Evaluation of the Microbial Profile on the Polydioxanone Membrane and the Collagen Membrane Exposed to Multi-Species Subgingival Biofilm: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes Preparation
2.1.1. Plenum Membranes
2.1.2. Bio-Guide Membrane
2.2. Membrane Stamps
2.3. DNA–DNA Hybridization (Checkboard DNA–DNA)
2.4. Statistical Analysis
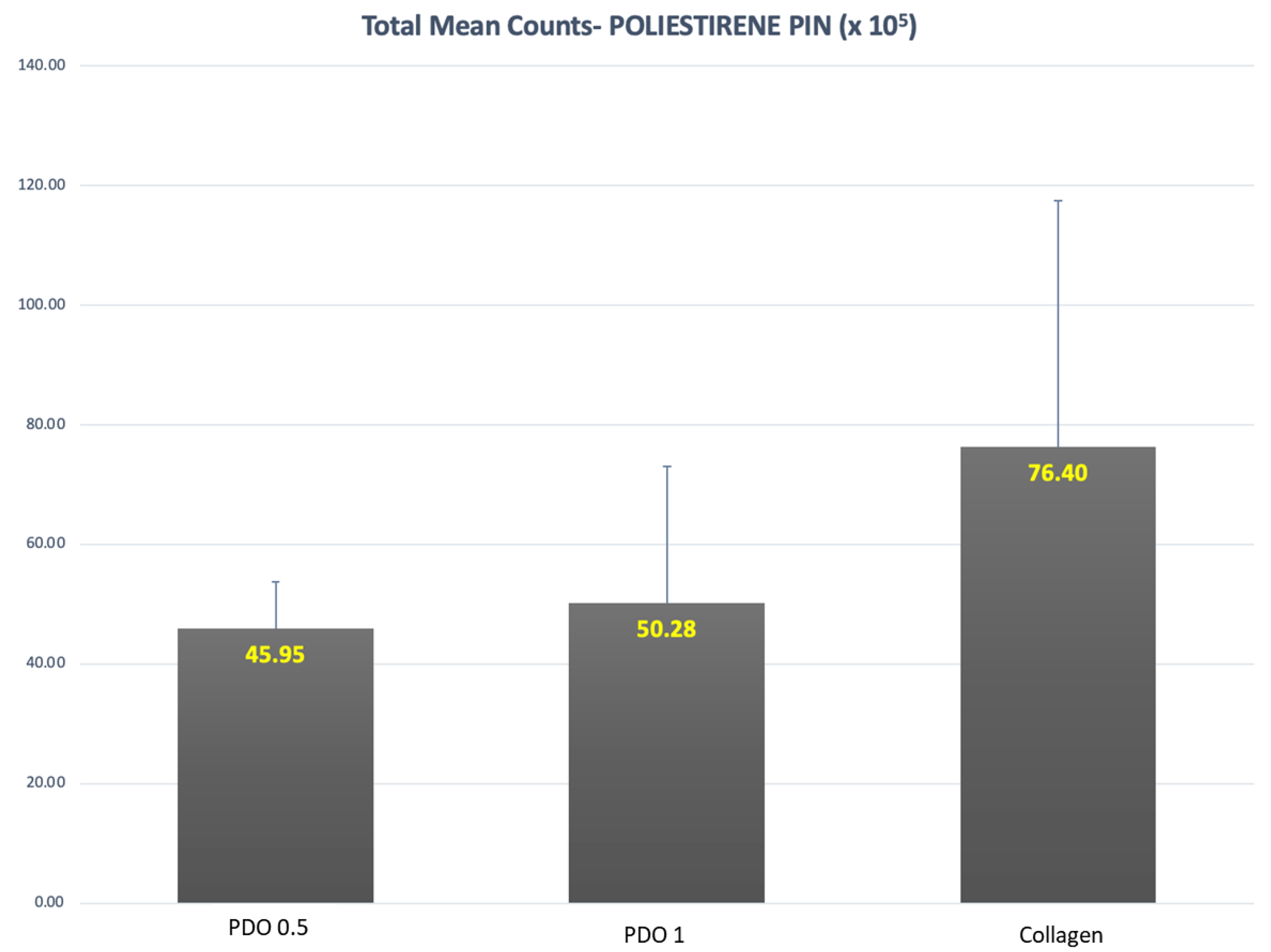
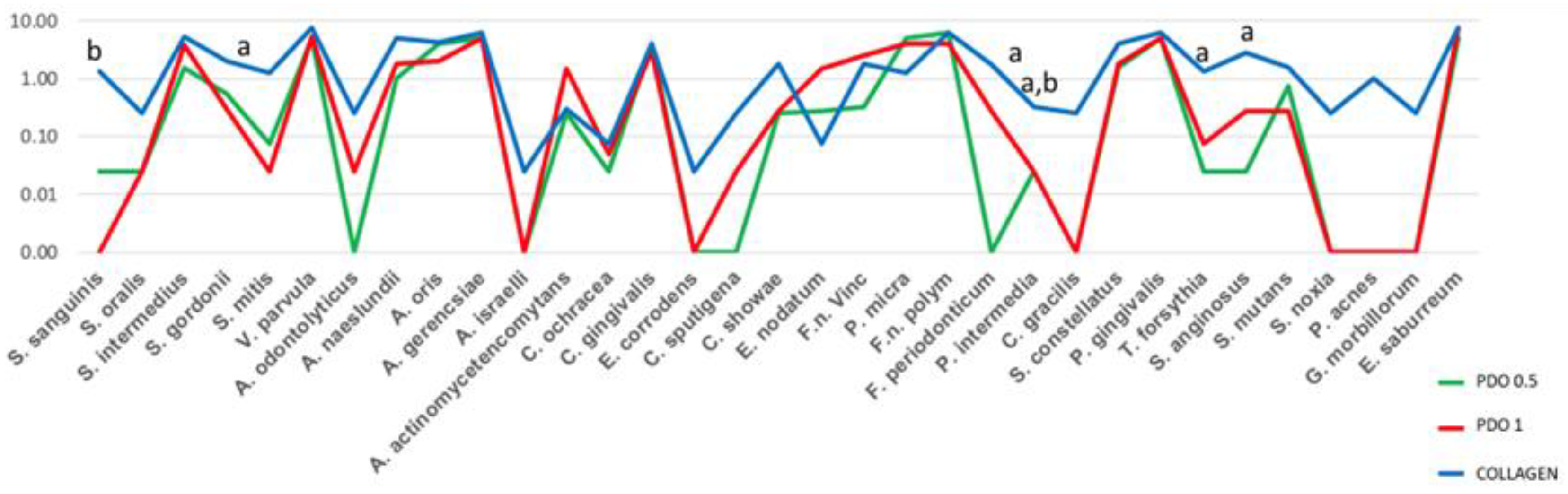
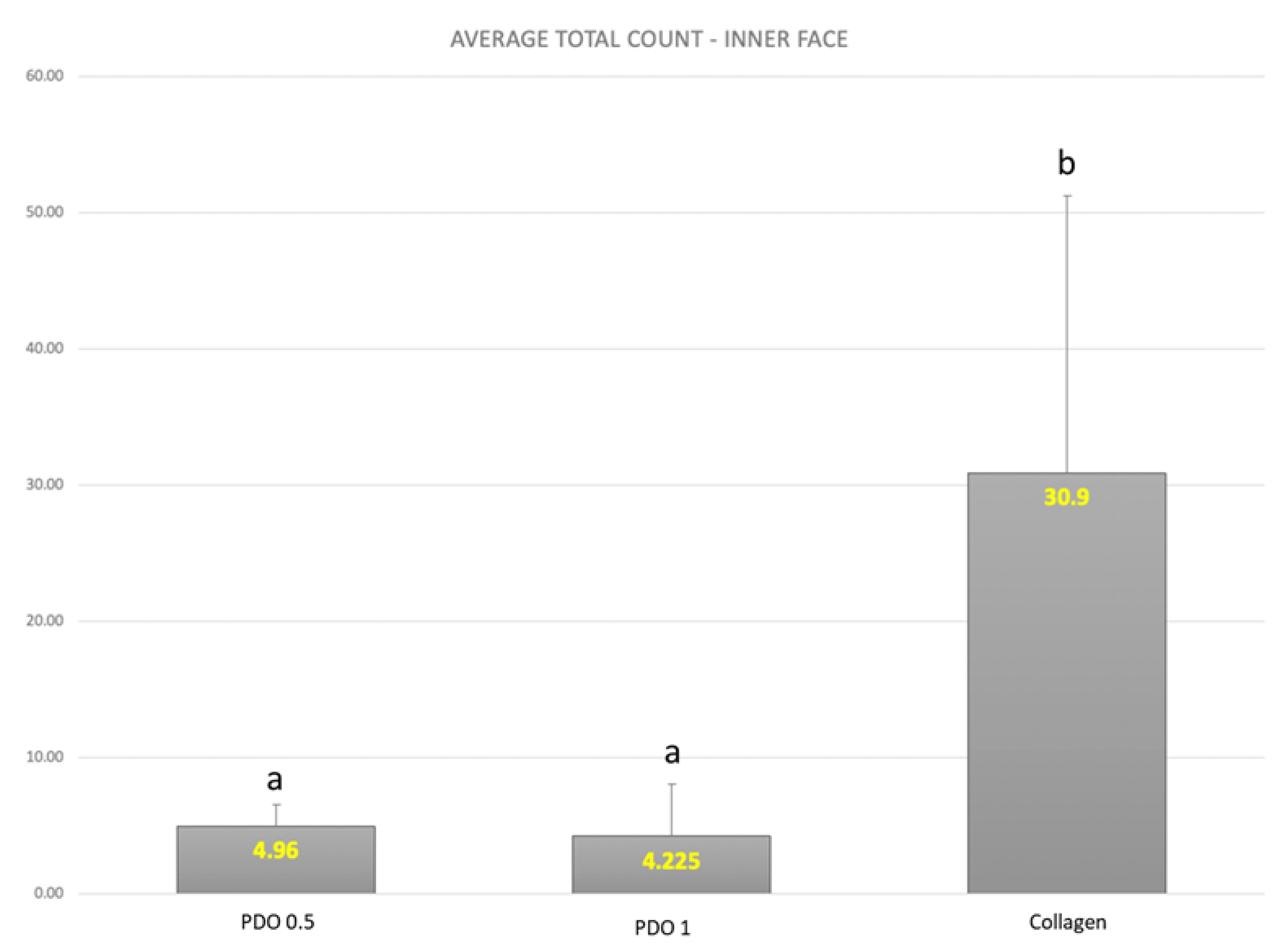
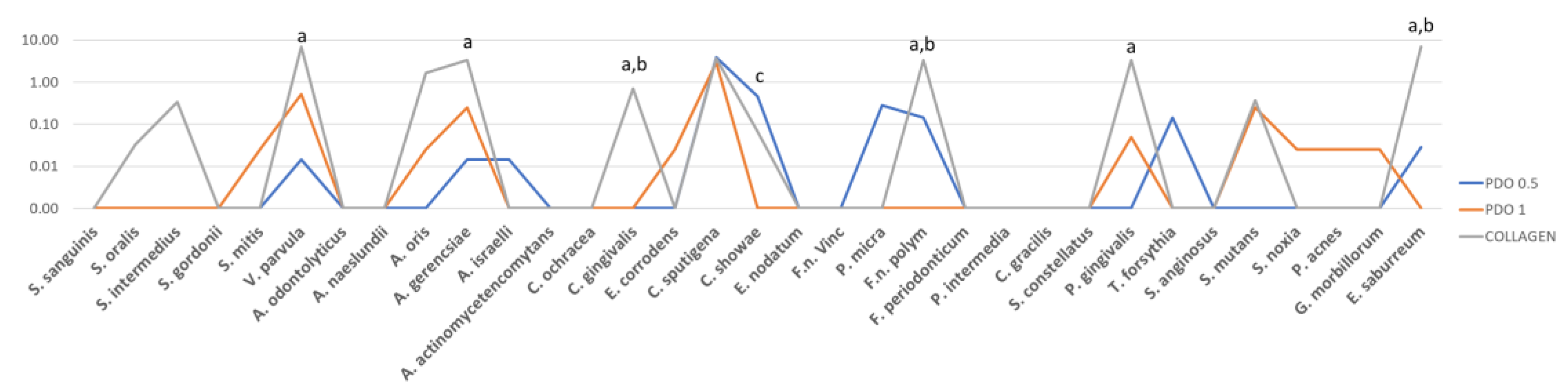
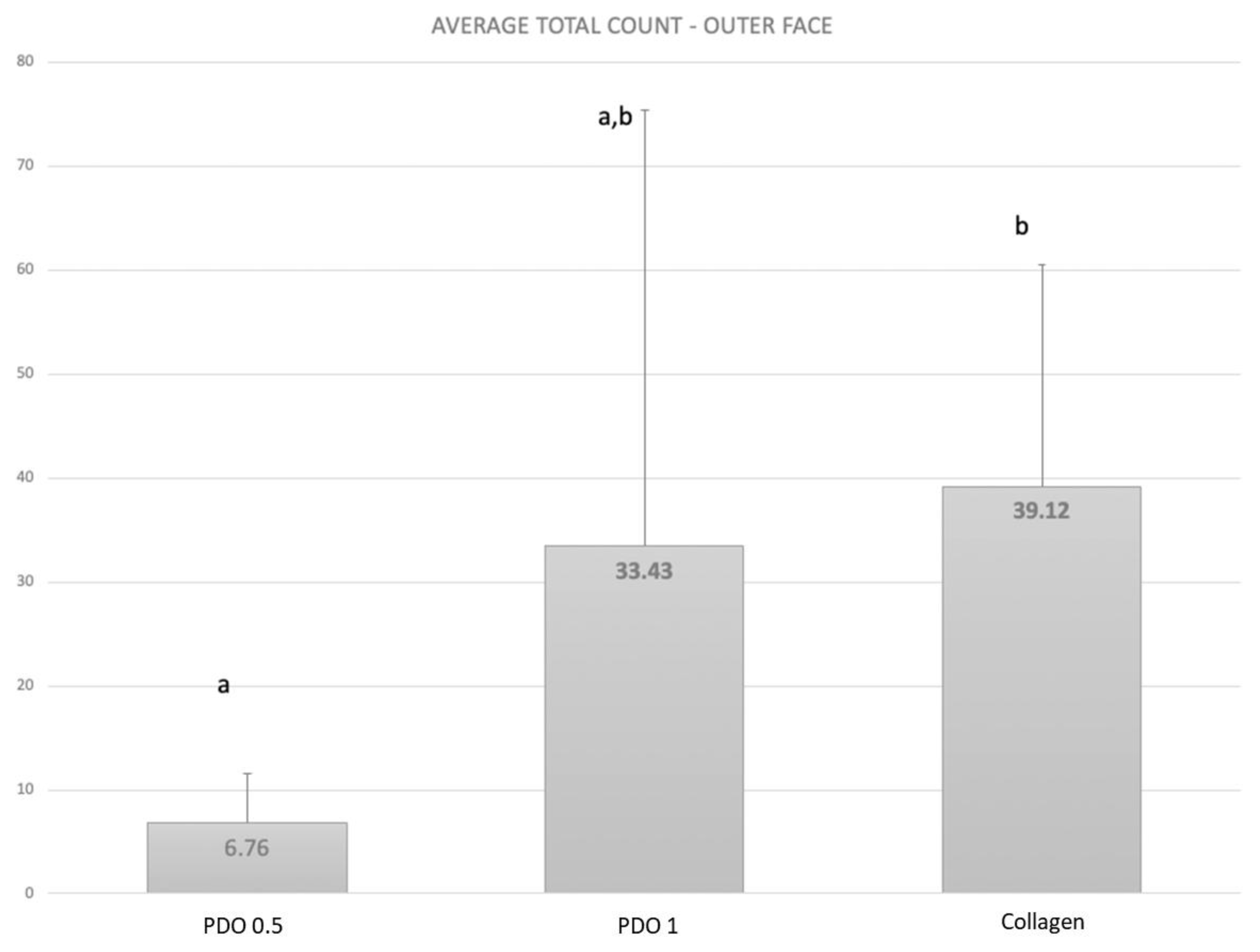
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fuller, J.; Donos, N.; Suvan, J.; Tsakos, G.; Nibali, L. Association of oral health-related quality of life measures with aggressive and chronic periodontitis. J. Periodontal Res. 2020, 55, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Micu, I.C.; Muntean, A.; Roman, A.; Stratul, Ș.I.; Pall, E.; Ciurea, A.; Soancă, A.; Negucioiu, M.; Barbu Tudoran, L.; Delean, A.G. A Local Desiccant Antimicrobial Agent as an Alternative to Adjunctive Antibiotics in the Treatment of Periodontitis: A Narrative Review. Antibiotics 2023, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Curtis, M.A. Oral biofilms revisited: A novel host tissue of bacteriological origin. Periodontology 2000 2021, 86, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 103–123. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Carli, E.; Bambini, F.; Mummolo, S.; Licini, C.; Memè, L. The Use of Nano-Hydroxyapatite (NH) for Socket Preservation: Communication of an Upcoming Multicenter Study with the Presentation of a Pilot Case Report. Medicina 2023, 59, 1978. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R.S. Oral microbiome research—A Beginner’s glossary. J. Oral Maxillofac. Pathol. 2022, 26, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Modoni, M.; Monterubbianesi, R.; Dallari, G.; Memè, L. The ‘Guided Tissue Regeneration (GTR) Effect’ of Guided Bone Regeneration (GBR) with the Use of Bone Lamina: A Report of Three Cases with More than 36 Months of Follow-Up. Appl. Sci. 2022, 12, 11247. [Google Scholar] [CrossRef]
- Memè, L.; Bambini, F.; Gallusi, G.; Sartini, D.; Pozzi, V.; Emanuelli, M.; Strappa, E.M.; Mummolo, S. The Effect and the Potential Use of Magnetic–Dam Barrier in Guided Bone Regeneration: A Laboratory Study. Appl. Sci. 2023, 13, 1625. [Google Scholar] [CrossRef]
- Rossi, R.; Memè, L.; Strappa, E.M.; Bambini, F. Restoration of Severe Bone and Soft Tissue Atrophy by Means of a Xenogenic Bone Sheet (Flex Cortical Sheet): A Case Report. Appl. Sci. 2023, 13, 692. [Google Scholar] [CrossRef]
- Abdo, V.L.; Suarez, L.J.; de Paula, L.G.; Costa, R.C.; Shibli, J.; Feres, M.; Barão, V.A.R.; Bertolini, M.; Souza, J.G.S. Underestimated microbial infection of resorbable membranes on guided regeneration. Colloids Surf. B Biointerfaces 2023, 226, 113318. [Google Scholar] [CrossRef]
- Tay, J.R.H.; Lu, X.J.; Lai, W.M.C.; Fu, J.H. Clinical and histological sequelae of surgical complications in horizontal guided bone regeneration: A systematic review and proposal for management. Int. J. Implant. Dent. 2020, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Zelikman, H.; Slutzkey, G.; Rosner, O.; Levartovsky, S.; Matalon, S.; Beitlitum, I. Bacterial Growth on Three Non-Resorbable Polytetrafluoroethylene (PTFE) Membranes—An In Vitro Study. Materials 2022, 15, 5705. [Google Scholar] [CrossRef] [PubMed]
- Trobos, M.; Juhlin, A.; Shah, F.A.; Hoffman, M.; Sahlin, H.; Dahlin, C. In vitro evaluation of barrier function against oral bacteria of dense and expanded polytetrafluoroethylene (PTFE) membranes for guided bone regeneration. Clin. Implant. Dent. Relat. Res. 2018, 20, 738–748. [Google Scholar] [CrossRef]
- Talon, I.; Schneider, A.; Ball, V.; Hemmerlé, J. Functionalization of PTFE Materials Using a Combination of Polydopamine and Platelet-Rich Fibrin. J. Surg. Res. 2020, 251, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Godovikova, V.; Qian, X.; Seshadrinathan, S.; Kapila, Y.L.; Fenno, J.C. Treponema denticola upregulates MMP-2 activation in periodontal ligament cells: Interplay between epigenetics and periodontal infection. Arch. Oral Biol. 2014, 59, 1056–1064. [Google Scholar] [CrossRef]
- Tan, K.H.; Seers, C.A.; Dashper, S.G.; Mitchell, H.L.; Pyke, J.S.; Meuric, V.; Slakeski, N.; Cleal, S.M.; Chambers, J.L.; McConville, M.J.; et al. Porphyromonas gingivalis and Treponema denticola exhibit metabolic symbioses. PLoS Pathog. 2014, 10, e1003955. [Google Scholar] [CrossRef]
- Jun, H.K.; Jung, Y.J.; Choi, B.K. Treponema denticola, Porphyromonas gingivalis, and Tannerella forsythia induce cell death and release of endogenous danger signals. Arch. Oral Biol. 2017, 73, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Radenković, M.; Alkildani, S.; Stoewe, I.; Bielenstein, J.; Sundag, B.; Bellmann, O.; Jung, O.; Najman, S.; Stojanović, S.; Barbeck, M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR). Membranes 2021, 11, 712. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S.; Gunsolley, J.C. Systemic anti-infective periodontal therapy. A systematic review. Ann. Periodontol. 2003, 8, 115–181. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Smith, C.; Haffajee, A.D. Subgingival microbial profiles in refractory periodontal disease. J. Clin. Periodontol. 2002, 29, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.Y.; Furtado Araujo, M.V.; Strausbaugh, L.D.; Terzi, E.; Ioannidou, E.; Diaz, P.I. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS ONE 2015, 10, e0127077. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Huang, J.M.; Chang, Y.Y.; Yuan, K.; Lin, W.C.; Ting, C.C. Comparative prevalence of oral bacteria and protozoa in patients with periodontitis in Taiwan. Oral Dis. 2023; early view. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Diaz, P.I. Porphyromonas gingivalis: Immune subversion activities and role in periodontal dysbiosis. Curr. Oral Health Rep. 2020, 7, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, R.M.; Ganvir, S.M.; Hazarey, V.K.; Qureshi, A.; Purohit, H.J. Detection of Porphyromonas gingivalis and Treponema denticola in chronic and aggressive periodontitis patients: A comparative polymerase chain reaction study. Contemp. Clin. Dent. 2016, 7, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Aronova, E.; Dmitrienko, M.; Ivanova, A.; Gaykova, Y.; Kurochkina, A.; Blinova, A.; Bazarnova, J.; Paponova, E. Express Diagnostics of Proteolytic Activity of Periodontopathogens-Methodological Approach. Dent. J. 2022, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.P.V.; do Souto, R.M.; Araújo, L.L.; Espíndola, L.C.P.; Hartenbach, F.; Magalhães, C.B.; da Silva Oliveira Alves, G.; Lourenço, T.G.B.; da Silva-Boghossian, C.M. Antimicrobial resistance and virulence of subgingival staphylococci isolated from periodontal health and diseases. Sci. Rep. 2023, 13, 11613. [Google Scholar] [CrossRef]
- Wang, H.L.; Yuan, K.; Burgett, F.; Shyr, Y.; Syed, S. Adherence of oral microorganisms to guided tissue membranes: An in vitro study. J. Periodontol. 1994, 65, 211–218. [Google Scholar] [CrossRef]
- Ricci, G.; Rasperini, G.; Silvestri, M.; Cocconcelli, P.S. In vitro permeability evaluation and colonization of membranes for periodontal regeneration by Porphyromonas gingivalis. J. Periodontol. 1996, 67, 490–496. [Google Scholar] [CrossRef]
- Tang, Z.; Cheng, X.; Su, X.; Wu, L.; Cai, Q.; Wu, H. Treponema denticola Induces Alzheimer-Like Tau Hyperphosphorylation by Activating Hippocampal Neuroinflammation in Mice. J. Dent. Res. 2022, 101, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Upara, C.; Ding, Q.; Zhu, M.; Zeng, E.; Banas, J.A.; Hong, L. Spent culture supernatant of Streptococcus gordonii mitigates inflammation of human periodontal cells and inhibits proliferation of pathogenic oral microbes. J. Periodontol. 2023, 94, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Gode, S.; Sarp, T.Z.; Saribas, S.; Ergin, S.; Kasnak, G.; Dinc, H.O.; Caliskan, R.; Akkus, S.; Tokman, H.B.; Kocak, B.T.; et al. The Prevalence of Periodontal Pathogenic Bacteria in Atherosclerotic Cardiovascular Disease. Clin. Lab. 2020, 66, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, P.; Mahmoudi, M.; Kheder, R.K.; Faraj, T.A.; Mollazadeh, S.; Abdulabbas, H.S.; Esmaeili, S.A. Impacts of Porphyromonas gingivalis periodontitis on rheumatoid arthritis autoimmunity. Int. Immunopharmacol. 2023, 118, 109936. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cintra Moreira, M.V.; Figueiredo, L.C.; da Cunha Melo, M.A.R.; Uyeda, F.H.; da Silva, L.D.A.; Macedo, T.T.; Sacco, R.; Mourão, C.F.; Shibli, J.A.; Bueno-Silva, B. Evaluation of the Microbial Profile on the Polydioxanone Membrane and the Collagen Membrane Exposed to Multi-Species Subgingival Biofilm: An In Vitro Study. Membranes 2023, 13, 907. https://doi.org/10.3390/membranes13120907
Cintra Moreira MV, Figueiredo LC, da Cunha Melo MAR, Uyeda FH, da Silva LDA, Macedo TT, Sacco R, Mourão CF, Shibli JA, Bueno-Silva B. Evaluation of the Microbial Profile on the Polydioxanone Membrane and the Collagen Membrane Exposed to Multi-Species Subgingival Biofilm: An In Vitro Study. Membranes. 2023; 13(12):907. https://doi.org/10.3390/membranes13120907
Chicago/Turabian StyleCintra Moreira, Marcus Vinícius, Luciene C. Figueiredo, Marcelo Augusto Ruiz da Cunha Melo, Fabio Hideaki Uyeda, Lucas Daylor Aguiar da Silva, Tatiane Tiemi Macedo, Roberto Sacco, Carlos Fernando Mourão, Jamil A. Shibli, and Bruno Bueno-Silva. 2023. "Evaluation of the Microbial Profile on the Polydioxanone Membrane and the Collagen Membrane Exposed to Multi-Species Subgingival Biofilm: An In Vitro Study" Membranes 13, no. 12: 907. https://doi.org/10.3390/membranes13120907
APA StyleCintra Moreira, M. V., Figueiredo, L. C., da Cunha Melo, M. A. R., Uyeda, F. H., da Silva, L. D. A., Macedo, T. T., Sacco, R., Mourão, C. F., Shibli, J. A., & Bueno-Silva, B. (2023). Evaluation of the Microbial Profile on the Polydioxanone Membrane and the Collagen Membrane Exposed to Multi-Species Subgingival Biofilm: An In Vitro Study. Membranes, 13(12), 907. https://doi.org/10.3390/membranes13120907







