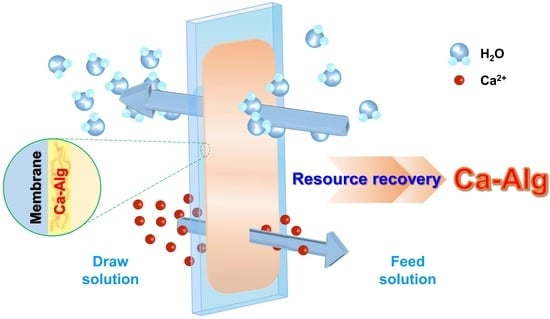
Calcium Alginate Production through Forward Osmosis with Reverse Solute Diffusion and Mechanism Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup and Technique
2.3. Preparation of TFC FO Membranes
2.4. Analytical Methods
2.5. Evaluation of Forward Osmosis Performance
3. Results and Discussion
3.1. Reverse Solute Diffusion of Calcium Ion for Producing Ca-Alg in Forward Osmosis
3.2. Calcium Ions in the Ca-Alg Recovery Production
3.3. Ca-Alg Production Using ENM-TFC FO Membranes
3.4. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Am | effective area of forward osmosis membrane | [m2] |
| Ct | concentration of Ca2+ in feed solution at time t | [kg/m2/s] |
| concentration of Ca2+ in feed solution at time (t + t0) | [kg/m2/s] | |
| JCa | reverse solute flux of Ca2+ without considering Ca2+ in Ca-Alg recovery production passing through the forward osmosis membrane per unit membrane area per unit time at time t | [kg/m2/s] |
| JCa,av | total average reverse solute flux of Ca2+ with considering Ca2+ in Ca-Alg recovery production passing through the forward osmosis membrane per unit membrane area from time t = 0 to t | [kg/m2/s] |
| Jw | volume of water passing through the forward osmosis membrane per unit membrane area per unit time at time t | [m/s] |
| Jw,av | average volume of water passing through the forward osmosis membrane per unit membrane area from time t = 0 to t | [m/s] |
| t | forward osmosis time | [s] |
| t0 | time interval between two consecutive samplings | [s] |
| VDS | volume of draw solution at time t | [m3] |
| Vt | volume of feed solution at time t | [m3] |
| volume of feed solution at time (t + t0) | [m3] | |
| WCa,r | content of Ca2+ in Ca-Alg recovery production formed on the forward osmosis membrane | [kg] |
| WCa | total Ca2+ content of reverse osmosis containing Ca2+ in Ca-Alg recovery production formed on the forward osmosis membrane | [kg] |
| WSA | content of SA in the feed solution | [kg] |
References
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- van Loosdrecht, M.C.M.; Brdjanovic, D. Anticipating the next century of wastewater treatment: Advances in activated sludge sewage treatment can improve its energy use and resource recovery. Science 2014, 344, 1452–1453. [Google Scholar]
- Cao, D.Q.; Hao, X.D.; Wang, Z.; Song, X.; Iritani, E.; Katagiri, N. Membrane recovery of alginate in an aqueous solution by the addition of calcium ions: Analyses of resistance reduction and fouling mechanism. J. Membr. Sci. 2017, 535, 312–321. [Google Scholar]
- van der Roest, H.; van Loosdrecht, M.C.M.; Langkamp, E.J.; Uijterlinde, C. Recovery and reuse of alginate from granular Nereda sludge. Water 2015, 21, 48. [Google Scholar]
- Amorim de Carvalho, C.D.; Ferreira dos Santos, A.; Tavares Ferreira, T.J.; Sousa Aguiar Lira, V.N.; Mendes Barros, A.R.; Bezerra dos Santos, A. Resource recovery in aerobic granular sludge systems: Is it feasible or still a long way to go? Chemosphere 2021, 274, 129881. [Google Scholar]
- Feng, C.J.; Lotti, T.; Canziani, R.; Lin, Y.M.; Tagliabue, C.; Malpei, F. Extracellular biopolymers recovered as raw biomaterials from waste granular sludge and potential applications: A critical review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar]
- Cao, D.Q.; Jin, J.Y.; Wang, Q.H.; Song, X.; Hao, X.D.; Iritani, E.; Katagiri, N. Ultrafiltration recovery of alginate: Membrane fouling mitigation by multivalent metal ions and properties of recycled materials. Chinese J. Chem. Eng. 2020, 28, 2881–2889. [Google Scholar]
- Cao, D.Q.; Sun, X.Z.; Jin, J.Y.; Fang, X.M.; Yang, X.X.; Hao, X.D. Recovery of extracellular polymeric substance: Impact factors in forward osmosis separation of sodium alginate. Environ. Eng. 2020, 38, 1–6. (In Chinese) [Google Scholar]
- Cao, D.Q.; Yang, X.X.; Jing, J.Y.; Sun, X.Z.; Hao, X.D. A Method for Alginate Concentration and Recovery via Forward Osmosis Using Reverse Diffusion of Draw Solute. C.N. Patent 202010189536.8, 18 March 2020. (In Chinese). [Google Scholar]
- Cao, D.Q.; Sun, X.Z.; Zhang, W.Y.; Ji, Y.T.; Yang, X.X.; Hao, X.D. News on alginate recovery by forward osmosis: Reverse solute diffusion is useful. Chemosphere 2021, 285, 131483. [Google Scholar]
- Ino, K.; Fukuda, M.T.; Hiramoto, K.; Taira, N.; Nashimoto, Y.J.; Shiku, H.S. Fabrication of three-dimensional calcium alginate hydrogels using sacrificial templates of sugar. J. Biosci. Bioeng. 2020, 130, 539–544. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application: A mini review. Carbohyd. Polym. 2020, 236, 116025. [Google Scholar]
- Cai, J.; Chen, X.J.; Wang, X.J.; Tan, Y.L.; Ye, D.D.; Jia, Y.T.; Liu, P.F.; Yu, H. High-water-absorbing calcium alginate fibrous scaffold fabricated by microfluidic spinning for use in chronic wound dressings. RSC ADV. 2018, 8, 39463–39469. [Google Scholar]
- Kashima, K.; Inage, T.; Yamaguchi, Y.; Imai, M. Tailorable regulation of mass transfer channel in environmentally friendly calcium alginate membrane for dye removal. J. Environ. Chem. Eng. 2021, 9, 105210. [Google Scholar] [CrossRef]
- Zhou, Q.Z.; Wang, W.Z.; Liu, F.M.; Chen, R. Removal of difenoconazole and nitenpyram by composite calcium alginate beads during apple juice clarification. Chemosphere 2022, 286, 131813. [Google Scholar] [CrossRef]
- Duc, T.H.; Vu, T.K.; Dang, C.T.; Nguyen, V.H.; La, D.D.; Kim, G.M.; Chang, S.W.; Bui, X.T.; Dang, T.D.; Nguyen, D.D. Synthesis and application of hydrogel calcium alginate microparticles as a biomaterial to remove heavy metals from aqueous media. Environ. Technol. Inno. 2021, 22, 101400. [Google Scholar] [CrossRef]
- Song, D.; Park, S.J.; Kang, H.W.; Park, S.B.; Han, J.I. Recovery of lithium(i), strontium(ii), and lanthanum(iii) using ca–alginate beads. J. Chem. Eng. Data. 2013, 58, 2455–2464. [Google Scholar] [CrossRef]
- Khajavi, P.; Keshtkar, A.R.; Moosavian, M.A. The optimization of U(VI) removal by a novel amidoximated modified calcium alginate gel bead with entrapped functionalized SiO2 nanoparticles. Prog. Nucl. Energ. 2021, 140, 103887. [Google Scholar]
- Isik, Z.; Saleh, M.; Dizge, N. Adsorption studies of ammonia and phosphate ions onto calcium alginate beads. Surf. Interfaces 2021, 26, 101330. [Google Scholar]
- Sun, C.; Zhan, H.H.; Wang, L.J. Enhanced aerobic sludge granulation by seeding concentrated activated sludge with ca-alginate gel. Water Environ. Res. 2017, 89, 2078–2087. [Google Scholar] [CrossRef]
- Meng, F.; Li, M.; Wang, H.; Xin, L.; Wu, X.; Liu, X. Encapsulating microscale zero valent iron-activated carbon into porous calcium alginate for the improvement on the nitrate removal rate and Fe0 utilization factor. Micropor. Mesopor. Mat. 2020, 307, 110522. [Google Scholar]
- Su, C.; Sun, X.J.; Mu, Y.Z.; Li, P.W.; Li, J.; Fan, P.S.; Zhang, M.X.; Wang, M.Y.; Chen, X.G.; Feng, C. Multilayer calcium alginate beads containing Diatom Biosilica and Bacillus subtilis as microecologics for sewage treatment. Carbohyd. Polym. 2021, 256, 117603. [Google Scholar]
- Zhang, Y.; Zhao, K.Y.; Zhang, Z.J.; Xie, H.K.; Li, Z.Y.; Lin, Z.; Mo, C.; Yang, W.D.; Wang, X.L.; Wei, J.F. Polypropylene non-woven supported calcium alginate hydrogel filtration membrane for efficient separation of dye/salt at low salt concentration. Desalination 2020, 500, 114845. [Google Scholar]
- Zhang, Y.; Zhao, K.Y.; Yang, Z.H.; Zhang, Z.J.; Guo, Z.L.; Chu, R.R.; Zhang, W.; Shi, W.X.; Li, J.C.; Li, Z.Y.; et al. Calcium alginate and barium alginate hydrogel filtration membrane coated on fibers for molecule/ion separation. Separ. Purif. Technol. 2021, 270, 118761. [Google Scholar]
- Xu, S.; Tabakovic, A.; Liu, X.; Schlangen, E. Calcium alginate capsules encapsulating rejuvenator as healing system for asphalt mastic. Constr. Build. Mater. 2018, 169, 379–387. [Google Scholar]
- Bao, S.W.; Liu, Q.T.; Rao, W.H.; Yu, X.; Zhang, L. Synthesis and characterization of calcium alginate-attapulgite composite capsules for long term asphalt self-healing. Constr. Build. Mater. 2020, 265, 120779. [Google Scholar]
- Rao, W.; Liu, Q.; Yu, X.; Wan, P.; Wang, H.; Song, J.; Ye, Q. Efficient preparation and characterization of calcium alginate-attapulgite composite capsules for asphalt self-healing. Constr. Build. Mater. 2021, 299, 123931. [Google Scholar] [CrossRef]
- Pfaff, N.M.; Kleijn, J.M.; van Loosdrecht, M.C.M.; Kemperman, A.J.B. Formation and ripening of alginate-like exopolymer gel layers during and after membrane filtration. Water Res. 2021, 195, 116959. [Google Scholar] [CrossRef]
- Kahrizi, M.; Lin, J.Y.; Ji, G.Z.; Kong, L.X.; Song, C.W.; Dumée, L.F.; Sahebi, S.; Zhao, S.F. Relating forward water and reverse salt fluxes to membrane porosity and tortuosity in forward osmosis: CFD modelling. Separ. Purif. Technol. 2020, 241, 11672. [Google Scholar] [CrossRef]
- Zou, S.Q.; Qin, M.H.; He, Z. Tackle reverse solute flux in forward osmosis towards sustainable water recovery: Reduction and perspectives. Water Res. 2019, 149, 362–374. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Sengupta, A.; Ang, M.B.M.Y.; Chen, S.T.; Haan, T.Y.; Almodovar, J.; Hung, W.S.; Wickramasinghe, S.R. Application of zwitterions in forward osmosis: A short review. Polymers 2021, 13, 583. [Google Scholar]
- Wu, Z.Y.; Zou, S.Q.; Zhang, B.; Wang, L.J.; He, Z. Forward osmosis promoted in-situ formation of struvite with simultaneous water recovery from digested swine wastewater. Chem. Eng. J. 2018, 342, 274–280. [Google Scholar]
- Wu, S.M.; Zou, S.Q.; Yang, Y.L.; Qian, G.R.; He, Z. Enhancing the performance of an osmotic microbial fuel cell through self-buffering with reverse-fluxed sodium bicarbonate. Chem. Eng. J. 2018, 349, 241–248. [Google Scholar] [CrossRef]
- Volpin, F.; Yu, H.; Cho, J.; Lee, C.; Phuntsho, S.; Ghaffour, N.; Vrouwenvelder, J.S.; Shon, H.K. Human urine as a forward osmosis draw solution for the application of microalgae dewatering. J. Hazard. Mater. 2019, 378, 120724. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right staff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar]
- Cao, D.Q.; Liu, X.D.; Han, J.L.; Zhang, W.Y.; Hao, X.D.; Iritani, E.; Katagiri, N. Recovery of extracellular polymeric substances from excess sludge using high-flux electrospun nanofiber membranes. Membranes 2023, 13, 74. [Google Scholar] [CrossRef]
- Bui, N.; Lind, M.; Hoek, E.; McCutcheon, J. Electrospun nanofiber supported thin film composite membrane for engineered osmosis. J. Membrane. Sci. 2011, 385, 10–19. [Google Scholar]
- Song, X.; Liu, Z.; Sun, D.D. Nano gives the answer: Breaking the bottleneck of internal concentration polarization with a nanofiber composite forward osmosis membrane for a high water production rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar]
- Pugua, J.; Kim, H.S.; Lee, K.J.; Kim, H. Low internal concentration polarization in forward osmosis membranes with hydrophilic crosslinked PVA nanofibers as porous support layer. Desalination 2014, 336, 24–31. [Google Scholar]
- Ndiaye, I.; Vaudreuil, S.; Bounahmidi, T. Forward osmosis process: State-of-the-art of membranes. Sep. Purif. Rev. 2021, 50, 53–73. [Google Scholar] [CrossRef]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of polyamide thin film composite forward osmosis membranes using electrospun polyvinylidene fluoride (PVDF) nanofibers as substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Wang, W.; Guo, Y.; Liu, M.; Song, X.; Duan, J. Porous nano-hydroxyapatites doped into substrate for thin film composite forward osmosis membrane to show high performance. Korean J. Chem. Eng. 2020, 37, 1573–1584. [Google Scholar] [CrossRef]
- Cao, D.Q.; Yang, X.X.; Yang, W.Y.; Wang, Q.H.; Hao, X.D. Separation of trace pharmaceuticals individually and in combination via forward osmosis. Sci. Total Environ. 2020, 718, 137366. [Google Scholar] [CrossRef]
- Cao, D.Q.; Hao, X.D.; Wang, Q.H.; Fang, X.M.; Song, X. A Novel Setup and Method of Forward Osmosis Concentration. C.N. Patent 201910619042.6, 10 July 2019. (In Chinese). [Google Scholar]
- Huang, J. Molecular sieving effect of a novel hyper-cross-linked resin. Chem. Eng. J. 2010, 165, 265–272. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Adha, R.S.; Lee, C.; Kim, D.H.; Kim, I.S. Quantifying the influence of divalent cations mass transport on critical flux and organic fouling mechanism of forward osmosis membrane. Desalination 2021, 512, 115146. [Google Scholar]
- Song, L.; Heiranian, M.; Elimelech, M. True driving force and characteristics of water transport in osmotic membranes. Desalination 2021, 520, 115360. [Google Scholar] [CrossRef]







| Conc. of DS | Conc. of SA | WCaa | WCa,rb | WCa,r/WCac | WCa/WSAd | JCa,ave | Jw,avf |
|---|---|---|---|---|---|---|---|
| [M] | [g/L] | [mg] | [mg] | [%] | [%] | [g/m2/h] | [L/m2/h] |
| 1 | 0 | 34.85 | - g | - | - | 1.66 ± 0.12 | 10.66 ± 0.28 |
| 1 | 25.55 | 4.19 | 16.40 | 5.11 | 1.22 ± 0.14 | 6.63 ± 0.23 | |
| 2 | 0 | 41.43 | - | - | 1.97 ± 0.11 | 15.52 ± 0.16 | |
| 1 | 16.99 | 3.13 | 18.42 | 3.40 | 0.81 ± 0.09 | 9.30 ± 0.09 | |
| 2 | 35.43 | 7.92 | 22.35 | 3.54 | 1.69 ± 0.07 | 7.45 ± 0.13 | |
| 3 | 44.30 | 16.07 | 36.28 | 2.95 | 2.11 ± 0.05 | 7.56 ± 0.15 | |
| 3 | 0 | 52.71 | - | - | - | 2.51 ± 0.21 | 19.29 ± 0.32 |
| 1 | 11.59 | 2.97 | 25.63 | 2.32 | 0.55 ± 0.02 | 9.58 ± 0.18 |
| FO Membranes | Conc. of DS | Conc. of SA | Jw,av | Jca,av |
|---|---|---|---|---|
| [M] | [g/L] | [L/(m2·h)] | [g/(m2·h)] | |
| CTA-ES | 2 | 1 | 4.22 ± 0.17 | 0.39 ± 0.06 |
| PVDF ENM-TFC | 2 | 1 | 3.98 ± 0.03 | 0.91 ± 0.09 |
| PSf ENM-TFC | 2 | 1 | 5.07 ± 0.14 | 1.13 ± 0.05 |
| 1 | 1 | 4.19 ± 0.04 | 0.91 ± 0.32 | |
| 3 | 1 | 5.18 ± 0.38 | 0.63 ± 0.08 | |
| 2 | 2 | 5.94 ± 0.02 | 1.30 ± 0.22 | |
| 2 | 3 | 4.82 ± 0.11 | 1.11 ± 0.18 |
| ENM Support Layer | Average Pore Size [μm] | Water Contact Angle [°] | Tensile Strength [MPa] | Elastic Modulus [MPa] |
|---|---|---|---|---|
| PSf | 3.90 ± 0.47 | 42.90 ± 0.54 | 6.79 ± 0.59 | 160.51 ± 3.08 |
| PVDF | 0.51 ± 0.04 | 61.60 ± 2.08 | 6.58 ± 0.80 | 79.56 ± 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, D.-Q.; Tang, K.; Zhang, W.-Y.; Chang, C.; Han, J.-L.; Tian, F.; Hao, X.-D. Calcium Alginate Production through Forward Osmosis with Reverse Solute Diffusion and Mechanism Analysis. Membranes 2023, 13, 207. https://doi.org/10.3390/membranes13020207
Cao D-Q, Tang K, Zhang W-Y, Chang C, Han J-L, Tian F, Hao X-D. Calcium Alginate Production through Forward Osmosis with Reverse Solute Diffusion and Mechanism Analysis. Membranes. 2023; 13(2):207. https://doi.org/10.3390/membranes13020207
Chicago/Turabian StyleCao, Da-Qi, Kai Tang, Wen-Yu Zhang, Cheng Chang, Jia-Lin Han, Feng Tian, and Xiao-Di Hao. 2023. "Calcium Alginate Production through Forward Osmosis with Reverse Solute Diffusion and Mechanism Analysis" Membranes 13, no. 2: 207. https://doi.org/10.3390/membranes13020207