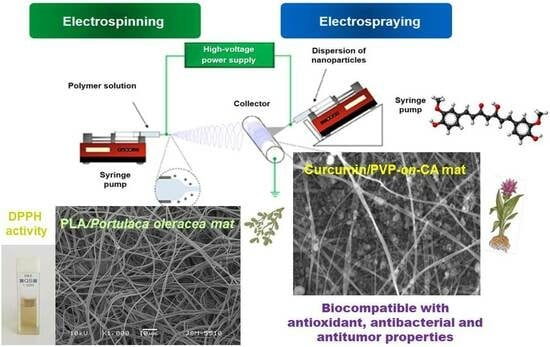
Innovative Bioactive Nanofibrous Materials Combining Medicinal and Aromatic Plant Extracts and Electrospinning Method
Abstract
:1. Introduction
2. Plant Extracts (Ple) as Medicinal Cures for Prevalent Diseases
3. Biopolymers and Natural Polymers Used as Carriers of Plant Extracts
- (1)
- Polymers that were directly extracted or separated from biomass, like starch, cellulose, arabinoxylan, and lignin;
- (2)
- Polymers that were synthesized from bio-derived monomers like polylactic acid (PLA) and cellulose acetate (CA);
- (3)
- Polymers that were produced by microorganisms such as polyhydroxyalkanoates (PHAs) and polysaccharides [51].
4. Electrospinning Method
4.1. Schematic Representation of the Electrospinning/Electrospraying Set-Up
4.2. Factors Influencing the Process
5. Plant Extracts Incorporated by Electrospinning
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrovska, B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yap, C.; He, J.; Chen, C.; Wong, S.; Li, X. Electrospinning: A Facile Technique for Fabricating Functional Nanofibers for Environmental Applications. Nanotechnol. Rev. 2016, 5, 51–73. [Google Scholar] [CrossRef]
- Aghababaei, F.; McClements, D.J.; Martinez, M.M.; Hadidi, M. Electrospun Plant Protein-Based Nanofibers in Food Packaging. Food Chem. 2024, 432, 137236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Z.; Low, S.C.; Xu, Z.; Tan, S.H. Electrospinning Technique Meets Solar Energy: Electrospun Nanofiber-Based Evaporation Systems for Solar Steam Generation. Adv. Fiber Mater. 2023, 5, 1318–1348. [Google Scholar] [CrossRef]
- Tipduangta, P.; Watcharathirawongs, W.; Waritdecha, P.; Sirithunyalug, B.; Leelapornpisid, P.; Chaiyana, W.; Goh, C.F. Electrospun Cellulose Acetate/Polyvinylpyrrolidone Fiber Mats as Potential Cosmetic Under-Eye Masks for Caffeine Delivery. J. Drug Deliv. Sci. Technol. 2023, 86, 104732. [Google Scholar] [CrossRef]
- Passaro, J.; Imparato, C.; Parida, D.; Bifulco, A.; Branda, F.; Aronne, A. Electrospinning of PVP-Based Ternary Composites Containing SiO2 Nanoparticles and Hybrid TiO2 Microparticles with Adsorbed Superoxide Radicals. Compos. B Eng. 2022, 238, 109874. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Wang, R.; Che, Y.; Han, C.; Feng, W.; Wang, C.; Zhao, W. Electrospun Nanofiber/Hydrogel Composite Materials and Their Tissue Engineering Applications. J. Mater. Sci. Technol. 2023, 162, 151–178. [Google Scholar] [CrossRef]
- Wei, M.; Pan, X.; Rong, L.; Dong, A.; He, Y.; Song, X.; Li, J. Polymer Carriers for Controlled Fragrance Release. Mater. Res. Express. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Fox, M.; Szoka, F.; Fréchet, J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef]
- Toncheva, A.; Spasova, M.; Paneva, D.; Manolova, N.; Rashkov, I. Drug-Loaded Electrospun Polylactide Bundles. J. Bioact. Compat. Polym. 2011, 26, 161–172. [Google Scholar] [CrossRef]
- Spasova, M.; Stoyanova, N.; Nachev, N.; Ignatova, M.; Manolova, N.; Rashkov, I.; Georgieva, A.; Toshkova, R.; Markova, N. Innovative Fibrous Materials Loaded with 5-Nitro-8-hydroxyquinoline via Electrospinning/Electrospraying Demonstrate Antioxidant, Antimicrobial and Anticancer Activities. Antioxidants 2023, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.; Afridi, S.; Shinwari, Z. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Luengo-Fernandez, R.; Leal, J.; Gray, A.; Sullivan, R. Economic Burden of Cancer Across the European Union: A Population-Based Cost Analysis. Lancet Oncol. 2013, 14, 1165–1174. [Google Scholar] [CrossRef]
- Jha, V.; Al-Ghamdi, S.; Li, G.; Wu, M.-S.; Stafylas, P.; Retat, L.; Card-Gowers, J.; Barone, S.; Cabrera, C.; Garcia Sanchez, J. Global Economic Burden Associated with Chronic Kidney Disease: A Pragmatic Review of Medical Costs for the Inside CKD Research Programme. Adv. Ther. 2023, 40, 4405–4420. [Google Scholar] [CrossRef]
- Srinivasan, D.; Nathan, S.; Suresh, T.; Perumalsamy, P. Antimicrobial Activity of Certain Indian Medicinal Plants used In Folkloric Medicine. J. Ethnopharmacol. 2001, 74, 217–220. [Google Scholar] [CrossRef]
- El Maaiden, E.; Bouzroud, S.; Nasser, B.; Moustaid, K.; El Mouttaqi, A.; Ibourki, M.; Boukcim, H.; Hirich, A.; Kouisni, L.; El Kharrassi, Y. A Comparative Study between Conventional and Advanced Extraction Techniques: Pharmaceutical and Cosmetic Properties of Plant Extracts. Molecules 2022, 27, 2074. [Google Scholar] [CrossRef]
- Marsoul, A.; Ijjaali, M.; Oumous, I.; Bennani, B.; Boukir, A. Determination of Polyphenol Contents in Papaver rhoeas L. Flowers Extracts (Soxhlet, Maceration), Antioxidant and Antibacterial Evaluation. Mater. Today Proc. 2020, 31, S183–S189. [Google Scholar] [CrossRef]
- Shirsath, S.; Sable, S.; Gaikwad, S.; Sonawane, S.H.; Saini, D.; Gogate, P. Intensification of Extraction of Curcumin from Curcuma amada Using Ultrasound Assisted Approach: Effect of Different Operating Parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef]
- Bachtler, S.; Bart, H.-J. Increase the Yield of Bioactive Compounds from Elder Bark and Annatto Seeds Using Ultrasound and Microwave Assisted Extraction Technologies. Food. Bioprod. Process. 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Bajpai, V.; Majumder, R.; Park, J. Isolation and Purification of Plant Secondary Metabolites Using Column-Chromatographic Technique. Bangladesh J. Pharmacol. 2016, 11, 844–848. [Google Scholar] [CrossRef]
- Ali, K.; Ali, A.; Khan, M.N.; Rahman, S.; Faizi, S.; Ali, M.; Khalifa, S.; El-Seedi, H.; Musharraf, S. Rapid Identification of Common Secondary Metabolites of Medicinal Herbs Using High-Performance Liquid Chromatography with Evaporative Light Scattering Detector in Extracts. Metabolites 2021, 11, 489. [Google Scholar] [CrossRef]
- Hassaan, Y.; Handoussa, H.; El-Khatib, A.; Linscheid, M.; El Sayed, N. Evaluation of Plant Phenolic Metabolites as a Source of Alzheimer’s Drug Leads. BioMed Res. Int. 2014, 2014, 843263. [Google Scholar] [CrossRef]
- Rahmati, B.; Kiasalari, Z.; Roghani, M.; Khalili, M.; Ansari, F. Antidepressant and Anxiolytic Activity of Lavandula officinalis Aerial Parts Hydroalcoholic Extract in Scopolamine-Treated Rats. Pharm. Biol. 2017, 55, 958–965. [Google Scholar] [CrossRef]
- Saleem, U.; Hussain, L.; Shahid, F.; Anwar, F.; Chauhdary, Z.; Zafar, A. Pharmacological Potential of the Standardized Methanolic Extract of Prunus armeniaca L. in the Haloperidol-Induced Parkinsonism Rat Model. Evid. Based Complement. Alternat. Med. 2022, 2022, 3697522. [Google Scholar] [CrossRef]
- Cao, Y.-L.; Lin, J.-H.; Hammes, H.-P.; Zhang, C. Flavonoids in Treatment of Chronic Kidney Disease. Molecules 2022, 27, 2365. [Google Scholar] [CrossRef]
- Khan, M.; Kassianos, A.; Hoy, W.; Alam, K.; Healy, H.; Gobe, G. Promoting Plant-Based Therapies for Chronic Kidney Disease. J. Evid. Based Integr. Med. 2022, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liwa, A.; Jaka, H. Renal Diseases and Use of Medicinal Herbal Extracts: A Concise Update of Reported Literature in Africa. J. Nephrol. Renal Ther. 2016, 2, 008. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Modaeinama, S.; Abasi, M.; Abbasi, M.; Jahanban-Esfahlan, R. Anti Proliferative Properties of Melissa officinalis in Different Human Cancer Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5703–5707. [Google Scholar] [CrossRef]
- Gull, N.; Arshad, F.; Naikoo, G.; Hassan, I.; Pedram, M.; Ahmad, A.; Aljabali, A.; Mishra, V.; Satija, S.; Charbe, N.; et al. Recent Advances in Anticancer Activity of Novel Plant Extracts and Compounds from Curcuma longa in Hepatocellular Carcinoma. J. Gastrointest. Cancer 2022, 54, 368–390. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Souza, P.; Rosenthal, A.; Ayres, E.; Teodoro, A. Potential Functional Food Products and Molecular Mechanisms of Portulaca oleracea L. on Anticancer Activity: A Review. Oxid. Med. Cell. Longev. 2022, 2022, 7235412. [Google Scholar] [CrossRef]
- Roozbeh, N.; Darvish, L.; Abdi, F. Hypoglycemic Effects of Acacia nilotica in Type Ii Diabetes: A Research Proposal. BMC Res. Notes 2017, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Mukundi, M.J.; Piero, N.M.; Mwaniki, N.E.N.; Murugi, N.J.; Daniel, A.S.; Peter, G.K.; Alice, M.N. Antidiabetic Effects of Aqueous Leaf Extracts of Acacia niloticain Alloxan Induced Diabetic Mice. J. Diabetes Metab. 2015, 6, 658–664. [Google Scholar] [CrossRef]
- Dulić, M.; Ciganović, P.; Vujić, L.; Zovko Končić, M. Antidiabetic and Cosmeceutical Potential of Common Barbery (Berberis vulgaris L.) Root Bark Extracts Obtained by Optimization of ‘Green’ Ultrasound-Assisted Extraction. Molecules 2019, 24, 3613. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Hussain, S.; Mahwi, T.; Ahmed, Z.; Rahman, H.; Rasedee, A. The Efficacy and Safety of Ginkgo Biloba Extract as an Adjuvant in Type 2 Diabetes Mellitus Patients Ineffectively Managed with Metformin: A Double-Blind, Randomized, Placebo-Controlled Trial. Drug Des. Devel. Ther. 2018, 12, 735–742. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.; Oprea, O.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Asadi, A.; Shidfar, F.; Safari, M.; Hosseini, A.; Huseini, H.; Heidari, I.; Rajab, A. Efficacy of Melissa officinalis L. (Lemon Balm) Extract on Glycemic Control and Cardiovascular Risk Factors in Individuals with Type 2 Diabetes: A Randomized, Double-Blind, Clinical Trial. Phytother. Res. 2019, 33, 651–659. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Xiao, W.; Zhu, J.-B. Investigation on the Active Ingredient and Mechanism of Cannabis sativa L. For Treating Epilepsy Based on Network Pharmacology. Biotechnol. Biotechnol. Equip. 2021, 35, 994–1009. [Google Scholar] [CrossRef]
- Jalali, J.; Rahbardar, M. Ameliorative Effects of Portulaca oleracea L. (Purslane) and Its Active Constituents on Nervous System Disorders: A Review. Iran. J. Basic. Med. Sci. 2023, 26, 2–12. [Google Scholar] [CrossRef]
- Kapoor, L. Handbook of Ayurvedic Medicinal Plants: Herbal Reference Library, 1st ed.; Routledge: Oxfordshire, UK, 2017. [Google Scholar] [CrossRef]
- Boskabady, M.; Shakeri, F.; Naghdi, F. Studies in Natural Products Chemistry Chapter 7—The Effects of Curcuma longa L. Its Constituents in Respiratory Disorders and Molecular Mechanisms of Their Action. In Bioactive Natural Products Editor: Atta-ur-Rahman; In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 65, pp. 239–269. [Google Scholar] [CrossRef]
- Londhe, V.; Gavasane, A.; Nipate, S.; Bandawane, D.; Chaudhari, P. Role of Garlic (Allium sativum) In Various Diseases: An Overview. Angiogenesis 2011, 12, 129–134. [Google Scholar]
- Sanna, C.; Marengo, A.; Acquadro, S.; Caredda, A.; Lai, R.; Corona, A.; Tramontano, E.; Rubiolo, P.; Esposito, F. In Vitro Anti-HIV-1 Reverse Transcriptase and Integrase Properties of Punica granatum L. Leaves, Bark, and Peel Extracts and Their Main Compounds. Plants 2021, 10, 2124. [Google Scholar] [CrossRef]
- Mahdavi, M.A.; Seyedsadjadi, N.; Javadivala, Z. Potential Effects of Pomegranate (Punica granatum) On Rheumatoid Arthritis: A Systematic Review. Int. J. Clin. Pract. 2021, 75, e13999. [Google Scholar] [CrossRef] [PubMed]
- Palshetkar, A.; Pathare, N.; Jadhav, N.; Pawar, M.; Wadhwani, A.; Kulkarni, S.; Singh, K. In Vitro Anti-HIV Activity of Some Indian Medicinal Plant Extracts. BMC Complement. Med. Ther. 2020, 20, 69. [Google Scholar] [CrossRef]
- Kumar, R.; Nair, V.; Singh, S.; Gupta, Y. In Vivo Antiarthritic Activity of Rosa centifolia L. Flower Extract. Ayu 2015, 36, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Mohan, O.; Oluwafemi, S.; Kalarikkal, N.; Thomas, S.; Songca, S.P. Biopolymers—Application in Nanoscience and Nanotechnology. In Recent Advances in Biopolymers; IntecOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Lisitsyn, A.; Semenova, A.; Nasonova, V.; Polishchuk, E.; Revutskaya, N.; Kozyrev, I.; Kotenkova, E. Approaches in Animal Proteins and Natural Polysaccharides Application for Food Packaging: Edible Film Production and Quality Estimation. Polymers 2021, 13, 1592. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.; Manan, S.; Ullah, M.; Ul-Islam, M. Plant Extract-Loaded Bacterial Cellulose Composite Membrane for Potential Biomedical Applications. J. Bioresour. Bioprod. 2021, 6, 6–32. [Google Scholar] [CrossRef]
- Rajeswari, A.; Christy, J.; Swathi, E.; Pius, A. Fabrication of Improved Cellulose Acetate-Based Biodegradable Films for Food Packaging Applications. Environ. Toxicol. Chem. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Hassanloofard, Z.; Gharekhani, M.; Zandi, M.; Ganjloo, A.; Roufegarinejad, L. Fabrication and Characterization of Cellulose Acetate Film Containing Falcaria vulgaris Extracta. Cellulose 2023, 30, 6833–6853. [Google Scholar] [CrossRef]
- Gradinaru, L.; Barbalata-Mandru, M.; Enache, A.; Rimbu, C.; Badea, G.; Aflori, M. Chitosan Membranes Containing Plant Extracts: Preparation, Characterization and Antimicrobial Properties. Int. J. Mol. Sci. 2023, 24, 8673. [Google Scholar] [CrossRef] [PubMed]
- Benković, M.; Sarić, I.; Jurinjak Tušek, A.; Jurina, T.; Gajdoš Kljusurić, J.; Valinger, D. Analysis of the Adsorption and Release Processes of Bioactives from Lamiaceae Plant Extracts on Alginate Microbeads. Food Bioprocess. Technol. 2021, 14, 1216–1230. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant Extract Synthesized PLA Nanoparticles for Controlled and Sustained Release of Quercetin: A Green Approach. PLoS ONE 2012, 7, e41230. [Google Scholar] [CrossRef]
- Lahmar, A.; Rjab, M.; Sioud, F.; Selmi, M.; Salek, A.; Kilani-Jaziri, S.; Ghedira, L.C. Design of 3D Hybrid Plant Extract/Marine and Bovine Collagen Matrixes as Potential Dermal Scaffolds for Skin Wound Healing. Sci. World J. 2022, 2022, 8788061. [Google Scholar] [CrossRef] [PubMed]
- Gavan, A.; Colobatiu, L.; Hanganu, D.; Bogdan, C.; Olah, N.K.; Achim, M.; Mirel, S. Development and Evaluation of Hydrogel Wound Dressings Loaded with Herbal Extracts. Processes 2022, 10, 242. [Google Scholar] [CrossRef]
- Wang, W.-C.; Cheng, Y.-T.; Estroff, B. Electrostatic Self-Assembly of Composite Nanofiber Yarn. Polymers 2021, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Wei, J.; Liang, W.; Zhang, J. Preparation of Mechanically Stable Superamphiphobic Coatings via Combining Phase Separation of Adhesive and Fluorinated SiO2 for Anti-Icing. Nanomaterials 2023, 13, 1872. [Google Scholar] [CrossRef]
- Yadavalli, N.; Asheghali, D.; Tokarev, A.; Zhang, W.; Xie, J.; Minko, S. Gravity Drawing of Micro- and Nanofibers for Additive Manufacturing of Well-Organized 3D-Nanostructured Scaffolds. Small 2020, 16, 1907422. [Google Scholar] [CrossRef]
- Botta, L.; Teresi, R.; Titone, V.; Salvaggio, G.; La Mantia, F.; Lopresti, F. Use of Biochar as Filler for Biocomposite Blown Films: Structure-Processing-Properties Relationships. Polymers 2021, 13, 3953. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Islam, S.; Ang, B.; Andriyana, A.; Afifi, A. A Review on Fabrication of Nanofibers via Electrospinning and Their Applications. SN Appl. Sci. 2019, 1, 1248–1263. [Google Scholar] [CrossRef]
- Reneker, D.; Yarin, A. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Bhushani, J.; Anandharamakrishnan, C. Electrospinning and Electrospraying Techniques: Potential Food Based Applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Fokin, N.; Grothe, T.; Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Döpke, C.; Blachowicz, T.; Hütten, A.; Ehrmann, A. Magnetic Properties of Electrospun Magnetic Nanofiber Mats after Stabilization and Carbonization. Materials 2020, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Rashkov, I.; Manolova, N. Drug-Loaded Electrospun Materials in Wound-Dressing Applications and in Local Cancer Treatment. Expert. Opin. Drug. Deliv. 2013, 10, 469–483. [Google Scholar] [CrossRef]
- Ignatova, M.; Nachev, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun 5-Chloro-7-iodo-8-hydroxyquinoline (Clioquinol)-Containing Poly(3-hydroxybutyrate)/Polyvinylpyrrolidone Antifungal Materials Prospective as Active Dressings against Esca. Polymers 2022, 14, 367. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Polymer Fibers with Magnetic Core Decorated with Titanium Dioxide Prospective for Photocatalytic Water Treatment. J. Environ. Chem. Eng. 2018, 6, 2075–2084. [Google Scholar] [CrossRef]
- Shenoy, S.; Bates, W.; Frisch, H.; Wnek, G. Role of Chain Entanglements on Fiber Formation During Electrospinning of Polymer Solutions: Good Solvent, Non-specific Polymer–Polymer Interaction Limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of Chitosan Containing Nanofibers by Electrospinning Chitosan/Poly(Ethylene Oxide) Mixed Solutions. e-Polymers 2004, 056, 1–12. [Google Scholar] [CrossRef]
- Stoyanova, N.; Paneva, D.; Mincheva, R.; Toncheva, A.; Manolova, N.; Dubois, P.; Rashkov, I. Poly(L-Lactide) And Poly(Butylene Succinate) Immiscible Blends: From Electrospinning to Biologically Active Materials. Mater. Sci. Eng. C 2014, 41, 119–126. [Google Scholar] [CrossRef]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of Polymer Concentration on the Morphology and Mechanical Characteristics of Electrospun Cellulose Acetate and Poly (Vinyl Chloride) Nanofiber Mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Abdulhamid, M.; Holtzl, T.; Szekely, G. Nanofiber Engineering of Microporous Polyimides Through Electrospinning: Influence of Electrospinning Parameters and Salt Addition. Mater. Des. 2021, 198, 109280. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Laudenslager, M.; Sigmund, W. Electrospinning. In Encyclopedia of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 769–777. [Google Scholar] [CrossRef]
- Angammana, C.; Jayaram, S. Fundamentals of Electrospinning and Processing Technologies. Part. Sci. Technol. 2015, 34, 72–82. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.; Chase, D.; Rabolt, J. Micro- And Nanostructured Surface Morphology on Electrospun Polymer Fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Ghim, H.; Kim, S.; Chun, D.; Kim, H.; Lyoo, W. Role of Molecular Weight of Atactic Poly(Vinyl Alcohol) (PVA) In the Structure and Properties of PVA Nanofabric Prepared by Electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646. [Google Scholar] [CrossRef]
- Jabur, A.; Abbas, L.; Aldain, S. Effects of Ambient Temperature and Needle to Collector Distance on PVA Nanofibers Diameter Obtained From Electrospinning Technique. Eng. Technol. J. 2017, 35, 340–347. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Li, H.-P.; Yang, J.-H.; Wan, J.; Yu, D.-G. Influence of Working Temperature on The Formation of Electrospun Polymer Nanofibers. Nanoscale Res. Lett. 2017, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Um, I.C. Effect of Relative Humidity on the Electrospinning Performance of Regenerated Silk Solution. Polymers 2021, 13, 2479. [Google Scholar] [CrossRef]
- Zander, N. Hierarchically Structured Electrospun Fibers. Polymers 2013, 5, 19–44. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R. Nano Fibres by Electro Spinning, Properties and Applications. J. Text. Eng. Fash. Technol. 2017, 2, 486–497. [Google Scholar] [CrossRef]
- Subrahmanya, T.; Arshad, A.; Lin, P.; Widakdo, J.; Makari, H.; Austria, H.; Hu, C.-C.; Lai, J.-Y.; Hung, W.-S. A Review of Recent Progress in Polymeric Electrospun Nanofiber Membranes in Addressing Safe Water Global Issues. RSC Adv. 2021, 11, 9638–9663. [Google Scholar] [CrossRef]
- Tsekova, P.; Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun Curcumin-Loaded Cellulose Acetate/Polyvinylpyrrolidone Fibrous Materials with Complex Architecture and Antibacterial Activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun Cellulose Acetate Fiber Mats Containing Curcumin and Release Characteristic of the Herbal Substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Tsekova, P.; Spasova, M.; Manolova, N.; Rashkov, I.; Markova, N.; Georgieva, A.; Toshkova, R. Electrospun Cellulose Acetate Membranes Decorated with Curcumin-PVP Particles: Preparation, Antibacterial and Antitumor Activities. J. Mater. Sci. Mater. Med. 2018, 29, 9. [Google Scholar] [CrossRef]
- Sun, X.-Z.; Williams, G.; Hou, X.-X.; Zhu, L.-M. Electrospun Curcumin-Loaded Fibers with Potential Biomedical Applications. Carbohydr. Polym. 2013, 94, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.; Zaman, S.; Perveen, A.; Jahan, R.; Islam, F.; Arafat, M. Controlled Release of Curcumin from Electrospun Fiber Mats with Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Shahid, A.; Ali, A.; Uddin, N.; Miah, S.; Islam, S.; Mohebbullah, M.; Jamal, M. Antibacterial Wound Dressing Electrospun Nanofibrous Material from Polyvinyl Alcohol, Honey and Curcumin longa Extract. J. Ind. Text. 2021, 51, 455–469. [Google Scholar] [CrossRef]
- Gaydhane, M.; Kanuganti, J.; Sharma, C. Honey and Curcumin Loaded Multilayered Polyvinylalcohol/Cellulose Acetate Electrospun Nanofibrous Mat for Wound Healing. J. Mater. Res. 2020, 35, 600–609. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The Use of Electrospun Curcumin-Loaded Poly(L-Lactic Acid) Fiber Mats as Wound Dressing Materials. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Dhurai, B.; Saraswathy, N.; Maheswaran, R.; Sethupathi, P.; Vanitha, P.; Vigneshwaran, S.; Rameshbabu, V. Electrospinning of Curcumin Loaded Chitosan/Poly (Lactic Acid) Nanofilm and Evaluation of Its Medicinal Characteristics. Front. Mater. Sci. 2013, 7, 350–361. [Google Scholar] [CrossRef]
- Yakub, G.; Toncheva, A.; Manolova, N.; Rashkov, I.; Danchev, D.; Kussovski, V. Electrospun Polylactide-Based Materials for Curcumin Release: Photostability, Antimicrobial Activity, and Anticoagulant Effect. J. Appl. Polym. Sci. 2016, 133, 5738. [Google Scholar] [CrossRef]
- Yakub, G.; Toncheva, A.; Kussovski, V.; Toshkova, R.; Georgieva, A.; Nikolova, E.; Manolova, N.; Rashkov, I. Curcumin-PVP Loaded Electrospun Membranes with Conferred Antibacterial and Antitumoral Activities. Fibers Polym. 2020, 21, 55–65. [Google Scholar] [CrossRef]
- Yakub, G.; Manolova, N.; Rashkov, I.; Markova, N.; Toshkova, R.; Georgieva, A.; Mincheva, R.; Toncheva, A.; Raquez, J.-M.; Dubois, P. Pegylated Curcumin Derivative: Water-Soluble Conjugates with Antitumor and Antibacterial Activity. ACS Omega 2022, 41, 36403–36414. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, R.; Biazar, E.; Taleghani, A.; Keshel, S. Design of Curcumin-Loaded Electrospun Polyhydroxybutyrate Mat as a Wound Healing Material. Nano Biomed. Eng. 2020, 12, 14–20. [Google Scholar] [CrossRef]
- Papoti, V.T.; Totomis, N.; Atmatzidou, A.; Zinoviadou, K.; Androulaki, A.; Petridis, D.; Ritzoulis, C. Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes 2019, 7, 88. [Google Scholar] [CrossRef]
- Râpa, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.-D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.-M.; Predescu, C. Bioactive Collagen Hydrolysate-Chitosan/Essential Oil Electrospun Nanofibers Designed for Medical Wound Dressings. Pharmaceutics 2021, 13, 1939. [Google Scholar] [CrossRef]
- Stoyanova, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Kamenova-Nacheva, M.; Staleva, P.; Tavlinova-Kirilova, M. Electrospun PLA-Based Biomaterials Loaded with Melissa officinalis Extract with Strong Antioxidant Activity. Polymers 2023, 15, 1070. [Google Scholar] [CrossRef]
- de Oliveira, J.; Camargo, S.E.; de Oliveira, L. Rosmarinus officinalis L. (Rosemary) As Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Borges, R.; Ortiz, B.L.; Pereira, A.C.; Keita, H.; Carvalho, J.C. Rosmarinus officinalis Essential Oil: A Review of Its Phytochemistry, Anti-inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A Novel Nano-Encapsulation Approach for Bioactive Compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Saad, E.; El Gohary, N.; El-Shenawy, B.; Handoussa, H.; Klingner, A.; Elwi, M.; Hamed, Y.; Khalil, I.; El Nashar, R.; Mizaikoff, B. Fabrication of Magnetic Molecularly Imprinted Beaded Fibers for Rosmarinic Acid. Nanomaterials 2020, 10, 1478. [Google Scholar] [CrossRef]
- Vatankhah, E. Rosmarinic Acid-Loaded Electrospun Nanofibers: In Vitro Release Kinetic Study and Bioactivity Assessment. Eng. Life Sci. 2018, 18, 732–742. [Google Scholar] [CrossRef]
- Spasova-Todorova, M.; Stoyanova, N.; Stoilova, O. Composition of non-woven fabric (mat) containing rosmarinic acid. No 5817/10/10/2023.
- Azuka, O.; Mary, A.; Abu, O. A Review on Portulaca oleracea (Purslane) Plant- Its Nature and Biomedical Benefits. Int. J. Biomed. Res. 2014, 5, 75–80. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.; Mahyari, S.; Hassani, F.; Karimi, G. Review of Traditional Uses, Phytochemistry and Pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Stoyanova, N.; Manolova, N.; Rashkov, I.; Taneva, S.; Momchilova, S.; Georgieva, A. Facile Preparation of Novel Antioxidant Fibrous Material Based on Natural Plant Extract from Portulaca oleracea and PLA by Electrospinning for Biomedical Applications. Polym. Int. 2022, 71, 689–696. [Google Scholar] [CrossRef]
- Stoyanova, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Taneva, S.; Momchilova, S.; Georgieva, A. Physico-Chemical, Mechanical, and Biological Properties of Polylactide/Portulaca oleracea Extract Electrospun Fibers. Membranes 2023, 13, 298. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Double-Layer PLLA/PEO_chitosan Nanofibrous Mats Containing Hypericum perforatum L. As an Effective Approach for Wound Treatment. Polym. Adv. Technol. 2020, 32, 1493–1506. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Emulsion Electrospinning of PLLA/PVA/Chitosan with Hypericum perforatum L. as an Antibacterial Nanofibrous Wound Dressing. Gels 2023, 9, 353. [Google Scholar] [CrossRef]
- García-Hernández, A.; Morales-Sánchez, E.; Berdeja-Martínez, B.; Escamilla-García, M.; Salgado-Cruz, M.P.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Vega-Cuellar, M.; Calderón-Domínguez, G. PVA-Based Electrospun Biomembranes with Hydrolyzed Collagen and Ethanolic Extract of Hypericum perforatum for Potential Use as Wound Dressing: Fabrication and Characterization. Polymers 2022, 14, 1981. [Google Scholar] [CrossRef] [PubMed]
- Beran, M.; Horna, A.; Vorisek, V.; Berkova, E.; Korinkova, R.; Trousil, V.; Hrubanova, M. Antimicrobial Polyhydroxybutyrate Submicron Fiber Mat Loaded with Extract of Hypericum perforatum. J. Plant Biotechnol. 2022, 49, 257–270. [Google Scholar] [CrossRef]
- Shokrollahi, M.; Hajir Bahrami, S.; Haghbin Nazarpak, M.; Solouk, A. Multilayer Nanofibrous Patch Comprising Chamomile Loaded Carboxyethyl Chitosan/Poly(Vinyl Alcohol) And Polycaprolactone as a Potential Wound Dressing. Int. J. Biol. Macromol. 2020, 147, 547–559. [Google Scholar] [CrossRef]
- Nikbakht, M.; Salehi, M.; Rezayat, S.M.; Majidi, R.F. Various Parameters in the Preparation of Chitosan/Polyethylene Oxide Electrospun Nanofibers Containing Aloe Vera Extract for Medical Applications. Nanomed. J. 2020, 7, 21–28. [Google Scholar]
- Baghersad, S.; Bahrami, H.; Mohammadi, M.; Mojtahedi, M.; Milan, P. Development of Biodegradable Electrospun Gelatin/Aloe-Vera/Poly(ε-Caprolactone) Hybrid Nanofibrous Scaffold for Application as Skin Substitutes. Mater. Sci. Eng. C 2018, 93, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.; Villarreal, A.; Rodriguez, C.; De Leon, H.; Gilkerson, R.; Lozano, K. Aloe Vera Extract-Based Composite Nanofibers for Wound Dressing Applications. Mater. Sci. Eng. C 2021, 124, 112061. [Google Scholar] [CrossRef]
- Pathalamuthu, P.; Siddharthan, A.; Giridev, V.R.; Victoria, V.; Thangam, R.; Sivasubramanian, S.; Savariar, V.; Hemamalini, T. Enhanced Performance of Aloe Vera Incorporated Chitosan-Polyethylene Oxide Electrospun Wound Scaffold Produced Using Novel Spirograph Based Collector Assembly. Int. J. Biol. Macromol. 2019, 140, 808–824. [Google Scholar] [CrossRef]
- Kharat, Z.; Goushki, M.A.; Sarvian, N.; Asad, S.; Dehghan, M.M.; Kabiri, M. Chitosan/PEO Nanofibers Containing Calendula officinalis Extract: Preparation, Characterization, In Vitro and In Vivo Evaluation for Wound Healing Applications. Int. J. Pharm. 2021, 609, 121132. [Google Scholar] [CrossRef]
- Azizi, M.; Azimzadeh, M.; Afzali, M.; Alafzadeh, M.; Mirhosseini, S.H. Characterization and Optimization of Using Calendula officinalis Extract in Fabrication of Polycaprolactone-Gelatin Electrospun Nanofibers for Wound Dressing Applications. J. Adv. Mater. Process. 2018, 6, 34–46. [Google Scholar]
- Tahami, S.; Nemati, N.; Keshvari, H.; Khorasani, M. Effect of Electrical Potential on the Morphology of Polyvinyl Alcohol/Sodium Alginate Electrospun Nanofibers, Containing Herbal Extracts of Calendula officinalis for Using in Biomedical Applications. J. Mod. Process. Manuf. Prod. 2020, 9, 43–56. [Google Scholar]
- Hidalgo-Báez, D.; Ricardi, M.; Gaviria, J.; Estrada, J. Aportes a la Etnofarmacología de Los Páramos Venezolanos. Ciencia 1999, 7, 23–32. [Google Scholar]
- Alerico, G.C.; Beckenkamp, A.; Vignoli-Silva, M.; Buffon, A.; von Poser, G.L. Proliferative Effect of Plants Used for Wound Healing in Rio Grande Do Sul State, Brazil. J. Ethnopharmacol. 2015, 176, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Espiña, D.C.; Carvalho, F.B.; Zanini, D.; Schlemmer, J.B.; Coracini, J.D.; Rubin, M.A.; Morsch, V.M.; Schetinger, M.R.C.; Leal, D.B.R.; Baiotto, C.R.; et al. A More Accurate Profile of Achyrocline satureioides Hypocholesterolemic Activity. Cell Biochem. Funct. 2012, 30, 347–353. [Google Scholar] [CrossRef]
- Obulesu, M.; Rao, D.M. Effect of Plant Extracts on Alzheimer’s Disease: An Insight into Therapeutic Avenues. J. Neurosci. Rural. Pract. 2011, 2, 56–61. [Google Scholar] [CrossRef]
- Chiari, M.E.; Joray, M.B.; Ruiz, G.; Palacios, S.M.; Carpinella, M.C. Tyrosinase Inhibitory Activity of Native Plants from Central Argentina: Isolation of an Active Principle from Lithrea Molleoides. Food Chem. 2010, 120, 10–14. [Google Scholar] [CrossRef]
- Carpinella, M.C.; Andrione, D.G.; Ruiz, G.; Palacios, S.M. Screening for Acetylcholinesterase Inhibitory Activity in Plant Extracts from Argentina. Phytother. Res. 2009, 24, 259–263. [Google Scholar] [CrossRef]
- Lamichhane, G.; Pandey, J.; Devkota, H.P. Bioactive Chemical Constituents and Pharmacological Activities of Ponciri fructus. Molecules 2023, 28, 255. [Google Scholar] [CrossRef]
- Kuete, V.; Metuno, R.; Ngameni, B.; Tsafack, A.M.; Ngandeu, F.; Fotso, G.W.; Bezabih, M.; Etoa, F.X.; Ngadjui, B.T.; Abegaz, B.M.; et al. Antimicrobial Activity of the Methanolic Extracts and Compounds from Treculia obovoidea (Moraceae). J. Ethnopharmacol. 2007, 112, 531–536. [Google Scholar] [CrossRef]
- Raafat, B.M.; Alsanie, W.F.; Thobaity, A.A.; Alamri, A.S.; Elesawy, B.H.; Dahlawi, H. A Combined Protective Dose of Angelica archangelica and Ginkgo biloba Restores Normal Functional Hemoglobin Derivative Levels in Rabbits after Oxidative Stress Induced by Gallium-68. Appl. Sci. 2021, 11, 4804. [Google Scholar] [CrossRef]
- Fraternale, D.; Teodori, L.; Rudov, A.; Prattichizzo, F.; Olivieri, F.; Guidarelli, A.; Albertini, M.C. The in Vitro Activity of Angelica archangelica L. Essential Oil on Inflammation. J. Med. Food 2018, 21, 1238–1243. [Google Scholar] [CrossRef]
- Drever, B.D.; Anderson, W.G.; Riedel, G.; Kim, D.H.; Ryu, J.H.; Choi, D.Y.; Platt, B. The Seed Extract of Cassia obtusifolia Offers Neuroprotection to Mouse Hippocampal Cultures. J. Pharmacol. Sci. 2008, 107, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Jannat, S.; Jung, H.A.; Min, B.-S.; Paudel, P.; Choi, J.S. Hepatoprotective Effect of Cassia obtusifolia Seed Extract and Constituents Against Oxidative Damage Induced by Tert -Butyl Hydroperoxide in Human Hepatic HEPG2 Cells. J. Food Biochem. 2018, 42, e12439. [Google Scholar] [CrossRef]
- Kitanaka, S.; Takido, M. Studies on the Constituents in the Roots of Cassia obtusifolia L. and the Antimicrobial Activities of Constituents of the Roots and the Seeds. Yakugaku Zasshi 1986, 106, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K. An Ethnomedicinal, Phytochemical and Pharmacological Profile of Desmodium gangeticum (L.) DC. and Desmodium adscendens (Sw.) DC. J. Ethnopharmacol. 2011, 136, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Bora, M.; Mandal, S.; Singh, P.K.; Das, H.; Bora, G.K.; Bora, D.; Baruah, D.; Gautam, M.K. Phytochemical and Pharmacological Profile of Desmodium gangeticum (L.) DC.: A Comprehensive Review. Curr. Tradit. Med. 2024, 10, e230523217246. [Google Scholar] [CrossRef]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef]
- Ezema, C.A.; Ezeorba, T.P.C.; Aguchem, R.N.; Okagu, I.U. Therapeutic Benefits of Salvia Species: A Focus on Cancer and Viral Infection. Heliyon 2022, 8, e08763. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.C.S.S.B.; de Paula, T.; Gonçalves, J.P.; da Silva Soley, B.; Cretella, A.B.M.; Otuki, M.F.; Cabrini, D.A. The Oil from Moringa oleifera Seeds Accelerates Chronic Skin Wound Healing. Phytomed. Plus 2021, 1, 100099. [Google Scholar] [CrossRef]
- Mahadevan, S.; Park, Y. Multifaceted Therapeutic Benefits of Ginkgo biloba L.: Chemistry, Efficacy, Safety, and Uses. J. Food Sci. 2008, 73, R14–R19. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K. Composition, Biological Properties and Therapeutic Effects of Lavender (Lavandula angustifolia L). A Review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Seidel, V. Anticancer Potential and Other Pharmacological Properties of Prunus armeniaca L.: An Updated Overview. Plants 2022, 11, 1885. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.-M.; Liu, G.-Y.; Wei, B.-L.; Wang, Y.; Cai, H.-Y.; Li, F.-S.; Xu, Y.-L.; Zheng, S.-P.; Wang, G. Chinese Herbs in Treatment of Influenza: A Randomized, Double-Blind, Placebo-Controlled Trial. Respir. Med. 2010, 104, 1362–1369. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Swain, K.C. Traditional Uses and Medicinal Potential of Cordyceps sinensis of Sikkim. J. Ayurveda Integr. Med. 2011, 44, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Wu, Q.; Yang, Y.; Lv, H.; Liu, D.; Gan, Z.; Zeng, N. Traditional Uses, Chemical Constituents, Biological Properties, Clinical Settings, and Toxicities of Abelmoschus manihot L.: A Comprehensive Review. Front. Pharmacol. 2020, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 9, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Sonfack, C.S.; Nguelefack-Mbuyo, E.P.; Kojom, J.J.; Lappa, E.L.; Peyembouo, F.P.; Fofié, C.K.; Nolé, T.; Nguelefack, T.B.; Dongmo, A.B. The Aqueous Extract from the Stem Bark of Garcinia lucida Vesque (Clusiaceae) Exhibits Cardioprotective and Nephroprotective Effects in Adenine-Induced Chronic Kidney Disease in Rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 5581041. [Google Scholar] [CrossRef]
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose Benefits of an Underexplored Species Purslane (Portulaca oleracea L.): A Critical Review. Environ. Manag. 2023, 72, 309–320. [Google Scholar] [CrossRef]
- Zam, W.; Quispe, C.; Sharifi-Rad, J.; López, M.D.; Schoebitz, M.; Martorell, M.; Sharopov, F.; Fokou, P.V.T.; Mishra, A.P.; Chandran, D.; et al. An Updated Review on The Properties of Melissa officinalis L.: Not Exclusively Anti-anxiety. Front. Biosci. 2022, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sharma, M.P.; Gupta, V.K. A Review on Pharmacological Aspects of Achyranthes aspera. Int. J. Pharmacogn. Phytochem. Res. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A Review Study on Punica granatum L. J. Evid. Based Complement. Altern. Med. 2016, 21, 221–227. [Google Scholar] [CrossRef] [PubMed]







| Plant Extract | Polymer | Reference |
|---|---|---|
| C. longa | Cellulose acetate/polyvinylpyrrolidone | [92,94] |
| C. longa | Cellulose acetate (CA) | [93] |
| C. longa | Polyvinyl alcohol (PVA) | [95,96,97] |
| C. longa | PVA/CA | [98] |
| C. longa | PLA | [99] |
| C. longa | Chitosan/PLA | [100] |
| C. longa | PLA and PVP or polyethylene glycol (PEG) | [101] |
| C. longa | Poly(L-co-D,L-lactic) acid and PVP | [102] |
| C. longa | Poly(ethylene glycol)diacid | [103] |
| C. longa | PHB | [104] |
| M. officinalis | Collagen hydrolysate-chitosan | [106] |
| M. officinalis | PLA and PLA/PEG | [107] |
| R. officinalis | Poly(ε-caprolactone) | [112] |
| R. officinalis | CA | [113] |
| R. officinalis | CA/PEG | [114] |
| P. oleracea | PLA | [118,119] |
| H. perforatum | PLA | [120,121] |
| H. perforatum | PVA | [122,123] |
| Chamomile | Chitosan/PVA and polycaprolactone | [124] |
| Aloe vera | Chitosan/polyethylene oxide | [125] |
| Aloe vera | Poly(ε-caprolactone) | [126] |
| Aloe vera | Chitosan and pullulan | [127] |
| Aloe vera | Chitosan/polyethylene oxide | [128] |
| C. officinalis | Chitosan/PEO | [129] |
| C. officinalis | Polycaprolactone/gelatin | [130] |
| C. officinalis | PVA/Sodium alginate | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, N.; Nachev, N.; Spasova, M. Innovative Bioactive Nanofibrous Materials Combining Medicinal and Aromatic Plant Extracts and Electrospinning Method. Membranes 2023, 13, 840. https://doi.org/10.3390/membranes13100840
Stoyanova N, Nachev N, Spasova M. Innovative Bioactive Nanofibrous Materials Combining Medicinal and Aromatic Plant Extracts and Electrospinning Method. Membranes. 2023; 13(10):840. https://doi.org/10.3390/membranes13100840
Chicago/Turabian StyleStoyanova, Nikoleta, Nasko Nachev, and Mariya Spasova. 2023. "Innovative Bioactive Nanofibrous Materials Combining Medicinal and Aromatic Plant Extracts and Electrospinning Method" Membranes 13, no. 10: 840. https://doi.org/10.3390/membranes13100840
APA StyleStoyanova, N., Nachev, N., & Spasova, M. (2023). Innovative Bioactive Nanofibrous Materials Combining Medicinal and Aromatic Plant Extracts and Electrospinning Method. Membranes, 13(10), 840. https://doi.org/10.3390/membranes13100840