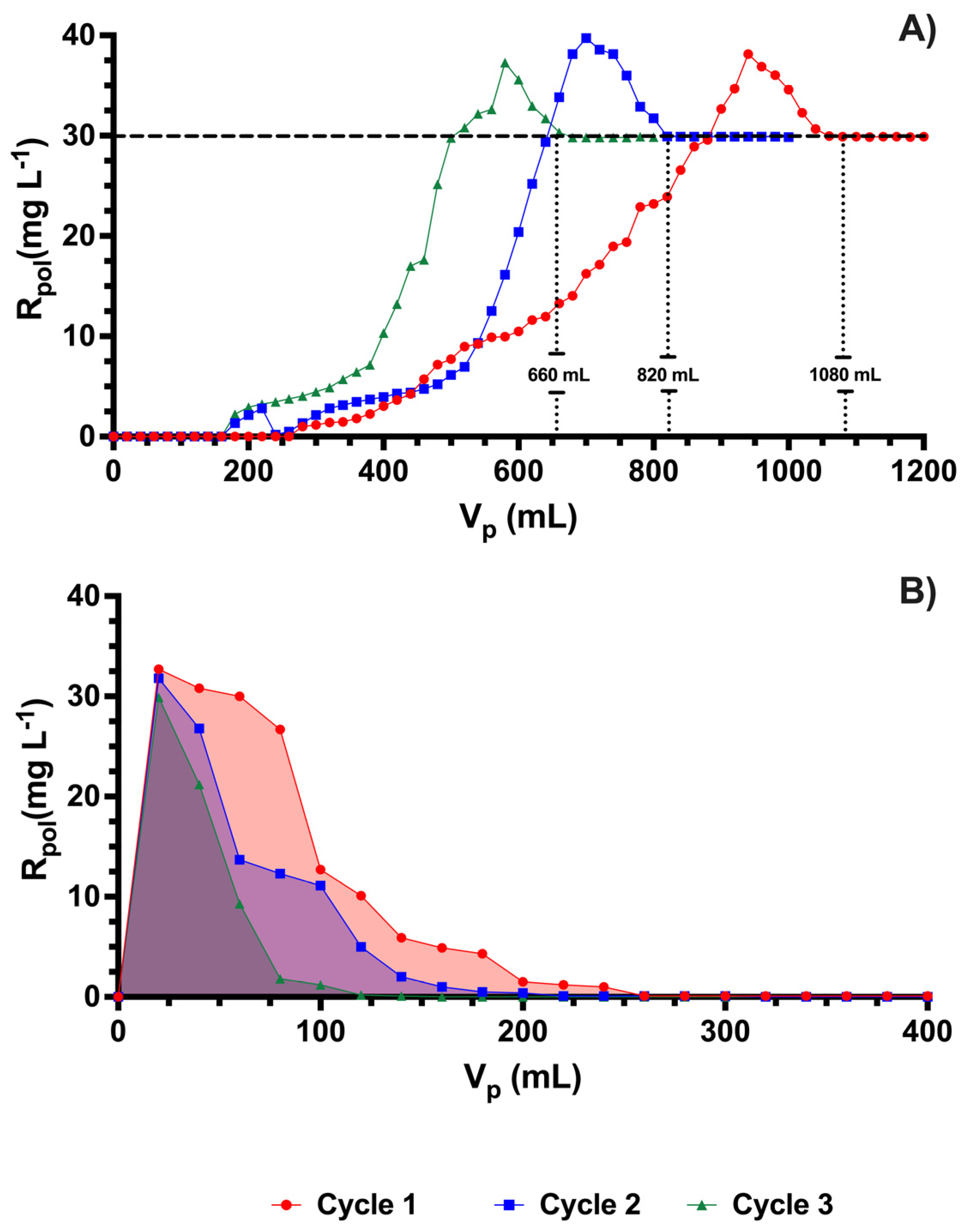
3.3. Charge–Discharge
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A Review on Emerging Water Contaminants and the Application of Sustainable Removal Technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Gomes, I.B.; Maillard, J.-Y.; Simões, L.C.; Simões, M. Emerging Contaminants Affect the Microbiome of Water Systems—Strategies for Their Mitigation. npj Clean Water 2020, 3, 39. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A Review on Effective Removal of Emerging Contaminants from Aquatic Systems: Current Trends and Scope for Further Research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef] [PubMed]
- Sversut, R.A.; da Silva, A.A.; Cardoso, T.F.M.; Kassab, N.M.; do Amaral, M.S.; Salgado, H.R.N. A Critical Review of Properties and Analytical Methods for the Determination of Oxytetracyline in Biological and Pharmaceutical Matrices. Crit. Rev. Anal. Chem. 2017, 47, 154–171. [Google Scholar] [CrossRef]
- Yu, J.; Kang, Y.; Yin, W.; Fan, J.; Guo, Z. Removal of Antibiotics from Aqueous Solutions by a Carbon Adsorbent Derived from Protein-Waste-Doped Biomass. ACS Omega 2020, 5, 19187–19193. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of Non-Steroidal Anti-Inflammatory Drugs from Aqueous Solution Using Activated Carbons: Review. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef]
- Giger, W.; Alder, A.C.; Golet, E.M.; Kohler, H.-P.E.; McArdell, C.S.; Molnar, E.; Siegrist, H.; Suter, M.J.-F. Occurrence and Fate of Antibiotics as Trace Contaminants in Wastewaters, Sewage Sludges, and Surface Waters. Chimia 2003, 57, 485. [Google Scholar] [CrossRef]
- Kwon, J.H.; Powderly, W.G. The Post-Antibiotic Era Is Here. Science 2021, 373, 471. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, Fate, and Risk Assessment of Typical Tetracycline Antibiotics in the Aquatic Environment: A Review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Blair-González, J.; Contreras-Villacura, E.; Carvajal Guevara, A.; Palma Toloza, C. Oxytetracycline Removal by Biological/Chemical Activated Mesoporous Carbon. Microporous Mesoporous Mater. 2021, 327, 111384. [Google Scholar] [CrossRef]
- Henríquez, P.; Kaiser, M.; Bohle, H.; Bustos, P.; Mancilla, M. Comprehensive Antibiotic Susceptibility Profiling of Chilean Piscirickettsia Salmonis Field Isolates. J. Fish Dis. 2016, 39, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.; Hernandez, N.; Osses, A.; Castillo, A.; Cancino, A.; Grothusen, H.; Navas, E.; Henriquez, P.; Bohle, H.; Bustamante, F.; et al. Prevalence, Geographic Distribution and Phenotypic Differences of Piscirickettsia Salmonis EM-90-like Isolates. J. Fish Dis. 2017, 40, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Lozano, I.; Díaz, N.F.; Muñoz, S.; Riquelme, C. Antibiotics in Chilean Aquaculture: A Review. In Antibiotic Use in Animals; InTech: Nappanee, IN, USA, 2018. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, M.; Zhang, Y.; Zhang, H.; Liu, J. Fabrication of a Novel “Loose” Nanofiltration Membrane by Facile Blending with Chitosan–Montmorillonite Nanosheets for Dyes Purification. Chem. Eng. J. 2015, 265, 184–193. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Nunes, A.V.M.; Barot, A.R.; Fernández-Castané, A.; Visak, Z.P.; Kiatkittipong, W.; Najdanovic-Visak, V. Extraction of Antibiotics Using Aqueous Two-Phase Systems Based on Ethyl Lactate and Thiosulphate Salts. Fluid Phase Equilibria 2021, 539, 113022. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar-Ayala, C.A. Emerging Contaminants and Their Removal from Aqueous Media Using Conventional/Non-Conventional Adsorbents: A Glance at the Relationship between Materials, Processes, and Technologies. Water 2023, 15, 1626. [Google Scholar] [CrossRef]
- Sengupta, A.; Jebur, M.; Kamaz, M.; Wickramasinghe, S.R. Removal of Emerging Contaminants from Wastewater Streams Using Membrane Bioreactors: A Review. Membranes 2021, 12, 60. [Google Scholar] [CrossRef]
- Sánchez, J.; Rivas, B.L. Liquid-phase polymer-based retention to remove arsenic from water. J. Chil. Chem. Soc. 2019, 64, 4513–4522. [Google Scholar] [CrossRef]
- Urbano, B.F.; Bustamante, S.; Palacio, D.A.; Vera, M.; Rivas, B.L. Polymer Supports for the Removal and Degradation of Hazardous Organic Pollutants: An Overview. Polym. Int. 2020, 69, 333–345. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Wang, R. The Coming of Age of Water Channels for Separation Membranes: From Biological to Biomimetic to Synthetic. Chem. Soc. Rev. 2022, 51, 4537–4582. [Google Scholar] [CrossRef]
- Madhura, L.; Kanchi, S.; Sabela, M.I.; Singh, S.; Bisetty, K.; Inamuddin. Membrane Technology for Water Purification. Environ. Chem. Lett. 2018, 16, 343–365. [Google Scholar] [CrossRef]
- Prapulla, S.G.; Karanth, N.G. FERMENTATION (INDUSTRIAL) | Recovery of Metabolites. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 822–833. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Z. Fe-Based Catalysts for Heterogeneous Catalytic Ozonation of Emerging Contaminants in Water and Wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Xie, Y.; Kong, F.; Mi, Z.; Huang, H.; Xia, C.; Ma, Z.; Li, S.; Zhang, Q.; Meng, Z. High-Efficiency Removal of Antibiotics through Self-Assembly Formation of Layered Double Hydroxides in Wastewater. J. Water Process Eng. 2023, 52, 103502. [Google Scholar] [CrossRef]
- Tessier, L.; Bouchard, P.; Rahni, M. Separation and Purification of Benzylpenicillin Produced by Fermentation Using Coupled Ultrafiltration and Nanofiltration Technologies. J. Biotechnol. 2005, 116, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; Teva, F. Removal of Emerging Contaminants from Secondary Effluents by Micellar-Enhanced Ultrafiltration. Sep. Purif. Technol. 2017, 181, 123–131. [Google Scholar] [CrossRef]
- Li, N.; Huo, L.; Shen, W.; Qiang, C.; Wu, M.; Sun, G.; Li, Q.; Shi, M.; Ma, J. Porous Organic Polymers for Superefficient Removal of Pollutants from Water: Design, Synthesis and Adsorption Performance. J. Clean. Prod. 2023, 396, 136558. [Google Scholar] [CrossRef]
- Jiang, D.; Song, X.; Zhang, H.; Yuan, M. Removal of Organic Pollutants with Polylactic Acid-Based Nanofiber Composites. Polymers 2022, 14, 4622. [Google Scholar] [CrossRef] [PubMed]
- Mosavi, S.S.; Zare, E.N.; Behniafar, H.; Tajbakhsh, M. Removal of Amoxicillin Antibiotic from Polluted Water by a Magnetic Bionanocomposite Based on Carboxymethyl Tragacanth Gum-Grafted-Polyaniline. Water 2023, 15, 202. [Google Scholar] [CrossRef]
- Leal, J.F.; Santos, E.B.H.; Esteves, V.I. Oxytetracycline in Intensive Aquaculture: Water Quality during and after Its Administration, Environmental Fate, Toxicity and Bacterial Resistance. Rev. Aquac. 2019, 11, 1176–1194. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, J.-M.; Lee, C.-G.; Park, S.-J.; Jho, E.H. Photodegradation Behavior of Agricultural Antibiotic Oxytetracycline in Water. Water 2022, 14, 3379. [Google Scholar] [CrossRef]
- Lundström, S.V.; Östman, M.; Bengtsson-Palme, J.; Rutgersson, C.; Thoudal, M.; Sircar, T.; Blanck, H.; Eriksson, K.M.; Tysklind, M.; Flach, C.-F.; et al. Minimal Selective Concentrations of Tetracycline in Complex Aquatic Bacterial Biofilms. Sci. Total Environ. 2016, 553, 587–595. [Google Scholar] [CrossRef]
- Al-Mohaimeed, A.M.; Abbasi, A.M.; Ali, M.A.; Dhas, D.S.D. Reduction of Multiple Antibiotics from the Waste Water Using Coated Glutathione S-Transferase Producing Biocatalyst. Env. Res 2022, 206, 112262. [Google Scholar] [CrossRef] [PubMed]
- Palacio, D.A.; Urbano, B.F.; Rivas, B.L. Application of Nanocomposite Polyelectrolytes for the Removal of Antibiotics as Emerging Pollutants in Water. J. Water Process Eng. 2022, 46, 102582. [Google Scholar] [CrossRef]
- Oyarce, E.; Santander, P.; Butter, B.; Pizarro, G.D.C.; Sánchez, J. Use of Sodium Alginate Biopolymer as an Extracting Agent of Methylene Blue in the Polymer-enhanced Ultrafiltration Technique. J. Appl. Polym. Sci. 2021, 138, 50844. [Google Scholar] [CrossRef]
- Sánchez, J.; Rivas, B.L. Cationic Hydrophilic Polymers Coupled to Ultrafiltration Membranes to Remove Chromium (VI) from Aqueous Solution. Desalination 2011, 279, 338–343. [Google Scholar] [CrossRef]
- Palacio, D.A.; Vásquez, V.; Rivas, B.L. N-Alkylated Chitosan Coupled to the Liquid-Phase Polymer-Based Retention (LPR) Technique to Remove Arsenic (V) from Aqueous Systems. J. Hazard. Mater. 2020, 400, 123216. [Google Scholar] [CrossRef] [PubMed]
- Palacio, D.A.; Urbano, B.F.; Rivas, B.L. Polyelectrolyte Nanocomposite Hydrogels Filled with Cationic and Anionic Clays. Carbohydr. Polym. 2020, 232, 115824. [Google Scholar] [CrossRef]
- Çelik, M.; Aydin, E.; Celik, A.; Gürtekin, E. Degradation of Oxytetracycline and Chlortetracycline by Fenton Process. Iğdır Üniversitesi Fen Bilimleri Enstitüsü Dergisi 2023, 13, 192–199. [Google Scholar] [CrossRef]
- He, T.; Pan, X.; Zhou, W.; Ding, H.; Liu, M.; Xiang, M.; Lou, Q.; Han, L.; Zhang, Y.; Wu, Y.; et al. Construction of High-Content α-Iron on Zero-Valent Iron @ Biochar Composite for the Ultra-Efficient Removal of Oxytetracycline Hydrochloride: A Key Step of Ammonium Bicarbonate Pretreatment. Sep. Purif. Technol. 2023, 323, 124378. [Google Scholar] [CrossRef]
- Li, Y.; Deng, M.; Wang, X.; Wang, Y.; Li, J.; Xia, S.; Zhao, J. In-Situ Remediation of Oxytetracycline and Cr(VI) Co-Contaminated Soil and Groundwater by Using Blast Furnace Slag-Supported Nanosized Fe0/FeSx. Chem. Eng. J. 2021, 412, 128706. [Google Scholar] [CrossRef]
- Leal, J.F.; Esteves, V.I.; Santos, E.B.H. Use of Sunlight to Degrade Oxytetracycline in Marine Aquaculture’s Waters. Environ. Pollut. 2016, 213, 932–939. [Google Scholar] [CrossRef]
- Harja, M.; Ciobanu, G. Removal of Oxytetracycline from Aqueous Solutions by Hydroxyapatite as a Low-Cost Adsorbent. E3S Web Conf. 2017, 22, 00062. [Google Scholar] [CrossRef]
- Lye, J.W.P.; Saman, N.; Sharuddin, S.S.N.; Othman, N.S.; Mohtar, S.S.; Md Noor, A.M.; Buhari, J.; Cheu, S.C.; Kong, H.; Mat, H. Removal Performance of Tetracycline and Oxytetracycline From Aqueous Solution Via Natural Zeolites: An Equilibrium and Kinetic Study. CLEAN-Soil Air Water 2017, 45, 1600260. [Google Scholar] [CrossRef]
- Liang, G.; Wang, Z.; Yang, X.; Qin, T.; Xie, X.; Zhao, J.; Li, S. Efficient Removal of Oxytetracycline from Aqueous Solution Using Magnetic Montmorillonite-Biochar Composite Prepared by One Step Pyrolysis. Sci. Total Environ. 2019, 695, 133800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, Z.; Hu, Y.; Lv, S.; Fan, H.; Zeng, Y.; Hu, J.; Wang, M. Removal of Tetracycline and Oxytetracycline from Water by Magnetic Fe3O4@graphene. Environ. Sci. Pollut. Res. 2017, 24, 2987–2995. [Google Scholar] [CrossRef]
- Solis, F.J.; de la Cruz, M.O. Flexible Polymers Also Counterattract. Phys. Today 2001, 54, 71–72. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, S.; Wang, Y. Mechanism of Oxytetracycline Removal by Coconut Shell Biochar Loaded with Nano-Zero-Valent Iron. Int. J. Environ. Res. Public Health 2021, 18, 13107. [Google Scholar] [CrossRef]
- El Hadki, A.; Ulucan-Altuntas, K.; El Hadki, H.; Ustundag, C.B.; Kabbaj, O.K.; Dahchour, A.; Komiha, N.; Zrineh, A.; Debik, E. Removal of Oxytetracycline by Graphene Oxide and Boron-Doped Reduced Graphene Oxide: A Combined Density Function Theory, Molecular Dynamics Simulation and Experimental Study. FlatChem 2021, 27, 100238. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Sadegh, N. Effective Removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from Aqueous Solutions Using Activated Carbon Nanoparticles Prepared from Vine Wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Mehdi, S.; Saeed, A.; Maleki, A.; Mansour, Z. Removal of chromium by using of adsorption onto strong base anion resin: Study of equilibrium and kinetic. J. Water Wastewater 2011, 22, 10–18. [Google Scholar]
- Hasan, I.; Ahamd, R. A Facile Synthesis of Poly (Methyl Methacrylate) Grafted Alginate@Cys-Bentonite Copolymer Hybrid Nanocomposite for Sequestration of Heavy Metals. Groundw. Sustain. Dev. 2019, 8, 82–92. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I. Retention of Metal Ions in Ultrafiltration of Mixtures of Divalent Metal Ions and Water-Soluble Polymers at Constant Ionic Strength Based on Freundlich and Langmuir Isotherms. J. Membr. Sci. 2003, 215, 195–202. [Google Scholar] [CrossRef]
- Oyarce, E.; Butter, B.; Santander, P.; Sánchez, J. Polyelectrolytes Applied to Remove Methylene Blue and Methyl Orange Dyes from Water via Polymer-Enhanced Ultrafiltration. J. Environ. Chem. Eng. 2021, 9, 106297. [Google Scholar] [CrossRef]
- Nasseh, N.; Barikbin, B.; Taghavi, L.; Nasseri, M.A. Adsorption of Metronidazole Antibiotic Using a New Magnetic Nanocomposite from Simulated Wastewater (Isotherm, Kinetic and Thermodynamic Studies). Compos. Part B Eng. 2019, 159, 146–156. [Google Scholar] [CrossRef]
- Chowdhury, A.; Kumari, S.; Khan, A.A.; Chandra, M.R.; Hussain, S. Activated Carbon Loaded with Ni-Co-S Nanoparticle for Superior Adsorption Capacity of Antibiotics and Dye from Wastewater: Kinetics and Isotherms. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125868. [Google Scholar] [CrossRef]
- Nayeri, D.; Mousavi, S.A.; Mehrabi, A. Oxytetracycline Removal from Aqueous Solutions Using Activated Carbon Prepared from Corn Stalks. J. Appl. Res. Water Wastewater 2019, 6, 67–72. [Google Scholar]
- Ramanayaka, S.; Sarkar, B.; Cooray, A.T.; Ok, Y.S.; Vithanage, M. Halloysite Nanoclay Supported Adsorptive Removal of Oxytetracycline Antibiotic from Aqueous Media. J. Hazard. Mater. 2020, 384, 121301. [Google Scholar] [CrossRef]
- Öztürk, D.; Mihçiokur, H. Production of Innovative Magnetic Adsorbent Fe3O4 @PEI®Tween 85 and Removal of Oxytetracycline from Aqueous Media. Sep. Sci. Technol. 2022, 57, 1030–1042. [Google Scholar] [CrossRef]
- Zafar, R.; Nabi, D.; Al-Huqail, A.A.; Jamil, U.; Khan, S.J.; Ahmed, Z.; Arshad, M. Efficient and Simultaneous Removal of Four Antibiotics with Silicone Polymer Adsorbent from Aqueous Solution. Emerg. Contam. 2023, 9, 100258. [Google Scholar] [CrossRef]
- Cha, B.; Kim, N.; Yea, Y.; Han, J.; Yoon, Y.; Kim, S.; Park, C.M. Comprehensive Evaluation of Antibiotic Tetracycline and Oxytetracycline Removal by Fe-Metal Organic Framework/Biopolymer-Clay Hydrogel. Ceram. Int. 2023, 49, 12201–12213. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption Characteristics of Molecular Oxytetracycline onto Alumina Particles: The Role of Surface Modification with an Anionic Surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Zaher, A.; Taha, M.; Farghali, A.A.; Mahmoud, R.K. Zn/Fe LDH as a Clay-like Adsorbent for the Removal of Oxytetracycline from Water: Combining Experimental Results and Molecular Simulations to Understand the Removal Mechanism. Environ. Sci. Pollut. Res. 2020, 27, 12256–12269. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Niu, G.; Hua, M.; Zhu, L.; Cui, P.; Li, X.; Chao, Y.; Zhu, W.; Liu, Z. Adsorptive Removal of Oxytetracycline in Wastewater by Cu/Al Doped Carbon Microspheres Prepared from Low-Molecular-Weight Chitosan. J. Environ. Chem. Eng. 2023, 11, 109496. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Torres, C.; González, F.; Shibue, T.; Nishide, H. Binding of Methylene Blue to Polyelectrolytes Containing Sulfonate Groups. Macromol. Chem. Phys. 2009, 210, 1167–1175. [Google Scholar] [CrossRef]
| Material | Retention Capacity of OXT (mg g−1) | Removal Efficiency (%) | Reference |
|---|---|---|---|
| Activated Carbon | 522.6 | 99.9 | [57] |
| Halloysite nanoclay | 52.4 | 68 | [58] |
| Iron oxide (Fe3O4) modified with polyethylenimine (PEI) and coated with polyoxyethylene sorbitan triolate (Tween 85). | 277.8 | 98 | [59] |
| Silicone-based polydimethylsiloxane (PDMS) | 8.38 | 99.58 | [60] |
| Fe-MetalOrganic Framework/Biopolymer-Clay Hydrogel. | 26.14 | 94 | [61] |
| Alumina modified with the surfactant sodium dodecyl sulfate (SDS) (SMA). | 143 | 97 | [62] |
| Zn/Fe double layer hydroxide hydroxide (LDH). | 90.3 | 77.23 | [63] |
| Cu/Al-doped nitrogen-containing carbon microspheres prepared from chitosan. | 1727.65 | 92.25 | [64] |
| Polycation-HPE | 1273.0 | 80 | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacio, D.A.; Oñate, P.; Esquivel, S.; Meléndrez, M.; Pereira, E.; Rivas, B.L. Study of the Efficiency of a Polycation Using the Diafiltration Technique in the Removal of the Antibiotic Oxytetracycline Used in Aquaculture. Membranes 2023, 13, 828. https://doi.org/10.3390/membranes13100828
Palacio DA, Oñate P, Esquivel S, Meléndrez M, Pereira E, Rivas BL. Study of the Efficiency of a Polycation Using the Diafiltration Technique in the Removal of the Antibiotic Oxytetracycline Used in Aquaculture. Membranes. 2023; 13(10):828. https://doi.org/10.3390/membranes13100828
Chicago/Turabian StylePalacio, Daniel A., Pablo Oñate, Samir Esquivel, Manuel Meléndrez, Eduardo Pereira, and Bernabé L. Rivas. 2023. "Study of the Efficiency of a Polycation Using the Diafiltration Technique in the Removal of the Antibiotic Oxytetracycline Used in Aquaculture" Membranes 13, no. 10: 828. https://doi.org/10.3390/membranes13100828
APA StylePalacio, D. A., Oñate, P., Esquivel, S., Meléndrez, M., Pereira, E., & Rivas, B. L. (2023). Study of the Efficiency of a Polycation Using the Diafiltration Technique in the Removal of the Antibiotic Oxytetracycline Used in Aquaculture. Membranes, 13(10), 828. https://doi.org/10.3390/membranes13100828







