Enhancing Palladium Recovery Rates in Industrial Residual Solutions through Electrodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Materials
2.3. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| pH 0 | pH 4 | pH 7 | pH 14 | |
|---|---|---|---|---|
| PdCl42− (%) | 99.739 | 99.794 | 99.794 | 99.738 |
| FPdCl3− (%) | 0.26 | 0.205 | 0.205 | 0.259 |
References
- Nakhjiri, A.T.; Sanaeepur, H.; Amooghin, A.E.; Shirazi, M.M.A. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: A critical review. Desalination 2022, 527, 115510. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, Y.; Wen, Q.; Liu, B.; Zhang, S. Separation and purification of platinum group metals from aqueous solution: Recent developments and industrial applications. Resour. Conserv. Recycl. 2021, 167, 105417. [Google Scholar] [CrossRef]
- Cowley, A. PGM Market Report May 2022. 2022. Available online: https://matthey.com/documents/161599/509428/PGM-market-report-May-2022.pdf/542bcada-f4ac-a673-5f95-ad1bbfca5106 (accessed on 6 September 2023).
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Perez, J.P.H.; Folens, K.; Leus, K.; Vanhaecke, F.; Van Der Voort, P.; Du Laing, G. Progress in hydrometallurgical technologies to recover critical raw materials and precious metals from low-concentrated streams. Resour. Conserv. Recycl. 2019, 142, 177–188. [Google Scholar] [CrossRef]
- Gervais, E.; Kleijn, R.; Nold, S.; van der Voet, E. Risk-based due diligence in supply chains: The case of silver for photovoltaics. Resour. Conserv. Recycl. 2023, 198, 107148. [Google Scholar] [CrossRef]
- Glaister, B.J.; Mudd, G.M. The environmental costs of platinum–PGM mining and sustainability: Is the glass half-full or half-empty? Miner. Eng. 2010, 23, 438–450. [Google Scholar] [CrossRef]
- Ayres, R.U. Metals recycling: Economic and environmental implications. Resour. Conserv. Recycl. 1997, 21, 145–173. [Google Scholar] [CrossRef]
- Hagelüken, C.; Lee-Shin, J.U.; Carpentier, A.; Heron, C. The EU circular economy and its relevance to metal recycling. Recycling 2016, 1, 242–253. [Google Scholar] [CrossRef]
- Oladeji, A.V.; Courtney, J.M.; Fernandez-Villamarin, M.; Rees, N.V. Electrochemical Metal Recycling: Recovery of Palladium from Solution and In Situ Fabrication of Palladium-Carbon Catalysts via Impact Electrochemistry. J. Am. Chem. Soc. 2022, 144, 18562–18574. [Google Scholar] [CrossRef]
- Cherkasov, R.; Garifzyanov, A.; Zakharov, S.; Vinokurov, A.; Galkin, V. Liquid extraction of noble metal ions with bis (α-aminophosphonates). Russ. J. Gen. Chem. 2006, 76, 417–420. [Google Scholar] [CrossRef]
- Cleare, M.J.; Charlesworth, P.; Bryson, D.J. Solvent extraction in platinum group metal processing. J. Chem. Technol. Biotechnol. 1979, 29, 210–224. [Google Scholar] [CrossRef]
- Levitin, G.; Schmuckler, G. Solvent extraction of rhodium chloride from aqueous solutions and its separation from palladium and platinum. React. Funct. Polym. 2003, 54, 149–154. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Y.; Zhou, Y.; Zhang, B.; Liu, G.; Li, Q.; Yang, Y.; Jiang, T. A Review of Recovery of Palladium from the Spent Automobile Catalysts. Metals 2022, 12, 533. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K. Recovery of Metals from Wastewater—State-of-the-Art Solutions with the Support of Membrane Technology. Membranes 2023, 13, 114. [Google Scholar] [CrossRef]
- Schmidt, V. Electrochemical Process Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Tekinalp, Ö.; Zimmermann, P.; Holdcroft, S.; Burheim, O.S.; Deng, L. Cation Exchange Membranes and Process Optimizations in Electrodialysis for Selective Metal Separation: A Review. Membranes 2023, 13, 566. [Google Scholar] [CrossRef] [PubMed]
- Smara, A.; Delimi, R.; Chainet, E.; Sandeaux, J. Removal of heavy metals from diluted mixtures by a hybrid ion-exchange/electrodialysis process. Sep. Purif. Technol. 2007, 57, 103–110. [Google Scholar] [CrossRef]
- Korngold, E.; Kock, K.; Strathmann, H. Electrodialysis in advanced waste water treatment. Desalination 1977, 24, 129–139. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Electrodialytic recovery of iron (III) with liquid membranes based on diphenylthiocarbamide in the presence of palladium (II). Russ. J. Appl. Chem. 2009, 82, 1949–1952. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Separation of platinum (IV) and iron (III) with liquid membranes under electrodialysis conditions. Russ. J. Appl. Chem. 2003, 76, 76–79. [Google Scholar] [CrossRef]
- Mohdee, V.; Ramakul, P.; Phatanasri, S.; Pancharoen, U. A numerical and experimental investigation on the selective separation of Pd (II) from wastewater using Aliquat 336 via hollow fiber supported liquid membrane. J. Environ. Chem. Eng. 2020, 8, 104234. [Google Scholar] [CrossRef]
- Pospiech, B. Highly efficient facilitated membrane transport of palladium (II) ions from hydrochloric acid solutions through plasticizer membranes with Cyanex 471X. Physicochem. Probl. Miner. Process. 2015, 51, 281–291. [Google Scholar]
- Fontàs, C.; Salvadó, V.; Hidalgo, M. Selective enrichment of palladium from spent automotive catalysts by using a liquid membrane system. J. Membr. Sci. 2003, 223, 39–48. [Google Scholar] [CrossRef]
- Ruhela, R.; Panja, S.; Sharma, J.; Tomar, B.; Tripathi, S.; Hubli, R.; Suri, A. Facilitated transport of Pd (II) through a supported liquid membrane (SLM) containing N, N, N, N-tetra-(2-ethylhexyl) thiodiglycolamide T (2EH) TDGA: A novel carrier. J. Hazard. Mater. 2012, 229, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Khusnun, N.F.; Hasan, N.S.; Amalina, I.; Jalil, A.A.; Firmansyah, M.L. Enhanced Recovery of Palladium from an Aqueous Solution Using an Ionic Liquid–Mesoporous Silica Composite in Batch and Fixed-Column Studies. Ind. Eng. Chem. Res. 2022, 61, 8634–8644. [Google Scholar] [CrossRef]
- Fajar, A.T.; Hanada, T.; Firmansyah, M.L.; Kubota, F.; Goto, M. Selective separation of platinum group metals via sequential transport through polymer inclusion membranes containing an ionic liquid carrier. ACS Sustain. Chem. Eng. 2020, 8, 11283–11291. [Google Scholar] [CrossRef]
- Wongkaew, K.; Pancharoen, U.; Phatanasri, S.; Leepipatpiboon, N.; Lothongkum, A.W. Effect of diluent polarity on membrane stability in the separation of trace Pd (II) from wastewater by HFSLM using LIX84-I. J. Ind. Eng. Chem. 2015, 21, 212–220. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Separation of palladium (II) and platinum (IV) by bulk liquid membranes during electrodialysis. Sep. Sci. Technol. 2006, 41, 3213–3228. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Extraction of Palladium (II) with Liquid Membranes Based on Tri-n-Octylammonium and Trialkylbenzylammonium Chlorides under Electrodialysis Conditions. Russ. J. Appl. Chem. 2021, 94, 986–995. [Google Scholar] [CrossRef]
- Zimmermann, P.; Tekinalp, Ö.; Deng, L.; Forsberg, K.; Wilhelmsen, Ø.; Burheim, O. Electrodialysis in hydrometallurgical processes. In Rare Metal Technology 2020; Springer: Heidelberg, Germany, 2020; pp. 159–167. [Google Scholar] [CrossRef]
- Okada, T.; Satou, H.; Okuno, M.; Yuasa, M. Ion and Water Transport Characteristics of Perfluorosulfonated Ionomer Membranes with H+ and Alkali Metal Cations. J. Phys. Chem. B 2002, 106, 1267–1273. [Google Scholar] [CrossRef]
- Solberg, S.B.; Zimmermann, P.; Wilhelmsen, Ø.; Bock, R.; Burheim, O.S. Analytical treatment of ion-exchange permselectivity and transport number measurements for high accuracy. J. Membr. Sci. 2023, 685, 121904. [Google Scholar] [CrossRef]
- Tekinalp, Ö.; Zimmermann, P.; Burheim, O.S.; Deng, L. Designing monovalent selective anion exchange membranes for the simultaneous separation of chloride and fluoride from sulfate in an equimolar ternary mixture. J. Membr. Sci. 2023, 666, 121148. [Google Scholar] [CrossRef]
- Zimmermann, P.; Solberg, S.B.B.; Tekinalp, Ö.; Lamb, J.J.; Wilhelmsen, Ø.; Deng, L.; Burheim, O.S. Heat to hydrogen by RED—Reviewing membranes and salts for the RED heat engine concept. Membranes 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Tekinalp, Ö.; Solberg, S.B.B.; Wilhelmsen, Ø.; Deng, L.; Burheim, O.S. Limiting current density as a selectivity factor in electrodialysis of multi-ionic mixtures. Desalination 2023, 558, 116613. [Google Scholar] [CrossRef]
- FUJIFILM. Products Catalogue. Available online: https://asset.fujifilm.com/www/us/files/2022-09/5be284eb385e7b72906880debde5dd50/IEM_brochure_version_2.2_final_September_2022.pdf (accessed on 6 September 2023).
- AGC. Products Catalogue. Available online: https://www.agec.co.jp/file.jsp?id=107824 (accessed on 6 September 2023).
- Kingsbury, R.; Zhu, S.; Flotron, S.; Coronell, O. Microstructure determines water and salt permeation in commercial ion-exchange membranes. ACS Appl. Mater. Interfaces 2018, 10, 39745–39756. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, K.; Murtomäki, L.; Manzanares, J.A. Ionic Transport Processes in Electrochemistry and Membrane Science; Oxford University Press Inc.: Oxford, UK, 2008. [Google Scholar] [CrossRef]
- Cherif, A.; Elmidaoui, A.; Gavach, C. Separation of Ag+, Zn2+ and Cu2+ ions by electrodialysis with monovalent cation specific membrane and EDTA. J. Membr. Sci. 1993, 76, 39–49. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ ver. 3.1 (Released 2014). Available online: https://vminteq.lwr.kth.se/ (accessed on 6 September 2023).
- Pankow, J.F. Aquatic Chemistry Concepts; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Irfan, M.; Wang, Y.; Xu, T. Novel electrodialysis membranes with hydrophobic alkyl spacers and zwitterion structure enable high monovalent/divalent cation selectivity. Chem. Eng. J. 2020, 383, 123171. [Google Scholar] [CrossRef]
- Ji, W.; Wu, B.; Zhu, Y.; Irfan, M.; Afsar, N.U.; Ge, L.; Xu, T. Self-organized nanostructured anion exchange membranes for acid recovery. Chem. Eng. J. 2020, 382, 122838. [Google Scholar] [CrossRef]
- Sosa-Fernández, P.A.; Post, J.W.; Nabaala, H.L.; Bruning, H.; Rijnaarts, H. Experimental evaluation of anion exchange membranes for the desalination of (Waste) water produced after polymer-flooding. Membranes 2020, 10, 352. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.; Biesheuvel, P. Nernst-Planck transport theory for (reverse) electrodialysis: II. Effect of water transport through ion-exchange membranes. J. Membr. Sci. 2017, 531, 172–182. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.; Biesheuvel, P. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membr. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
- Rumble, J.R. (Ed.) CRC Handbook of Chemistry and Physics, 102nd Edition (Internet Version 2021), 102nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Cieszynska, A.; Wieczorek, D. Extraction and separation of palladium (II), platinum (IV), gold (III) and rhodium (III) using piperidine-based extractants. Hydrometallurgy 2018, 175, 359–366. [Google Scholar] [CrossRef]
- Belloň, T.; Slouka, Z. Overlimiting behavior of surface-modified heterogeneous anion-exchange membranes. J. Membr. Sci. 2020, 610, 118291. [Google Scholar] [CrossRef]
- Rubinstein, I.; Staude, E.; Kedem, O. Role of the membrane surface in concentration polarization at ion-exchange membrane. Desalination 1988, 69, 101–114. [Google Scholar] [CrossRef]
- Krol, J.; Wessling, M.; Strathmann, H. Concentration polarization with monopolar ion exchange membranes: Current–voltage curves and water dissociation. J. Membr. Sci. 1999, 162, 145–154. [Google Scholar] [CrossRef]
- Simons, R. Water splitting in ion exchange membranes. Electrochim. Acta 1985, 30, 275–282. [Google Scholar] [CrossRef]
- Oda, Y.; Yawataya, T. Neutrality-disturbance phenomenon of membrane-solution systems. Desalination 1968, 5, 129–138. [Google Scholar] [CrossRef]
- Mavrov, V.; Pusch, W.; Kominek, O.; Wheelwright, S. Concentration polarization and water splitting at electrodialysis membranes. Desalination 1993, 91, 225–252. [Google Scholar] [CrossRef]
- Kniaginicheva, E.; Pismenskaya, N.; Melnikov, S.; Belashova, E.; Sistat, P.; Cretin, M.; Nikonenko, V. Water splitting at an anion-exchange membrane as studied by impedance spectroscopy. J. Membr. Sci. 2015, 496, 78–83. [Google Scholar] [CrossRef]
- Tanaka, Y. Water dissociation in ion-exchange membrane electrodialysis. J. Membr. Sci. 2002, 203, 227–244. [Google Scholar] [CrossRef]
- Kang, M.S.; Choi, Y.J.; Lee, H.J.; Moon, S.H. Effects of inorganic substances on water splitting in ion-exchange membranes: I. Electrochemical characteristics of ion-exchange membranes coated with iron hydroxide/oxide and silica sol. J. Colloid Interface Sci. 2004, 273, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Asraf-Snir, M.; Gilron, J.; Oren, Y. Gypsum scaling of anion exchange membranes in electrodialysis. J. Membr. Sci. 2016, 520, 176–186. [Google Scholar] [CrossRef]
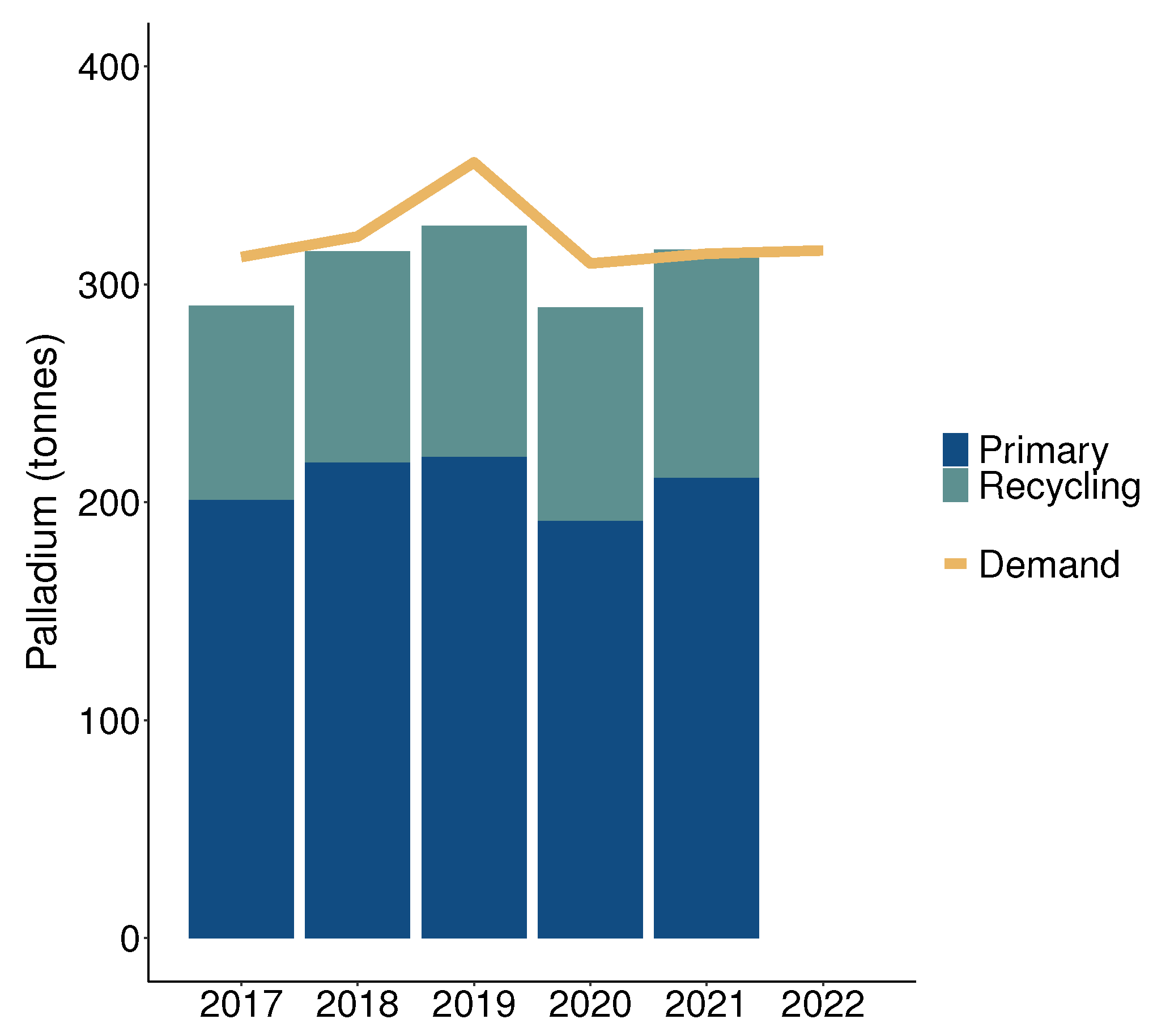






| Pd (mg/L) | Pt (mg/L) | Rh (mg/L) | Fe (mg/L) | Cl (g/L) | pH |
|---|---|---|---|---|---|
| 1060 | 24 | <5 | 13 | ∼50 | 0.63 |
| Membrane | Thickness Dry, (m) | Reinforcement | Resistance (cm) | Permselectivity | Water Permeation (mL/(bar m h) | Burst Strength kg/cm | Ion Exchange Capacity (meq/g) |
|---|---|---|---|---|---|---|---|
| Fujifilm Type 12, AEM | 110 | polyolefin | 6.0 | 95 | 2 | 373 | 1.1 |
| Fujifilm Type 12, CEM | 110 | polyolefin | 6.0 | 99 | 2.5 | 373 | 1.0 |
| Selemion AMVN | 100 | poly- (vinylchloride) | 2.0 | >95 e | - | 250 | 2.02 |
| Selemion CMVN | 100 | poly- (vinylchloride) | 2.0 | >97 e | - | 200 | 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, P.; Tekinalp, Ö.; Wilhelmsen, Ø.; Deng, L.; Burheim, O.S. Enhancing Palladium Recovery Rates in Industrial Residual Solutions through Electrodialysis. Membranes 2023, 13, 859. https://doi.org/10.3390/membranes13110859
Zimmermann P, Tekinalp Ö, Wilhelmsen Ø, Deng L, Burheim OS. Enhancing Palladium Recovery Rates in Industrial Residual Solutions through Electrodialysis. Membranes. 2023; 13(11):859. https://doi.org/10.3390/membranes13110859
Chicago/Turabian StyleZimmermann, Pauline, Önder Tekinalp, Øivind Wilhelmsen, Liyuan Deng, and Odne Stokke Burheim. 2023. "Enhancing Palladium Recovery Rates in Industrial Residual Solutions through Electrodialysis" Membranes 13, no. 11: 859. https://doi.org/10.3390/membranes13110859
APA StyleZimmermann, P., Tekinalp, Ö., Wilhelmsen, Ø., Deng, L., & Burheim, O. S. (2023). Enhancing Palladium Recovery Rates in Industrial Residual Solutions through Electrodialysis. Membranes, 13(11), 859. https://doi.org/10.3390/membranes13110859











