Application of Metal Oxide Nanoparticles in the Field of Potentiometric Sensors: A Review
Abstract
:1. Introduction
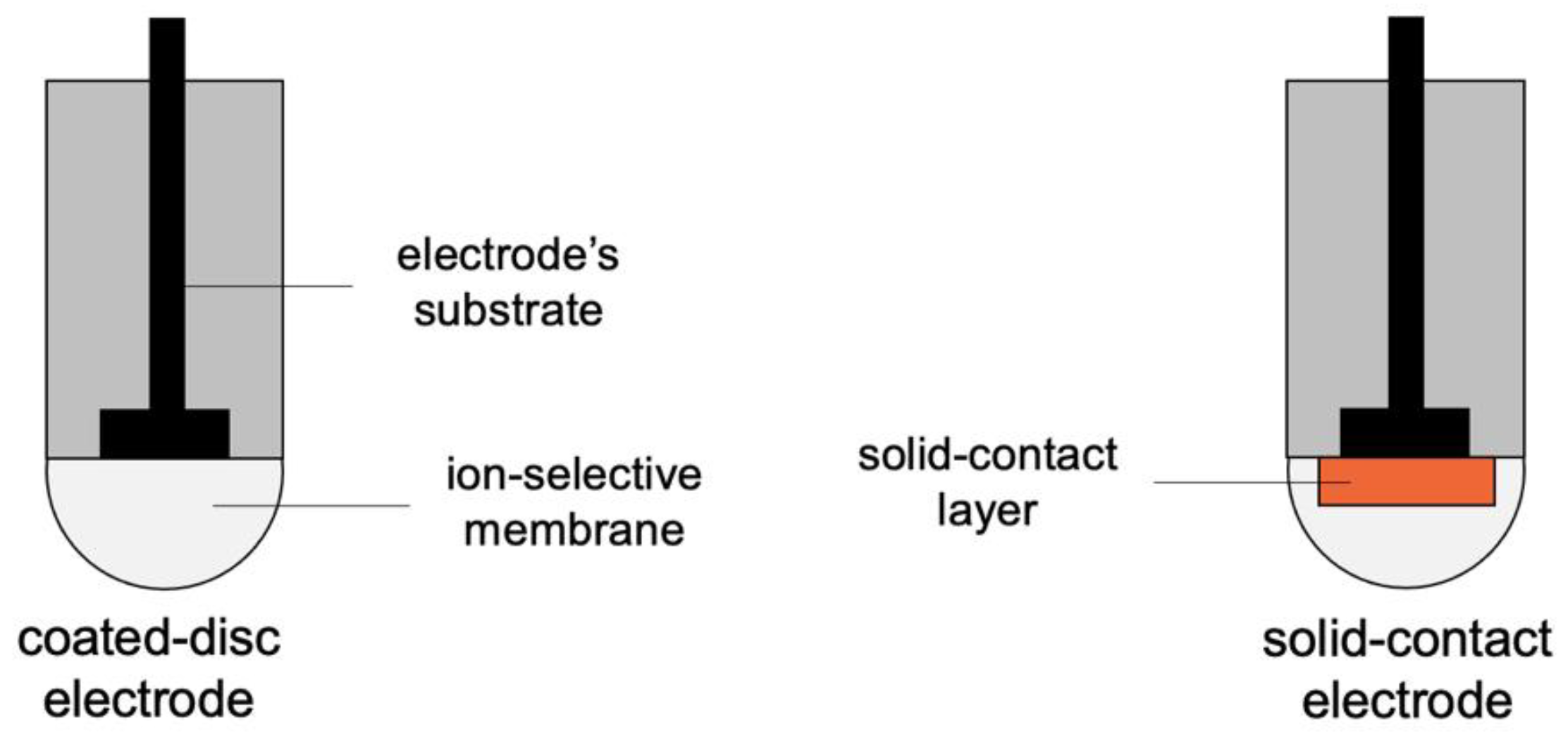
2. Potentiometric Sensors
3. Metal Oxides
3.1. Characterization of Metal Oxides as Electrode Materials
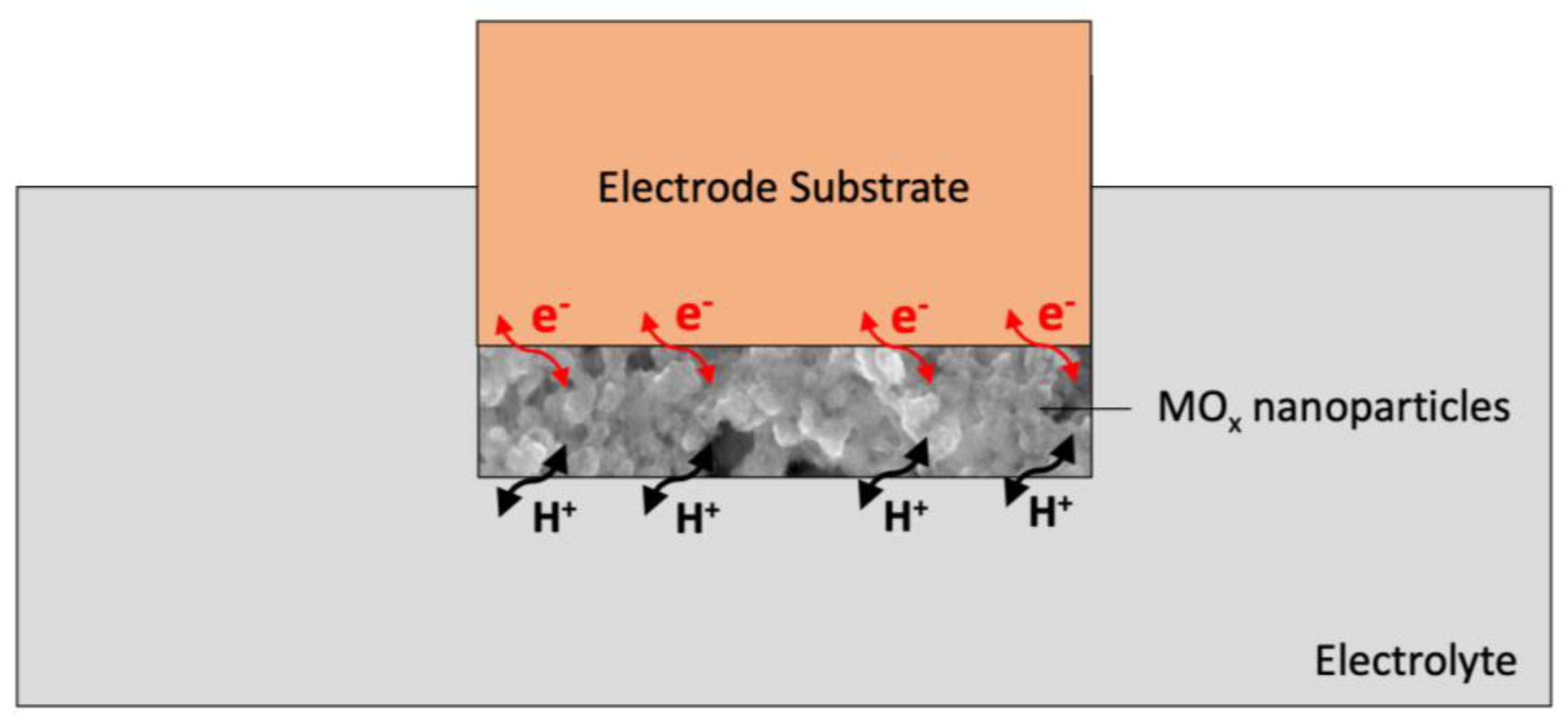
3.2. The Action Mechanism of Metal Oxides
4. Metal Oxide Nanoparticles as a Sensing Material
5. Metal Oxide Nanoparticles as Solid-Contact Layers
Metal Oxide Nanoparticles-Based Hybrid Materials as Solid-Contact Layers
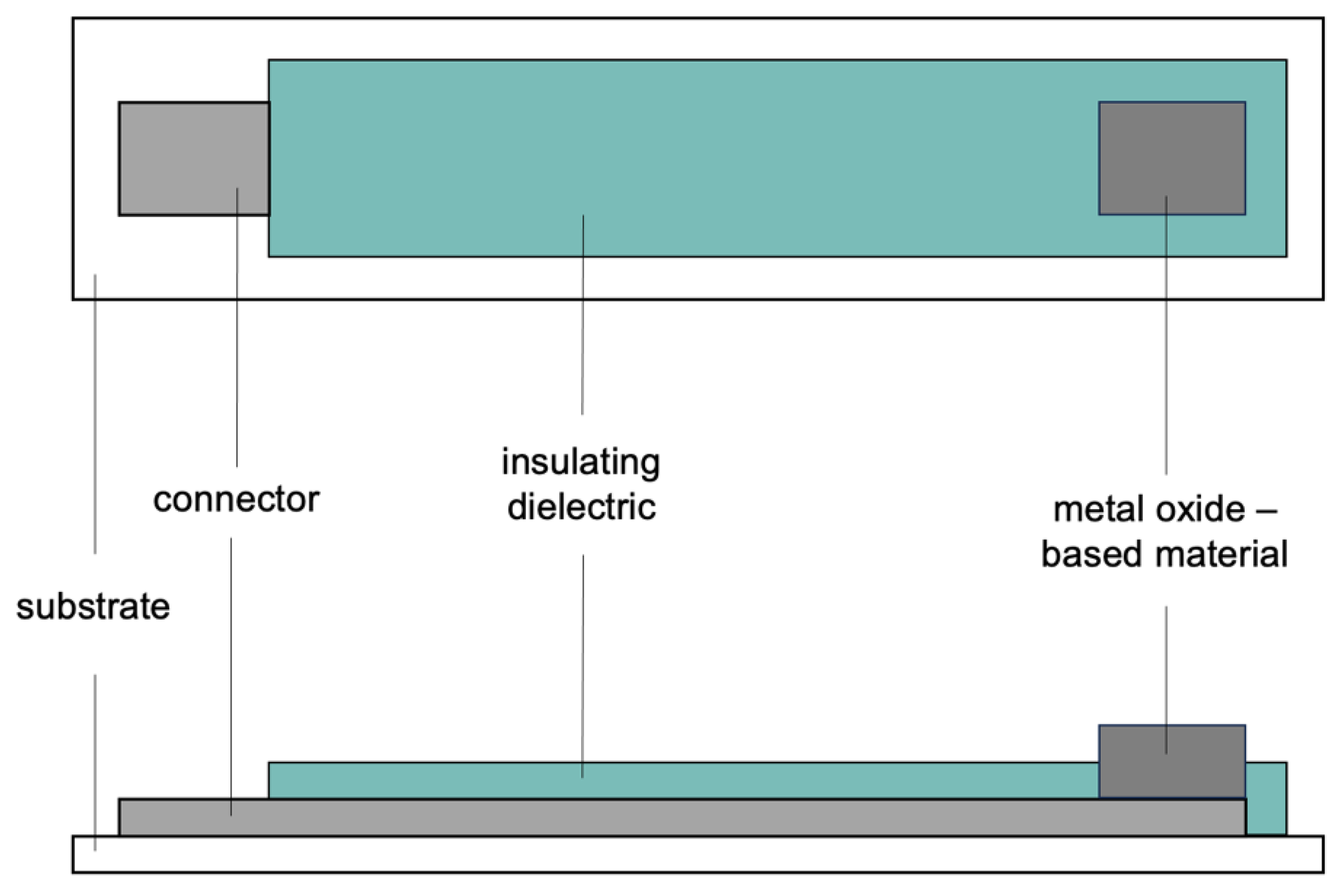
6. Metal Oxides Nanoparticles in Paste Electrodes
7. Comparative Study
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Karimi-Maleh, H.; Liu, Y.; Li, Z.; Darabi, R.; Orooji, Y.; Karaman, C.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Fu, L.; et al. Calf Thymus Ds-DNA Intercalation with Pendimethalin Herbicide at the Surface of ZIF-8/Co/rGO/C3N4/Ds-DNA/SPCE; A Bio-Sensing Approach for Pendimethalin Quantification Confirmed by Molecular Docking Study. Chemosphere 2023, 332, 138815. [Google Scholar] [CrossRef] [PubMed]
- Raccary, B.; Loubet, P.; Peres, C.; Sonnemann, G. Evaluating the environmental impacts of analytical chemistry methods: From a critical review towards a proposal using a life cycle approach. TrAC-Trends Anal. Chem. 2022, 147, 116525. [Google Scholar] [CrossRef]
- Yin, T.; Yu, H.; Ding, J.; Qin, W. An integrated screen-printed potentiometric strip for determination of Ca2+ in seawater. J. Electrochem. Soc. 2019, 166, B589–B593. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E. Potentiometric sensors for trace-level analysis. TrAC-Trends Anal. Chem. 2005, 24, 199–207. [Google Scholar] [CrossRef]
- Rousseau, C.R.; Bühlmann, P. Calibration-free potentiometric sensing with solidcontact ion-selective electrodes. TrAC-Trends Anal. Chem. 2021, 140, 116277. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC-Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Prataap, R.K.V.; Arunachalam, R.; Pavul Raj, R.; Mohan, S.; Peter, L. Effect of electrodeposition modes on ruthenium oxide electrodes for supercapacitors. Curr. Appl. Phys. 2018, 18, 1143–1148. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2019, 109, 100635. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical Sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Simões, F.R.; Xavier, M.G. Nanoscience and Its Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–178. [Google Scholar]
- van de Velde, L.; d’Angremont, E.; Olthuis, W. Solid contact potassium selective electrodes for biomedical applications—A review. Talanta 2016, 160, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.R.; Li, X.G. Highly sensing and transducing materials for potentiometric ion sensors with versatile applicability. Prog. Mater. Sci. 2022, 125, 100885. [Google Scholar] [CrossRef]
- Bakker, E. Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 231–251. [Google Scholar]
- Haber, F.; Klemensiewicz, Z.Z.F. Über elektrische Phasengrenzkräfte. Phys. Chem. 1909, 67, 385. [Google Scholar] [CrossRef]
- Graham, D.J.; Jaselskis, B.; Moore, C.E. Development of the glass electrode and the pH response. J. Chem. Educ. 2013, 90, 345–351. [Google Scholar] [CrossRef]
- Dole, M.J. The theory of the glass electrode. Am. Chem. Soc. 1931, 53, 4260–4280. [Google Scholar] [CrossRef]
- Rechnitz, G.A.; Hameka, H.F. A theory of glass electrode response. Z. Analyt. Chem. 1965, 1059, 252–257. [Google Scholar] [CrossRef]
- Nikolskii, B.P.; Materova, E. Solid contact in membrane ion-selective electrodes. Ion. Select. Electr. Rev. 1985, 7, 3–39. [Google Scholar]
- Cattrall, R.W.; Freiser, H. Coated wire ion-selective electrodes. Anal. Chem. 1971, 43, 1905–1906. [Google Scholar] [CrossRef]
- Cadogan, A.; Gao, Z.; Lewenstam, A.; Diamond, D. All-solid-state sodium-selective electrode based on a calixarene ionophore in a poly(vinyl chloride) membrane with a polypyrrole solid contact. Anal. Chem. 1992, 64, 2496–2501. [Google Scholar] [CrossRef]
- Bobacka, J.; McCarrick, M.; Lewenstam, A.; Ivaska, A. All solid-state poly(vinyl chloride) membrane ion-selective electrodes with poly(3-octylthiophene) solid internal contact. Analyst 1994, 119, 1985–1991. [Google Scholar] [CrossRef]
- Bobacka, J.; Lindfors, T.; McCarrick, M.; Ivaska, A.; Lewenstam, A. Single-piece all-solid-state ion-selective electrode. Anal. Chem. 1995, 67, 3819–3823. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Meruva, R.K.; Brown, R.B.; Meyerhoff, M.E. Enhancing EMF stability of solid-state ion-selective sensors by incorporating lipophilic silver-ligand complexes within polymeric films. Anal. Chim. Acta 1996, 321, 173–183. [Google Scholar] [CrossRef]
- Grygołowicz-Pawlak, E.; Wygla̧dacz, K.; Sȩk, S.; Bilewicz, R.; Brzózka, Z.; Malinowska, E. Studies on ferrocene organothiol monolayer as an intermediate phase of potentiometric sensors with gold inner contact. Sens. Actuators B Chem. 2005, 111–112, 310–316. [Google Scholar] [CrossRef]
- Gabrielli, C.; Hémery, P.; Liatsi, P.; Masure, M.; Perrot, H. An Electrogravimetric Study of an All-Solid-State Potassium Selective Electrode with Prussian Blue as the Electroactive Solid Internal Contact. J. Electrochem. Soc. 2005, 152, H219. [Google Scholar] [CrossRef]
- Zou, X.U.; Cheong, J.H.; Taitt, B.J.; Buhlmann, P. Solid Contact Ion-Selective Electrodes with a Well-Controlled Co(II)/Co(III) Redox Buffer Layer. Anal. Chem. 2013, 85, 9350–9355. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Pięk, M.; Piech, R. Application of Nanostructured TCNQ to Potentiometric Ion-Selective K+ and Na+ Electrodes. Anal. Chem. 2015, 87, 1718–1725. [Google Scholar] [CrossRef]
- Pięk, M.; Piech, R.; Paczosa-Bator, B. Improved Nitrate Sensing Using Solid Contact Ion Selective Electrodes Based on TTF and Its Radical Salt. J. Electrochem. Soc. 2015, 162, B257–B263. [Google Scholar] [CrossRef]
- Pięk, M.; Piech, R.; Paczosa-Bator, B. TTF-TCNQ solid contact layer in all-solid-state ion-selective electrodes for potassium or nitrate determination. J. Electrochem. Soc. 2018, 165, B60–B65. [Google Scholar] [CrossRef]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; De Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion- selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Rius, F.X. Ion-selective electrodes using carbon nanotubes as ion-to-electron transducers. Anal. Chem. 2008, 80, 1316–1322. [Google Scholar] [CrossRef]
- Liu, X.; Ying, Y.; Ping, J. Structure, synthesis, and sensing applications of single-walled carbon nanohorns. Biosens. Bioelectron. 2020, 167, 112495. [Google Scholar] [CrossRef]
- Jiang, C.; Yao, Y.; Cai, Y.; Ping, J. All-solid-state potentiometric sensor using single-walled carbon nanohorns as transducer. J. Sens. Actuators B Chem. 2019, 283, 284–289. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an All-Solid-State Potassium Ion-Selective Electrode Using Graphene as the Solid-Contact Transducer. Electrochem. Commun. 2011, 13, 1529–1532. [Google Scholar] [CrossRef]
- Paczosa-Bator, B. All-solid-state selective electrodes using carbon black. Talanta 2012, 93, 424–427. [Google Scholar] [CrossRef]
- Lai, C.Z.; Fierke, M.A.; Stein, A.; Bühlmann, P. Ion-selective electrodes with three-dimensionally ordered macroporous carbon as the solid contact. Anal. Chem. 2007, 79, 4621–4626. [Google Scholar] [CrossRef]
- Fouskaki, M.; Chaniotakis, N. Fullerene-Based Electrochemical Buffer Layer for Ion-Selective Electrodes. Analyst 2008, 133, 1072–1075. [Google Scholar] [CrossRef]
- Hu, J.; Zou, X.U.; Stein, A.; Bühlmann, P. Ion-selective electrodes with colloid-imprinted mesoporous carbon as solid contact. Anal. Chem. 2014, 86, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, E.; Wójcik, M.; Kisiel, A.; Mieczkowski, J.; Michalska, A. Gold Nanoparticles Solid Contact for Ion-Selective Electrodes of Highly Stable Potential Readings. Talanta 2011, 85, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Paczosa-Bator, B.; Cabaj, L.; Piech, R.; Skupien, K. Potentiometric sensors with carbon black supporting platinum nanoparticles. Anal. Chem. 2013, 85, 10255–10261. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Pan, D.; Qin, W. All-solid-state polymeric membrane ion-selective miniaturized electrodes based on a nanoporous gold film as solid contact. Anal. Chem. 2014, 86, 11038–11044. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Piech, R.; Wardak, C.; Cabaj, L. Application of Graphene Supporting Platinum Nanoparticles Layer in Electrochemical Sensors with Potentiometric and Voltammetric Detection. Ionics 2018, 24, 2455–2464. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Yan, R.; Gao, Y.; Wang, P. Bimetallic AuCu Nanoparticles Coupled with Multi-Walled Carbon Nanotubes as Ion-to-Electron Transducers in Solid-Contact Potentiometric Sensors. Electrochim. Acta 2019, 331, 135370. [Google Scholar] [CrossRef]
- Fog, A.; Buck, R.P. Electronic semiconducting oxides as pH sensors. Sens. Actuators 1984, 5, 137–146. [Google Scholar] [CrossRef]
- Adams, R.N. Carbon Paste Electrodes. Anal. Chem. 1958, 30, 1576. [Google Scholar] [CrossRef]
- Radić, J.; Perović, D.; Gričar, E.; Kolar, M. Potentiometric Determination of Maprotiline Hydrochloride in Pharmaceutical and Biological Matrices Using a Novel Modified Carbon Paste Electrode. Sensors 2022, 22, 9201. [Google Scholar] [CrossRef]
- Radić, J.; Buljac, M.; Genorio, B.; Gričar, E.; Kolar, M. A Novel Reduced Graphene Oxide Modified Carbon Paste Electrode for Potentiometric Determination of Trihexyphenidyl Hydrochloride in Pharmaceutical and Biological Matrices. Sensors 2021, 21, 2955. [Google Scholar] [CrossRef]
- Zayed, M.A.; Mahmoud, W.H.; Abbas, A.A.; Ali, A.E.; Mohamed, G.G. A highly sensitive, selective and renewable carbon paste electrode based on a unique acyclic diamide ionophore for the potentiometric determination of lead ions in polluted water samples. RSC Adv. 2020, 10, 17552–17560. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Bolado, P.; Hernández-Santos, D.; Lamas-Ardisana, P.J.; Martín-Pernía, A.; Costa-García, A. Electro-chemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim. Acta 2008, 53, 3635–3642. [Google Scholar] [CrossRef]
- Rice, M.E.; Galus, Z.; Adams, R.N. Graphite paste electrodes: Effects of paste composition and surface states on electron-transfer rates. J. Electroanal. Chem. 1983, 143, 89–102. [Google Scholar] [CrossRef]
- Martínez-Máñez, R.; Soto, J.; García-Breijo, E.; Gil, L.; Ibáñez, J.; Gadea, E. A multisensor in thick-film technology for water quality control. Sens. Actuators A Phys. 2005, 120, 589–595. [Google Scholar] [CrossRef]
- Zhuiykov, S. Morphology of Pt-doped RuO2 nanocomposited and their application in water quality monitoring sensors. Sens. Actuators B Chem. 2009, 136, 248–256. [Google Scholar] [CrossRef]
- Manjakkal, L.; Zaraska, K.; Cvejin, K.; Kulawik, J.; Szwagierczak, D. Potentiometric RuO2–Ta2O5 pH sensors fabricated using thick film and LTCC technologies. Talanta 2016, 147, 233–240. [Google Scholar] [CrossRef]
- Labrador, R.H.; Soto, J.; Martínez-Máñez, R.; Coll, C.; Benito, A.; Ibáñez, J.; García-Breijo, E.; Gil, L. An electrochemical characterization of thick-film electrodes based on RuO2-containing resistive pastes. J. Electroanal. Chem. 2007, 611, 175–180. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chou, J.C. Preparation and characteristics of ruthenium dioxide for pH array sensors with real-time measurement system. Sens. Actuators B Chem. 2008, 128, 603–612. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cho, B.W.; Kim, C.S.; Sohn, B.K. Effects of heat treatment on Ta2O5 sensing membrane for low drift and high sensitivity pH-ISFET. Sens. Actuators B Chem. 1996, 34, 441–445. [Google Scholar] [CrossRef]
- Chou, J.C.; Wang, Y.F. Preparation and study on the drift and hysteresis properties of the tin oxide gate ISFET by the sol-gel method. Sens. Actuators B Chem. 2002, 86, 58–62. [Google Scholar] [CrossRef]
- Huang, W.D.; Cao, H.; Deb, S.; Chiao, M.; Chiao, J.C. A Flexible pH Sensor Based on the Iridium Oxide Sensing Film. Sens. Actuators A Phys. 2011, 169, 1–11. [Google Scholar] [CrossRef]
- Santos, L.; Neto, J.P.; Crespo, A.; Nunes, D.; Costa, N.; Fonseca, I.M.; Barquinha, P.; Pereira, L.; Silva, J.; Martins, R.; et al. WO3 Nanoparticle-Based Conformable pH Sensor. ACS Appl. Mater. Interfaces 2014, 6, 12226–12234. [Google Scholar] [CrossRef] [PubMed]
- Prats-Alfonso, E.; Abad, L.; Casañ-Pastor, N.; Gonzalo-Ruiz, J.; Baldrich, E. Iridium oxide pH sensor for biomedical applications. Case urea-urease in real urine samples. Biosens. Bioelectron. 2013, 39, 163–169. [Google Scholar] [CrossRef]
- Mihell, J.A.; Atkinson, J.K. Planar thick-film pH electrodes based on ruthenium dioxide hydrate. Sens. Actuators B. Chem. 1998, 48, 505–511. [Google Scholar] [CrossRef]
- Glanc-Gostkiewicz, M.; Sophocleous, M.; Atkinson, J.K.; Garcia-Breijo, E. The effect on performance of fabrication parameter variations of thick-film screen printed silver/silver chloride potentiometric reference electrodes. Sens. Actuators A Phys. 2013, 202, 2–7. [Google Scholar] [CrossRef]
- McMurray, H.N.; Douglas, P.; Abbot, D. Novel thick film pH sensor based on Ruthenium dioxide glass composites. Sens. Actuators B. Chem. 1995, 28, 9–15. [Google Scholar] [CrossRef]
- Parr, R.A.; Wilson, J.C.; Kelly, R.G. A hybrid microelectronic pH sensor. J. Phys. E Sci. Instrum. 1986, 19, 1070. [Google Scholar] [CrossRef]
- Koncki, R.; Mascini, M. Screen-printed ruthenium dioxide electrodes for pH measurements. Anal. Chim. Acta 1997, 351, 143–149. [Google Scholar] [CrossRef]
- Goldberg, H.D.; Brown, R.B.; Liu, D.P.; Meyerhoff, M.E. Screen printing: A technology for the batch fabrication of integrated chemical-sensor arrays. Sens. Actuators B. Chem. 1994, 21, 171–183. [Google Scholar] [CrossRef]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. TrAC-Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- De Marco, R.; Veder, J.-P.; Clarke, G.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Evidence of a water layer in solid-contact polymeric ion sensors. Phys. Chem. Chem. Phys. 2008, 10, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Papp, S.; Bojtár, M.; Gyurcsányi, R.E.; Lindfors, T. Potential Reproducibility of Potassium-Selective Electrodes Having Perfluorinated Alkanoate Side Chain Functionalized Poly(3,4-ethylenedioxytiophene) as a Hydrophobic Solid Contact. Anal. Chem. 2019, 91, 9111–9118. [Google Scholar] [CrossRef] [PubMed]
- Guzinski, M.; Jarvis, J.M.; D’Orazio, P.; Izadyar, A.; Pendley, B.D.; Lindner, E. Solid-Contact pH Sensor without CO2 Interference with a Superhydrophobic PEDOT-C14 as Solid Contact: The Ultimate “Water Layer” Test. Anal. Chem. 2017, 89, 8468–8475. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B.; Piech, R.; Paczosa-Bator, B. All-Solid-State Carbon Black Paste Electrodes Modified by Poly(3-octylthiophene-2,5-diyl) and Transition Metal Oxides for Determination of Nitrate Ions. Molecules 2023, 28, 4313. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent Progress in Ruthenium Oxide-Based Composites for Supercapacitor Applications. ChemElectroChem 2019, 6, 4343–4372. [Google Scholar] [CrossRef]
- Wen, S.; Lee, J.; Yeo, I.; Park, J.; Mho, S. The role of cations of the electrolyte for the pseudocapacitive behavior of metal oxide electrodes, MnO2 and RuO2. Electrochim. Acta 2004, 50, 849–855. [Google Scholar] [CrossRef]
- Lenar, N.; Paczosa-Bator, B.; Piech, R. Ruthenium Dioxide as High-Capacitance Solid-Contact Layer in K+-Selective Electrodes Based on Polymer Membrane. J. Electrochem. Soc. 2019, 166, B1470–B1476. [Google Scholar] [CrossRef]
- Edwall, G. Improved antimony-antimony (III) oxide pH electrodes. Med. Biol. Eng. Comput. 1978, 16, 661–669. [Google Scholar] [CrossRef]
- Kurzweil, P. Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook. Sensors 2009, 9, 4955–4985. [Google Scholar] [CrossRef]
- Głąb, S.; Hulanicki, A.; Edwall, G.; Folke, F.; Ingman, I.; Koch, W.F. Metal-metal oxide and metal oxide electrodes as pH sensors. Crit. Rev. Anal. Chem. 1989, 21, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, S.A.M.; Ufer, S.; Buck, R.P.; Johnson, T.A.; Dunlap, L.A.; Cascio, W.E. Electrodeposited iridium oxide pH electrode for measurement of extracellular myocardial acidosis during acute ischemia. Anal. Chem. 1998, 70, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Shervedani, R.K.; Mehrdjardi, H.R.Z.; Ghahfarokhi, S.H.K. Electrochemical characterization and application of Ni-RuO2 as a pH sensor for determination of petroleum oil acid number. J. Iran. Chem. Soc. 2007, 4, 221–228. [Google Scholar] [CrossRef]
- da Silva, G.M.; Lemos, S.G.; Pocrifka, L.A.; Marreto, P.D.; Rosario, A.V.; Pereira, E.C. Development of low-cost metal oxide pH electrodes based on the polymeric precursor method. Anal. Chim. Acta 2008, 616, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, W. Modification of vertically aligned carbon nanotubes with RuO2 for a solid-state pH sensor. Electrochim. Acta 2010, 55, 2859–2864. [Google Scholar] [CrossRef]
- Manjakkal, L.; Cvejin, K.; Kulawik, J.; Zaraska, K.; Szwagierczak, D.; Stojanovic, G. Sensing mechanism of RuO2–SnO2 thick film pH sensors studied by potentiometric method and electrochemical impedance spectroscopy. J. Electroanal. Chem. 2015, 759, 82–90. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chou, J.C. Preparation and characterization of the titanium dioxide thin films used for pH electrode and procaine drug sensor by sol–gel method. Mater. Chem. Phys. 2009, 114, 542–548. [Google Scholar] [CrossRef]
- Pocrifka, L.A.; Gonçalves, C.; Grossi, P.; Colpa, P.C.; Pereira, E.C. Development of RuO2–TiO2 (70–30) mol% for pH measurements. Sens. Actuators B Chem. 2006, 113, 1012–1016. [Google Scholar] [CrossRef]
- Tsai, C.N.; Chou, J.C.; Sun, T.P.; Hsiung, S.K. Study on the time-dependent slow response of the tin oxide pH electrode. IEEE Sens. J. 2006, 6, 1243–1249. [Google Scholar] [CrossRef]
- Chiang, J.L.; Jan, S.S.; Chou, J.C.; Chen, Y.C. Study on the temperature effect, hysteresis and drift of pH-ISFET devices based on amorphous tungsten oxide. Sens. Actuators B Chem. 2001, 76, 624–628. [Google Scholar] [CrossRef]
- Zhang, W.D.; Xu, B. A solid-state pH sensor based on WO3-modified vertically aligned multiwalled carbon nanotubes. Electrochem. Commun. 2009, 11, 1038–1041. [Google Scholar] [CrossRef]
- Betelu, S.; Polychronopoulou, K.; Rebholz, C.; Ignatiadis, I. Novel CeO2-based screen-printed potentiometric electrodes for pH monitoring. Talanta 2011, 87, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Razmi, H.; Heidari, H.; Habibi, E. pH-sensing properties of PbO2 thin film electrodeposited on carbon ceramic electrode. J. Solid State Electrochem. 2008, 12, 1579–1587. [Google Scholar] [CrossRef]
- Telli, L.; Brahimi, B.; Hammouche, A. Study of a pH sensor with MnO2 and montmorillonite-based solid-state internal reference. Solid State Ionics 2000, 128, 255–259. [Google Scholar] [CrossRef]
- Qingwen, L.; Guoan, L.; Youqin, S. Response of nanosized cobalt oxide electrodes as pH sensors. Anal. Chim. Acta 2000, 409, 137–142. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Willander, M. A Selective Iodide Ion Sensor Electrode Based on Functionalized ZnO Nanotubes. Sensors 2013, 13, 1984–1997. [Google Scholar] [CrossRef]
- Khun, K.; Ibupoto, Z.H.; Willander, M. Urea Assisted Synthesis of Flower Like CuO Nanostructures and Their Chemical Sensing Application for the Determination of Cadmium Ions. Electroanalysis 2013, 25, 1425–1432. [Google Scholar] [CrossRef]
- Zeng, X.; Qin, W. A solid-contact potassium-selective electrode with MoO2 microspheres as ion-to-electron transducer. Anal. Chim. Acta 2017, 982, 72–77. [Google Scholar] [CrossRef]
- Yang, C.; Song, C.Q.; Zhang, Y.Q.; Qu, Y. Determination of Concentration of Serum Potassium Ion Using All-Solid-State Potassium Ion Selective Electrode Based on Two-Dimensional Manganese Dioxide Nanosheet. Chin. J. Anal. Chem. 2019, 47, 765–771. [Google Scholar]
- Lenar, N.; Piech, R.; Wyrwa, J.; Paczosa-Bator, B. Potassium-Selective Solid-Contact Electrode with High-Capacitance Hydrous Iridium Dioxide in the Transduction Layer. Membranes 2021, 11, 259. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Hydrous Cerium Dioxide-Based Materials as Solid-Contact Layers in Potassium-Selective Electrodes. Membranes 2022, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Krstulović, N.; Blažeka, D.; Car, J.; Malinowski, S.; Wardak, C. Metal oxide nanoparticles as solid contact in ion-selective electrodes sensitive to potassium ions. Talanta 2022, 243, 123335. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Jiang, W.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. Stable Pb2+ ion-selective electrodes based on polyaniline-TiO2 solid contacts. Anal. Chim. Acta 2020, 1094, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lenar, N.; Paczosa-Bator, B.; Piech, R.; Królicka, A. Poly(3-octylthiophene-2,5-diyl)-nanosized ruthenium dioxide composite material as solid-contact layer in polymer membrane-based K+-selective electrodes. Electrochim. Acta 2019, 322, 134718. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Potentiometric Sensor with High Capacity Composite Composed of Ruthenium Dioxide and Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate. Materials 2021, 14, 1891. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. High Capacity Nanocomposite Layers Based on Nanoparticles of Carbon Materials and Ruthenium Dioxide for Potassium Sensitive Electrode. Materials 2021, 14, 1308. [Google Scholar] [CrossRef]
- Su, Y.; Liu, T.; Song, C.; Fan, A.; Zhu, N.; Sun, B.; Yang, C. Using MoS2/Fe3O4 as Ion-Electron Transduction Layer to Manufacture All-Solid-State Ion-Selective Electrode for Determination of Serum Potassium. Chemosensors 2021, 9, 155. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Carbon Nanomaterials-Poly(3-octylthiophene-2,5-diyl)–Hydrous Iridium Dioxide Triple Composite Materials as Superhydrophobic Layers for Ion-Selective Electrodes. J. Electrochem. Soc. 2022, 169, 127508. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. The New Reliable pH Sensor Based on Hydrous Iridium Dioxide and Its Composites. Materials 2023, 16, 192. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Morawska, K. Nanocomposite of copper oxide nanoparticles and multi-walled carbon nanotubes as a solid contact of a copper-sensitive ion-selective electrode: Intermediate layer or membrane component–comparative studies. Appl. Nanosci. 2023, 13, 7017–7028. [Google Scholar] [CrossRef]
- Lenar, N.; Paczosa-Bator, B.; Piech, R. Ruthenium dioxide nanoparticles as a high-capacity transducer in solid-contact polymer membrane-based pH-selective electrodes. Microchim. Acta 2019, 186, 777. [Google Scholar] [CrossRef] [PubMed]
- Lenar, N.; Paczosa-Bator, B.; Piech, R. Optimization of ruthenium dioxide solid contact in ion-selective electrodes. Membranes 2020, 10, 182. [Google Scholar] [CrossRef] [PubMed]


| Material | pH Range | Slope [mV/pH] | Response Time | Reference |
|---|---|---|---|---|
| RuO2 | 1–13 | 55.64 | - | [60] |
| RuO2-TiO2 | 2–12 | 56.03 | - | [90] |
| RuO2-Ni | 1.5–12.5 | 52 | - | [86] |
| RuO2-NT | 2–12 | 55 | <40 s | [87] |
| RuO2-Ta2O5 | 2–12 | 56 | <8 s | [58] |
| RuO2-SnO2 | 2–12 | 56.5 | <5 s | [88] |
| IrO2 | 2–10 | 63.5 | ~1 min | [84] |
| IrO2-TiO2 | 1–13 | 59.1 | 120 s | [85] |
| Ta2O5 | 2–12 | 58–59 | <0.3 s | [61] |
| SnO2 | 2–12 | 58.1 | - | [91] |
| TiO2 | 1–11 | 58.73 | - | [89] |
| WO3 | 1–7 | 44.85 | - | [92] |
| WO3-NT | 2–12 | 41 | <90 s | [93] |
| CeO2 | 7.2–10.8 | 38 | - | [94] |
| PbO2 | 1.5–12.5 | 64.82 | <1 s | [95] |
| MnO2 | 2–12 | 78.3 | few seconds | [96] |
| CoO2 | 1–12 | 54.9 | <1 min | [97] |
| Solid-Contact | Ion | Slope, pX/dec | Linear Range, M | Potential Drift *, mV/h | Electrical Capacity **, μF | Reference |
|---|---|---|---|---|---|---|
| RuO2 | H+ | 59.31 | 10−12–10−2 | 0.15 | 1120 | [113] |
| IrO2 | H+ | 54.12 | 10−11–10−2 | 0.1 | 66 | [111] |
| IrO2-NT | H+ | 54.40 | 10−11–10−2 | 0.077 | 174 | [111] |
| IrO2-NT-POT | H+ | 57.18 | 10−11.5–10−2 | 0.036 | 387 | [111] |
| MoO2 microspheres | K+ | 55.0 | 10−3–10−5 | 0.012 | 86 | [100] |
| MnO2 nanosheets | K+ | 51.85 | 10−2–10−5 | - | 29 | [96] |
| CuO | K+ | 56.68 | 10−1–10−5 | 0.54 | 0.104 | [104] |
| ZnO | K+ | 56.18 | 10−1–10−5 | 0.16 | 0.026 | [104] |
| Fe2O3 | K+ | 55.11 | 10−1–10−5 | 1.40 | 0.010 | [104] |
| RuO2 | K+ | 57.37 | 10−1–10−6 | 0.0015 | 1233 | [114] |
| RuO2-POT | K+ | 58.64 | 10−6–10−1 | 0.028 | 1170 | [106] |
| RuO2- PEDOT:PSS | K+ | 58.93 | 10−6–10−1 | 0.077 | 7200 | [107] |
| RuO2-GR | K+ | 58.95 | 10−6–10−1 | - | 2600 | [108] |
| RuO2-NT | K+ | 58.25 | 10−6–10−1 | - | 1050 | [108] |
| RuO2-CB | K+ | 58.03 | 10−6–10−1 | - | 1080 | [108] |
| IrO2 | K+ | 59.29 | 10−6–10−1 | 0.063 | 920 | [102] |
| IrO2-POT-NT | K+ | 57.33 | 10−6–10−1 | 0.043 | 1500 | [110] |
| CeO2 | K+ | 55.32 | 10−5–10−1 | 0.086 | 9 | [103] |
| CeO2-NT | K+ | 58.90 | 10−6–10−1 | 0.095 | 610 | [103] |
| CeO2-POT | K+ | 58.21 | 10−6–10−1 | 0.24 | 96 | [103] |
| ZnO nanorods | Cr2+ | 28.65 | 10−6–10−1 | - | - | [98] |
| ZnO nanotubes | I− | 62 | 10−6–10−1 | - | - | [98] |
| CuO nanoflowers | Cd2+ | 29.3 | 10−9–10−1 | - | - | [99] |
| TiO2-PANI | Pb2+ | 29 | 10−9–10−1 | - | 8.1 | [105] |
| MoS2-Fe3O4 | K+ | 55.2 | 10−5–10−2 | - | 350 | [109] |
| CuO-NT | Cu2+ | 30.1 | 5 × 10−8–3 × 10−2 | - | - | [112] |
| CB-IrO2 (paste) | NO3− | −57.2 | 10−5–10−1 | 0.11 | 86 | [76] |
| CB-RuO2 (paste) | NO3− | −57.2 | 10−5–10−1 | 0.11 | 98 | [76] |
| CB-RuO2-POT (paste) | NO3− | −56.9 | 10−5–10−1 | 0.02 | 130 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenar, N.; Piech, R.; Wardak, C.; Paczosa-Bator, B. Application of Metal Oxide Nanoparticles in the Field of Potentiometric Sensors: A Review. Membranes 2023, 13, 876. https://doi.org/10.3390/membranes13110876
Lenar N, Piech R, Wardak C, Paczosa-Bator B. Application of Metal Oxide Nanoparticles in the Field of Potentiometric Sensors: A Review. Membranes. 2023; 13(11):876. https://doi.org/10.3390/membranes13110876
Chicago/Turabian StyleLenar, Nikola, Robert Piech, Cecylia Wardak, and Beata Paczosa-Bator. 2023. "Application of Metal Oxide Nanoparticles in the Field of Potentiometric Sensors: A Review" Membranes 13, no. 11: 876. https://doi.org/10.3390/membranes13110876







