Quercetin-Loaded Polycaprolactone-Polyvinylpyrrolidone Electrospun Membranes for Health Application: Design, Characterization, Modeling and Cytotoxicity Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Quercetin-Loaded Membranes
2.3. Methods
2.3.1. Morphological Analysis
2.3.2. Contact Angle and Liquid Retention Tests
2.3.3. Release Kinetic of Quercetin
2.3.4. Photostability
2.4. Computational Work
2.5. Biological Activity
2.5.1. Cells Culture
2.5.2. Cell Viability Assay
2.6. Statistical Analysis
3. Results
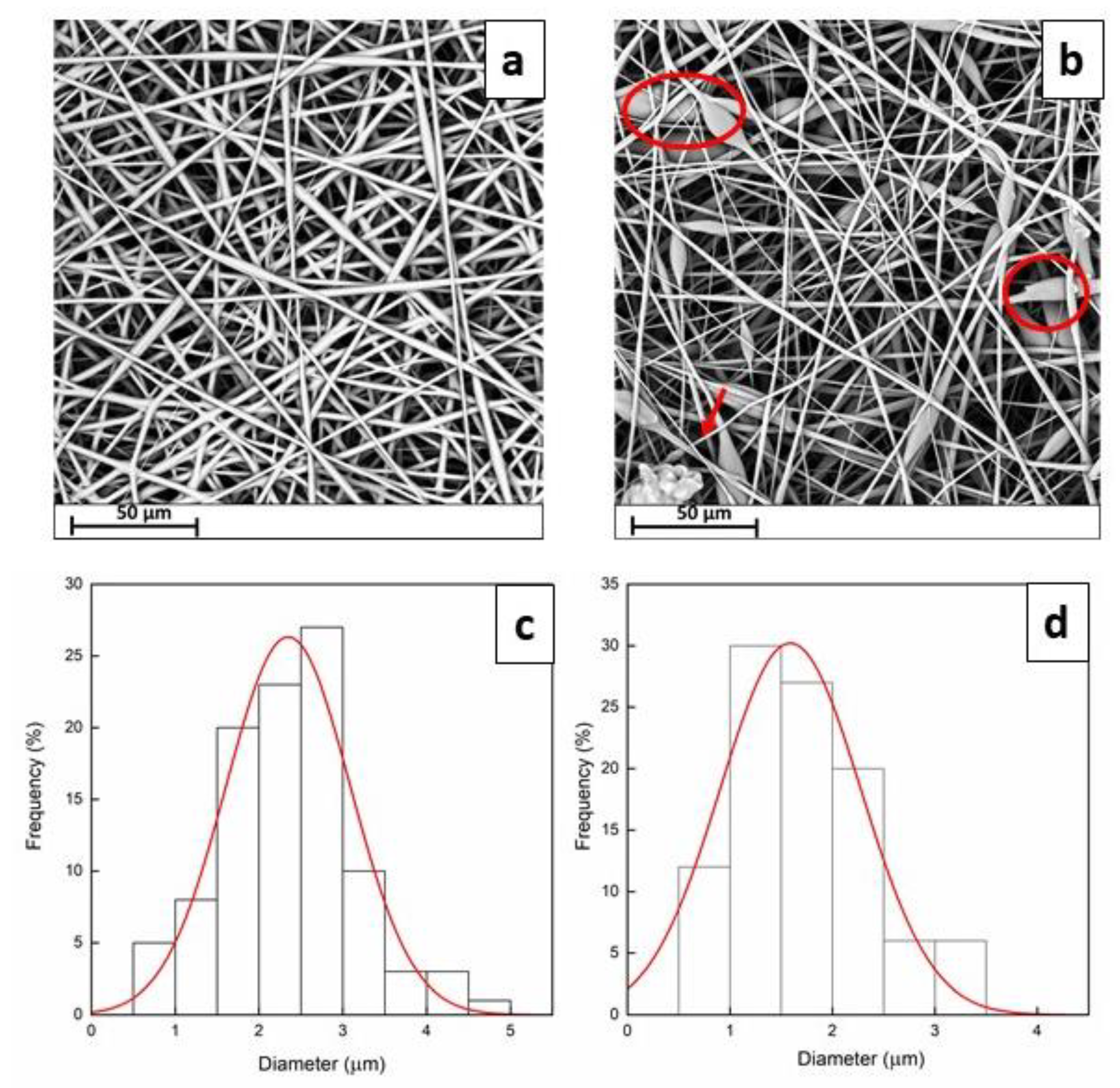
3.1. Morphological Analysis
3.2. Computational Analysis
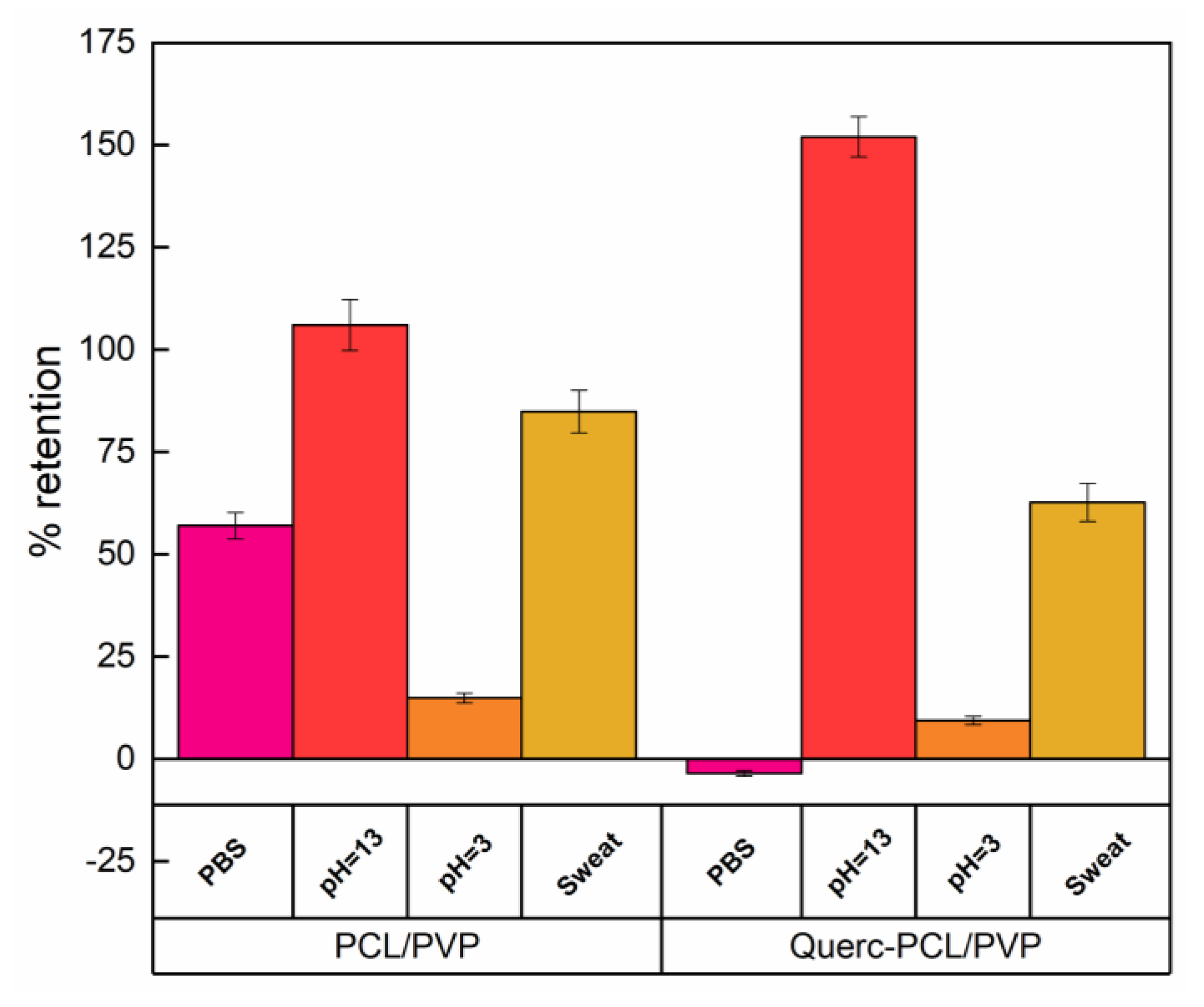
3.3. Liquid Retention Tests
3.4. Release Kinetics of Quercetin
3.5. Photostability
3.6. Effects of Quercetin-Loaded Membrane on Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of Electrospinning Technique for Biomedical Applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Longo, R.; Viscusi, G. Fabrication and Characterization of Electrospun Membranes Based on “Poly(ε-Caprolactone)”, “Poly(3-Hydroxybutyrate)” and Their Blend for Tunable Drug Delivery of Curcumin. Polymers 2020, 12, 2239. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent Advancements in Electrospinning Design for Tissue Engineering Applications: A Review. J. Biomed. Mater. Res. Part A 2017, 105, 2892–2905. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending Instability of Electrically Charged Liquid Jets of Polymer Solutions in Electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef] [Green Version]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun Nanofiber Scaffolds: Engineering Soft Tissues. Biomed. Mater 2008, 3, 34002. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of Nanofiber Matrix as Tissue-Engineering Scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable Electrospun Poly (l-Lactide) Fibers Containing Antibacterial Silver Nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T.; Supaphol, P. Preparation and Characterization of Chitosan-Hydroxybenzotriazole/Polyvinyl Alcohol Blend Nanofibers by the Electrospinning Technique. Carbohydr. Polym. 2010, 81, 675–680. [Google Scholar] [CrossRef]
- Chegoonian, P.; Feiz, M.; Ravandi, S.A.H.; Mallakpour, S. Preparation of Sulfonated Poly(Ethylene Terephthalate) Submicron Fibrous Membranes for Removal of Basic Dyes. J. Appl. Polym. Sci. 2012, 124, E190–E198. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Ribelles, J.L.G.; Lanceros-Méndez, S. Influence of Processing Conditions on Polymorphism and Nanofiber Morphology of Electroactive Poly (Vinylidene Fluoride) Electrospun Membranes. Soft Mater. 2010, 8, 274–287. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H. Electrospinning, Mechanical Properties, and Cell Behavior Study of Chitosan/PVA Nanofibers. J. Biomed. Mater. Res. Part A 2015, 103, 3081–3093. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A. On the Way to Clean and Safe Electrospinning-Green Electrospinning: Emulsion and Suspension Electrospinning. Polym. Adv. Technol. 2011, 22, 372–378. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Vittoria, V.; Gorrasi, G. Coaxial Electrospun Membranes of Poly (ε-Caprolactone)/Poly (Lactic Acid) with Reverse Core-Shell Structures Loaded with Curcumin as Tunable Drug Delivery Systems. Polym. Adv. Technol. 2021, 32, 4005–4013. [Google Scholar] [CrossRef]
- Meng, Z.X.; Xu, X.X.; Zheng, W.; Zhou, H.M.; Li, L.; Zheng, Y.F.; Lou, X. Preparation and Characterization of Electrospun PLGA/Gelatin Nanofibers as a Potential Drug Delivery System. Colloids Surf. B Biointerfaces 2011, 84, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, E.; Rajiv, S.; Natarajan, T.S. Comparison of Preparation and Characterization of Water-Bath Collected Porous Poly L–Lactide Microfibers and Cellulose/Silk Fibroin Based Poly L-Lactide Nanofibers for Biomedical Applications. J. Polym. Res. 2015, 22, 1–9. [Google Scholar] [CrossRef]
- Liparoti, S.; Mottola, S.; Viscusi, G.; Belvedere, R.; Petrella, A.; Gorrasi, G.; Pantani, R.; De Marco, I. Production of Mesoglycan/PCL Based Composites through Supercritical Impregnation. Molecules 2022, 27, 5800. [Google Scholar] [CrossRef]
- Bugatti, V.; Vertuccio, L.; Viscusi, G.; Gorrasi, G. Antimicrobial Membranes of Bio-Based Pa 11 and Hnts Filled with Lysozyme Obtained by an Electrospinning Process. Nanomaterials 2018, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Croitoru, A.M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly (Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Bugatti, V.; Sorrentino, A.; Gorrasi, G. Encapsulation of Lysozyme into Halloysite Nanotubes and Dispersion in PLA: Structural and Physical Properties and Controlled Release Analysis. Eur. Polym. J. 2017, 93, 495–506. [Google Scholar] [CrossRef]
- Xu, H.; Cui, W.; Chang, J. Fabrication of Patterned PDLLA/PCL Composite Scaffold by Electrospinning. J. Appl. Polym. Sci. 2013, 127, 1550–1554. [Google Scholar] [CrossRef]
- Di Salle, A.; Viscusi, G.; Di Cristo, F.; Valentino, A.; Gorrasi, G.; Lamberti, E.; Vittoria, V.; Calarco, A.; Peluso, G. Antimicrobial and Antibiofilm Activity of Curcumin-Loaded Electrospun Nanofibers for the Prevention of the Biofilm-Associated Infections. Molecules 2021, 26, 4866. [Google Scholar] [CrossRef] [PubMed]
- García Cruz, D.M.; Coutinho, D.F.; Mano, J.F.; Gómez Ribelles, J.L.; Salmerón Sánchez, M. Physical Interactions in Macroporous Scaffolds Based on Poly (ε-Caprolactone)/Chitosan Semi-Interpenetrating Polymer Networks. Polymer 2009, 50, 2058–2064. [Google Scholar] [CrossRef]
- Kim, G.M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP Blends for Tissue Engineering Scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fang, D.; Wang, N.; Jiang, S.; Nie, J.; Yu, Q.; Ma, G. Preparation of PVDF/PVP Core–Shell Nanofibers Mats via Homogeneous Electrospinning. Polymer 2014, 55, 2188–2196. [Google Scholar] [CrossRef]
- Contardi, M.; Russo, D.; Suarato, G.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Penna, I.; Margaroli, N.; Summa, M.; Spanò, R.; Tassistro, G.; et al. Polyvinylpyrrolidone/Hyaluronic Acid-Based Bilayer Constructs for Sequential Delivery of Cutaneous Antiseptic and Antibiotic. Chem. Eng. J. 2019, 358, 912–923. [Google Scholar] [CrossRef]
- Cui, L.; Li, Z.; Chang, X.; Cong, G.; Hao, L. Quercetin Attenuates Vascular Calcification by Inhibiting Oxidative Stress and Mitochondrial Fission. Vascul. Pharmacol. 2017, 88, 21–29. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Rodríguez, B.A.; Lagaron, J.M.; López-Rubio, A. Improved Antioxidant Capacity of Quercetin and Ferulic Acid during In-Vitro Digestion through Encapsulation within Food-Grade Electrospun Fibers. J. Funct. Foods 2015, 12, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Vyas, V.S.; Vishwakarma, M.; Moudrakovski, I.; Haase, F.; Savasci, G.; Ochsenfeld, C.; Spatz, J.P.; Lotsch, B.V.; Vyas, V.S.; Moudrakovski, I.; et al. Exploiting Noncovalent Interactions in an Imine-based Covalent Organic Framework for Quercetin Delivery. Adv. Mater. 2016, 28, 8749–8754. [Google Scholar] [CrossRef]
- Chu, J.; Shi, P.; Yan, W.; Fu, J.; Yang, Z.; He, C.; Deng, X.; Liu, H. PEGylated Graphene Oxide-Mediated Quercetin-Modified Collagen Hybrid Scaffold for Enhancement of MSCs Differentiation Potential and Diabetic Wound Healing. Nanoscale 2018, 10, 9547–9560. [Google Scholar] [CrossRef]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shabani Shayeh, J. Electrospun Poly-Caprolactone/Graphene Oxide/Quercetin Nanofibrous Scaffold for Wound Dressing: Evaluation of Biological and Structural Properties. Life Sci. 2020, 257, 118062–118072. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Hu, T.G.; Li, L.; Zong, M.H.; Wu, H. A Colon-Specific Delivery System for Quercetin with Enhanced Cancer Prevention Based on Co-Axial Electrospinning. Food Funct. 2018, 9, 5999–6009. [Google Scholar] [CrossRef] [PubMed]
- Visser, Z.B.; Kumar Verma, S.; Rainey, J.K.; Frampton, J.P. Loading and Release of Quercetin from Contact-Drawn Polyvinyl Alcohol Fiber Scaffolds. ACS Pharmacol. Transl. Sci. 2022, 2022, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Nikhil, K.; Pemmaraju, S.C.; Pruthi, P.A.; Mallick, V.; Singh, H.; Patel, A.; Mishra, N.C.; Singh, R.P.; Pruthi, V. Antibiofilm Activity of Quercetin-Encapsulated Cytocompatible Nanofibers against Candida albicans. J. Bioact. Compat. Polym. 2013, 28, 652–665. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, W.; Liu, L.; Wang, M.; Li, F.; Shen, W. Characterization and Bacteriostatic Effects of β-Cyclodextrin/Quercetin Inclusion Compound Nanofilms Prepared by Electrospinning. Food Chem. 2021, 338, 127980. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Krupa, I.; Nedelčev, T.; Chorvát, D.; Račko, D.; Lacík, I. Glucose Diffusivity and Porosity in Silica Hydrogel Based on Organofunctional Silanes. Eur. Polym. J. 2011, 47, 1477–1484. [Google Scholar] [CrossRef]
- Martucciello, S.; Paolella, G.; Romanelli, A.M.; Sposito, S.; Meola, L.; Cerulli, A.; Masullo, M.; Piacente, S.; Caputo, I. Pro-Apoptotic and Pro-Autophagic Properties of Cardenolides from Aerial Parts of Pergularia tomentosa. Molecules 2022, 27, 4874. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Dong, Y.; Chaudhary, D.; Haroosh, H.; Bickford, T. Development and Characterisation of Novel Electrospun Polylactic Acid/Tubular Clay Nanocomposites. J. Mater. Sci. 2011, 46, 6148–6153. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, N.K.; Tomar, V.R.; Ishika; Kishor, S.; Deep, S. Effect of PH and Temperature on Physicochemical Properties, Aggregation Behaviour and Degradation Kinetics of Quercetin and Baicalein in Nearly Aqueous Media. J. Mol. Liq. 2022, 366, 120236. [Google Scholar] [CrossRef]
- Eskitoros-Togay, M.; Bulbul, Y.E.; Dilsiz, N. Quercetin-Loaded and Unloaded Electrospun Membranes: Synthesis, Characterization and in Vitro Release Study. J. Drug Deliv. Sci. Technol. 2018, 47, 22–30. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Lee, D.S.; Zhu, X.; Yam, K.L. Release Kinetics of Tocopherol and Quercetin from Binary Antioxidant Controlled-Release Packaging Films. J. Agric. Food Chem. 2012, 60, 3492–3497. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Carlotti, M.E.; Sapino, S.; Ugazio, E.; Caron, G. On the Complexation of Quercetin with Methyl-β-Cyclodextrin: Photostability and Antioxidant Studies. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 81–90. [Google Scholar] [CrossRef]
- Smith, G.J.; Thomsen, S.J.; Markham, K.R.; Andary, C.; Cardon, D. The Photostabilities of Naturally Occurring 5-Hydroxyflavones, Flavonols, Their Glycosides and Their Aluminium Complexes. J. Photochem. Photobiol. A Chem. 2000, 136, 87–91. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Szalla, A.; Kamińska, A. Study of Poly (Acrylic Acid)–Poly (Vinylpyrrolidone) Complexes and Their Photostability. Polymer 2001, 42, 6057–6069. [Google Scholar] [CrossRef]
- Preethi, A.M.; Bellare, J.R. Concomitant Effect of Quercetin- and Magnesium-Doped Calcium Silicate on the Osteogenic and Antibacterial Activity of Scaffolds for Bone Regeneration. Antibiotics 2021, 10, 1170. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, W.; Sun, M.; He, P.; Lv, H.; Wang, Q.; Zhang, S.; Wu, Q.; Ling, P.; Chen, S.; et al. Novel Bi-Layered Dressing Patches Constructed with Radially-Oriented Nanofibrous Pattern and Herbal Compound-Loaded Hydrogel for Accelerated Diabetic Wound Healing. Appl. Mater. Today 2022, 28, 101542. [Google Scholar] [CrossRef]
- Li, M.; Qiu, W.; Wang, Q.; Li, N.; Liu, L.; Wang, X.; Yu, J.; Li, X.; Li, F.; Wu, D. Nitric Oxide-Releasing Tryptophan-Based Poly (Ester Urea)s Electrospun Composite Nanofiber Mats with Antibacterial and Antibiofilm Activities for Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 15911–15926. [Google Scholar] [CrossRef] [PubMed]








| Model | PBS (pH = 7.4) | HCl SLN (pH = 3) | ||
|---|---|---|---|---|
| Values | Values | |||
| Zeroth | K0 | 0.66 | K0 | 0.56 |
| R2 | <0 | R2 | <0 | |
| Gompertz | ||||
| α | 0.83 | α | 0.97 | |
| β | −2.7 | β | −1.5 | |
| R2 | 0.99 | R2 | 0.98 | |
| Higuchi | ||||
| KH | 9.49 | KH | 7.93 | |
| R2 | <0 | R2 | <0 | |
| Korsmeyer–Peppas | ||||
| KR | 48.60 | KR | 37.41 | |
| n | 0.13 | n | 0.14 | |
| R2 | 0.58 | R2 | 0.73 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viscusi, G.; Paolella, G.; Lamberti, E.; Caputo, I.; Gorrasi, G. Quercetin-Loaded Polycaprolactone-Polyvinylpyrrolidone Electrospun Membranes for Health Application: Design, Characterization, Modeling and Cytotoxicity Studies. Membranes 2023, 13, 242. https://doi.org/10.3390/membranes13020242
Viscusi G, Paolella G, Lamberti E, Caputo I, Gorrasi G. Quercetin-Loaded Polycaprolactone-Polyvinylpyrrolidone Electrospun Membranes for Health Application: Design, Characterization, Modeling and Cytotoxicity Studies. Membranes. 2023; 13(2):242. https://doi.org/10.3390/membranes13020242
Chicago/Turabian StyleViscusi, Gianluca, Gaetana Paolella, Elena Lamberti, Ivana Caputo, and Giuliana Gorrasi. 2023. "Quercetin-Loaded Polycaprolactone-Polyvinylpyrrolidone Electrospun Membranes for Health Application: Design, Characterization, Modeling and Cytotoxicity Studies" Membranes 13, no. 2: 242. https://doi.org/10.3390/membranes13020242
APA StyleViscusi, G., Paolella, G., Lamberti, E., Caputo, I., & Gorrasi, G. (2023). Quercetin-Loaded Polycaprolactone-Polyvinylpyrrolidone Electrospun Membranes for Health Application: Design, Characterization, Modeling and Cytotoxicity Studies. Membranes, 13(2), 242. https://doi.org/10.3390/membranes13020242








