A Guide to Your Desired Lipid-Asymmetric Vesicles
Abstract
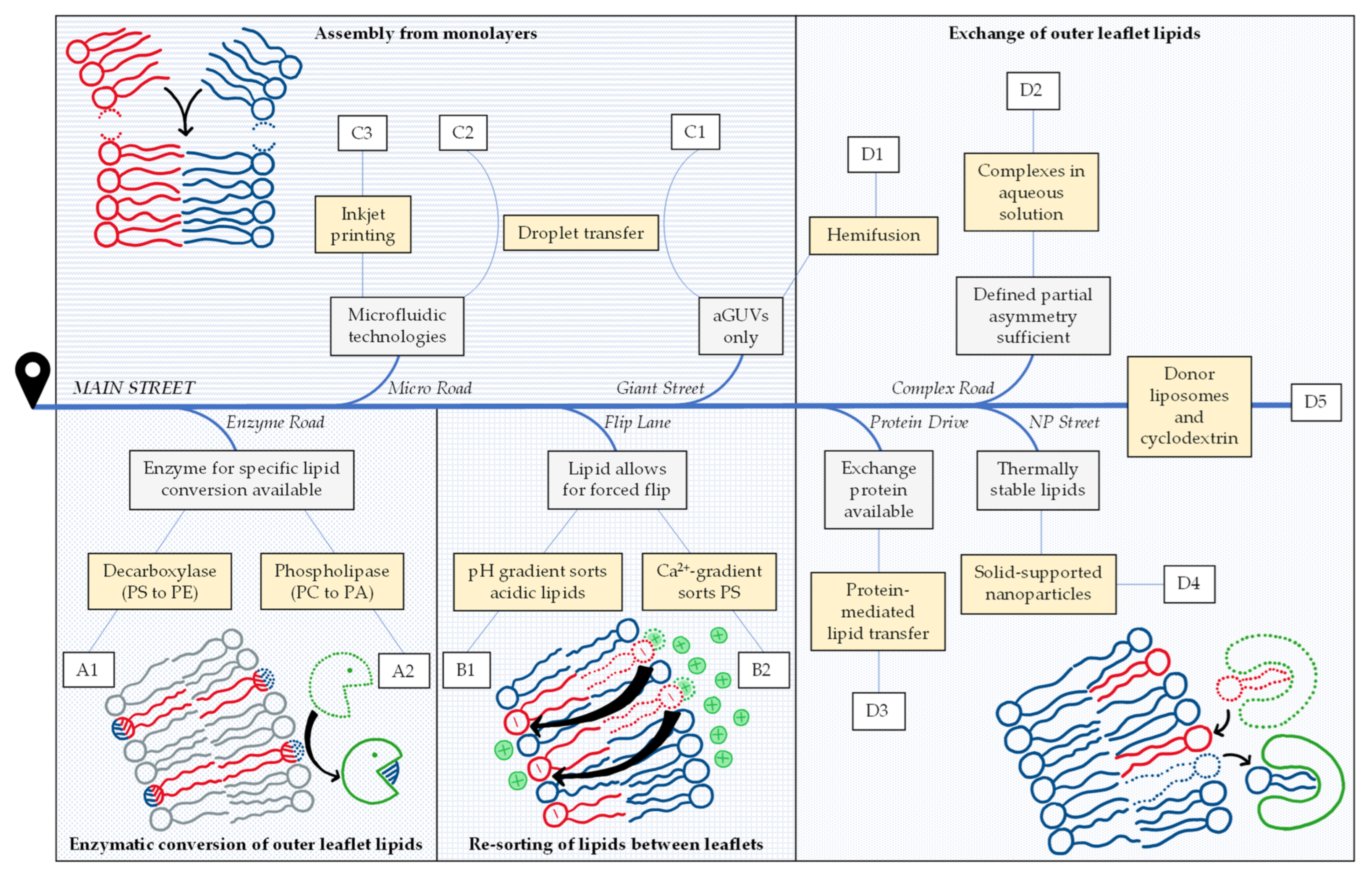
1. Aims and Content of This Review
2. Navigating the Preparation of Asymmetric Model Membranes
2.1. Enzymatic Conversion of Outer Leaflet Lipids
2.2. Re-Sorting of Lipids between Leaflets
2.3. Assembly from Monolayers
2.4. Exchange of Outer Leaflet Lipids
| Vesicle Type | Asy | Outer Leaflet | Inner Leaflet | Verification of Asy | Short Description | Ref. |
|---|---|---|---|---|---|---|
| A. Enzymatic conversion of outer leaflet lipids | ||||||
| A.1. Decarboxylase | ||||||
| LUV | 97% 1 | DOPC/NBD-PE | NBD-PS | FRET, trinitrophenylation | one-step method, enzyme conversion of PS to PE by PS-decarboxylase | [16] |
| LUV | a = −0.5 (PS), a ≈ 1 (PE) 2 | ePC/PE PC/chol/eSM/PE PC/PE PC/PE/PG/PE | POPS POPG POPS/POPG | ζ-potential, HPTLC | PS-decarboxylase converts PS to PE, aLUVs mimic PS-asymmetry of eukaryotic plasma membranes | [15] |
| A.2. Phospholipase D | ||||||
| LUV | 49% 1 | PA | PC/PE/N-NBD-PE/N-Rho-PE | F (N-Rho-PE, N-NBD-PE) | outer lipid conversion to PA, influenza-induced fusion between viral and liposome membrane | [17] |
| LUV | >95% 3 | POPS/POPE | POPC± chol | enzymatic assay/optical absorption, HPLC | enzymatic conversion of PC in the presence of serine and ethanolamine | [14] |
| B. Re-sorting of lipids between leaflets | ||||||
| B.1. pH gradient | ||||||
| LUV | 80–90% 10 | DOPC | ePG DOPA | ion-exchange C, 13C NMR, periodate oxidation | asymmetric distributions of PA in aLUVs via pH gradients | [21] |
| LUV | 50% 13 | DPPC DPoPC DOPC ePC ePC/chol ePC/PS | ePG DOPG MOPG | periodate oxidation | mechanism of pH-induced PG trans-bilayer transport | [22] |
| LUV | >80% | PA PC CL | PC PG SA | two-phase polymer partition, 3H-radioactivity | effect of temperature and lipid composition on formation and extent of asymmetry | [23] |
| LUV | >95% 13 | DOPA DOPE/DOPC/PI | DOPE/DOPC/PI DOPA | F (TNS) | influence of lipid asymmetry on Ca2+-stimulated vesicles fusion | [19] |
| GUV | n.a. | ePG | ePC | phase contrast M | influence of lipid redistribution on the shape of GUVs | [24] |
| LUV | n.a. | ePC/chol ePC/DOPE/chol | amino lipids AL1-AL6 | F (TNS) | pH gradient induced fusion of liposomes containing synthetic amino lipids | [20] |
| LUV | >80% 13 | DOPC ePC | DOPA ePA | NMR | NMR observation on transbilayer distribution of Chlorpromazine | [25] |
| B.2. Ca2+-ions | ||||||
| LUV | ≤30% 13 | DPPC | DOPS | FQ (NBD-PS), nanoDSC | Ca2+-induced inward flip of PS for controlled production of aLUVs | [26] |
| LUV | 38.5–52.3% 14 | DPPC | DOPS | FQ (NBD-PS), nanoDSC | effect of size, temperature and lipid composition on Ca2+-induced PS inward flip | [27] |
| C. Assembly from monolayers | ||||||
| C.1. Droplet transfer/emulsion phase transfer | ||||||
| GUV | ≤95% | POPC ePC | POPS polystyrene-polyacrylic acid | FQ (NBD-PE, NBD-PS) | engineering aGUVs with two independently prepared monolayers | [28] |
| GUV | n.a. | POPC/py-16-PC POPC/POPE/CL | POPC/POPE/CL POPC/py-16-P | F (pyrene) | membrane − protein interactions between Bax and liposomes of size 0.3-1.5 µm | [85] |
| GUV | n.a. | DOPC | ePC DOPC/DPPC/chol | FM (Rho-PE, NBD-PE) | cell-sized aGUVs, control over vesicle size via sugar gradient | [41] |
| GUV | n.a. | ePC DOPG | DOPE ePC DOPG | n.a. | reconstitution of the potassium channel KcsA into aGUVs | [54] |
| GUV | n.a. | POPC DOPC | DOPC POPC | FM of hemifused GUVs (Rho-PE, NBD-PE) | effects of lipid asymmetry on membrane bending rigidity | [44] |
| GUV | n.a. | DOPC/chol NBPC/chol | NBPC/chol DOPC/chol | FM (Rho-DHPE) | asymmetric distribution of photocleavable lipid, photoinduced pinocytosis behaviour | [86] |
| GUV | n.a. | DSPE/DSPG | DSPG DSPE DOPG DOPC | FQ (NBD-PE) | influence of lipid head group and acyl chain on Daptomycin-induced membrane permeability | [87] |
| GUV | n.a. | DOPC DOPC/DOPS DOPC/DOPG | DOPC/DOPS DOPC/DOPG DOPC | F Annexin V (Alexa Fluor 488) | protein translocation via cell-penetrating peptides, start of enzymatic reactions in aGUVs | [88] |
| C.2. Droplet transfer/microfluidic technologies | ||||||
| GUV | 85% | DPPC DOPC PS | DPPC DOPC PS | FQ (Texas Red (TR)-modified DPPE), biotin-binding (biotin-DPPE, avidin), F Annexin V (Alexa Fluor 488) | two-step fabrication of monodisperse and unilamellar aGUVs | [30] |
| GUV | 100% | NBD-DOPC | DOPC | F (NBD-DOPC) | controlled construction of uni- or multilamellar aGUVs using layer-by-layer membrane assembly | [31] |
| GUV | 90–95% | DOPC | DOPE | FM, FQ (NBD-DOPC, TR-DOPE) | continuous fabrication of aGUVs via double emulsions with customized membrane composition, size and luminal content | [32] |
| GUV | 95% | DMPC DOPC | DOPC DMPC | FQ (NBD-PC) | influence of asymmetry on area expansion modulus, customized micropipette aspiration system | [42] |
| GUV | n.a. | DOPC | POPC | F click chemistry (DSPE-DBCO, 3-azido-7-hydroxycoumarin) | high-throughput fabrication of aGUVs from aqueous lipid dispersions | [43] |
| GUV | ≤70% | DOPC DOPE-biotinyl | DOPC DOPE-biotinyl/DOPC | F/biotin-streptavidin (DOPE-biotinyl, streptavidin fluorescein isothiocyanate ST-FITC) | continuous single-step fabrication in a glass device using triple emulsion drops | [33] |
| GUV, LUV | 79% | POPS | POPC | FM (carboxy-fluorescein), FQ (NBD-PC) | preparation of liposomes with desired diameters using a tunable microfluidic device including a polycarbonate filter | [34] |
| GUV | 83% | POPC | DOPC | FQ (NBD-DHPE) | aGUVs with precisely modulated size and minimal oil contamination | [35] |
| C.3. Inkjet printing | ||||||
| GUV | n.a. | DPhPC/TMR-PIP2 PE-PEG2000 DPhPC/DGS-NTA-Ni DPhPC DPhPC/DPhPS/chol | DPhPC/TMR-PIP2 PE-PEG2000 DPhPC/DGS-NTA-Ni DPhPC DPhPC/DPhPS/chol | FM (TMR-PIP2, His-GFP) | separate vesicle and bilayer formation allows for monitoring and minimizing oil contamination | [36] |
| GUV | n.a. | DOPC DOPS | DOPC DOPS DOPS/DOPE/DOPC | F Annexin V (Alexa Fluor 488, 546) | membrane dynamics and protein interactions, use of little organic solvent | [37] |
| GUV | n.a. | DOPC DOPC/DOPE | DOPC/DOPS | FQ (Rho-DOPE) | device for sequentially generating aGUVs, influence of the peptide Cinnamycin on lipid dynamics | [38] |
| LUV | n.a. | DOPC biotin-DOPE | biotin-DOPE DOPC | Biotin-streptavidin (biotin-PEG(2000)-DSPE, Streptavidin-conjugated gold) and TEM, FM (Rho-DOPE) | fabricating nano-sized liposomes from a planar lipid bilayer by applying a pulsed-jet flow with optimized duration and pressure | [39] |
| GUV | n.a. | DOPC DOPC/DOPS | DOPC/DOPS DOPC/DOTAP | FM (Rho-DOPE) | fusion between LUVs and a monolayer, followed by application of a pulsed jet flow | [40] |
| D. Exchange of outer leaflet lipids | ||||||
| D.1. Hemifusion | ||||||
| GUV | 50–99% 12 | DOPC/chol | DSPC | FM (TRPC, DiD), FQ (NBD-PE) | hemifusion of giant vesicles and a supported lipid bilayer | [45] |
| GUV | ≤86% 12 | DOPC/chol | DSPC/POPC | FM (TRPC, DiD) | systematic study of aGUVs to investigate modulated phases | [46] |
| GUV | >70% 12 | DOPC DOPC/chol | DSPC bSM DSPC | FM (TRPC, DiD) | phase behavior and cholesterol movement in aGUVs | [47] |
| D.2. Complexes in aqueous solution | ||||||
| LUV | 0.05–0.45 9 | POPG | POPC | ζ-potential | PG-loaded mβCD-lipid-complexes in solution replace PC by PG | [48] |
| LUV | 0.2 9 | POPG | POPE/POPC/TOCL | ζ-potential | five-step protocol to proteoliposomes with incorporated ST-NhaA | [49] |
| LUV | 0.25 9 | POPG | POPC POPC/POPE | ζ-potential | phospholipid modulation of ELIC in PG-asymmetric proteoliposomes | [50] |
| D.3. Protein-mediated lipid transfer | ||||||
| SUV | 10–20% 4 | [N-13CH3]-DOPC [N-13CH3]-DMPC | DMPC DOPC | 13C-NMR | transfer of PC between acceptor and donor PC vesicles | [56] |
| MV | 62% 4 | [3H]-DOPC [3H]-DOPE | DOPC/DOPE/CL | radioactivity, TNBS-labeling | lipid transfer between isotopically asymmetric vesicles and chinese hamster fibroblasts | [58] |
| SUV | ≤59.1% 4 | BRPC POPC | POPC BRPC | F (CUGA), GC | studies on the membrane-binding domain of cytochrome b5 in brominated aSUVs | [57] |
| LUV | 60% 13 or 3 mol% 5 | ePG | ePC | FFE | pro-SCP2 mediated EPG transfer, separation of donor and acceptor vesicles via FFE | [59] |
| D.4. Solid-supported nanoparticles | ||||||
| SUV | 75.6 mol% 5, 24.4 mol% 10 | hDPPC | d62DPPC d75DPPC | SANS, 1H-NMR | lipid exchange via lipid-coated silica nanoparticles | [53] |
| D.5. Donor liposomes and cyclodextrin | ||||||
| SUV | 75–82% 4 | bSM | DOPC POPC POPS/POPE DOPC/chol POPE/POPS/chol | FA (DPH, TMA-DPH), HPTLC, pL4A18 peptide binding | mβCD-mediated lipid exchange, ha-strategy | [61] |
| GUV | 60% 4 | bSM | DOPC bPC bPC/bPE ± chol | FCS (Nile-red NR12S, NBD-PE) | solvent free method for mβCD-induced lipid exchange to prepare aGUVs, ha-strategy | [73] |
| LUV | 80–100% 4 | bSM bSM/POPC | DOPE/POPS ± chol | FA (DPH, TMA-DPH), HPTLC, pL4A18 peptide binding | mβCD-mediated exchange (ha-strategy), investigation of interleaflet coupling | [74] |
| GUV, SUV | 20–80 mol% 4 | bSM mSM C24:0-SM/bSM | DOPC POPC/bSM SOPC/bSM OMPC/bSM | FLIM (NBD-DPPE, NBD-DOPE, TMA-DPH) | mβCD-induced exchange (ha-strategy) with lipids of various acyl chains, investigation of interleaflet coupling | [75] |
| SUV | 62–96% 4 | bSM | di14:1PC di16:1PC di18:1PC di20:1PC di22:1PC diphyPC 16:01-18:2PC 16:0-20:4PC di18:2PC di18:3PC di20:4PC | FA (TMA-DPH), HPTLC | mβCD-mediated lipid exchange (ha-strategy), effects of PC acyl chain structure | [76] |
| LUV | >90% 4 | bSM PC bSM/PC | PE/PS/chol | HPTLC, pL4A18 peptide binding, TNBS-labeling | HPαCD-mediated exchange (ha-strategy) with controlled amount of cholesterol (0-50 mol%) | [78] |
| LUV | 80–90% 4 | POPC | POPE/POPS/chol | HPTLC, pL4A18 peptide binding, TNBS-labeling | HPαCD-mediated exchange (ha-strategy), influence of lipid composition and asymmetry on Perfringolysin O | [79] |
| GUV, LUV | 50 mol% 5 | eSM mSM | DOPC/chol | F (Rho-DOPE, NBD-DOPE), FA (TMA-DPH), HPTLC | solvent-free method for HPαCD-induced lipid exchange (ha-strategy) and control of cholesterol | [80] |
| LUV | n.a. | bSM | DOPE/POPS | F (Topfluor-PC) | mβCD-induced exchange (ha-strategy), antibody-decorated aLUVs bind HIV-1 virus-like particles | [77] |
| LUV | ≤0.95 6 | POPC-dHC POPC DPPC-dC | POPC POPC-dH POPC-dHC | GC-MS, 1H-NMR, SANS | solvent-free and sucrose-free aLUVs prepared via mβCD-mediated exchange, hd-strategy | [62] |
| GUV | n.a. | bSM 24:1-SM 16:0-SM 18:0-SM | POPC/SM POPC ± chol | TLC, FA (DPH, TMA-SPH) | mβCD-induced exchange (ha-strategy), influence of lipid composition on AChR distribution in symmetric and asymmetric liposomes | [55] |
| LUV | n.a. | py-PG py-PG py-PI | POPC | F (pyrene) | kinetic analysis of mβCD-mediated exchange via real-time monitoring of intervesicular lipid transfer | [64] |
| LUV | 60% 4 | DPPC DPPC-d62 POPC-d44 POPC | POPC POPC-d44 POPC-d31 | GC-MS, UPLC-MS, 1H-NMR, SANS | SANS and SAXS analysis of aLUVs prepared via mβCD-induced lipid exchange, hd-strategy | [65] |
| LUV | 59% 4 | DPPC-dC | DPPC-dH | 1H-NMR, GC | mβCD-mediated exchange (hd-strategy), lipid flip-flop in gel and fluid bilayers | [60] |
| LUV | 0.48–0.67 7 | POPC POPE | POPE POPC | DSC, UPLC-MS, 1H-NMR | mβCD-induced exchange (hd-strategy), leaflet-specific lipid packing and melting | [66] |
| LUV, SUV | 0.34–0.45 8 | DMPC-d54 eSM DPPC DPPC | POPC-d13 POPC POPE ± chol | GC-MS, 1H-NMR | detailed protocol for the preparation of asymmetric vesicles via mβCD-mediated lipid exchange | [63] |
| LUV | ≤85.9 4 | mSM bSM eSM DMPC DPPC diC(15:0)PC DSPC | DOPC ± chol | 1H-NMR, HPTLC, FQ (Rho-DMPE, Rho-DOPE) | HPαCD-induced lipid exchange (ha-strategy), domain formation and interleaflet coupling using FRET | [81] |
| GUV, LUV | 30–40% 4 | C24-SM C18-SM C16-SM C16-SM/C14-SM PC | DPPC/DOPC/chol POPC/POPS/POPE POPC/POPS/DOPE | FM (NBD-DPPE, Rho-DPPE), MS FA (TMA-DPH) | mβCD-induced exchange (ha-strategy), influence of C24 sphingolipids on cholesterol and membrane microdomains | [67] |
| LUV | ≤75% 4 | bSM | DOPC sterol (chol, epichol, lanosterol, 7-dehydrochol, 4-cholesten-3-one) | FRET (Rho-DOPE, DPH), HPTLC | HPαCD-induced exchange (ha-strategy), incorporation of different sterol structures into aLUVs | [82] |
| LUV | 0.32–0.45 9 | POPC-d31 DMPC-d54 | POPC POPC-d13 | GC-MS, 1H-NMR | mβCD-mediated exchange (hd-strategy), influence of Gramicidin on lipid flip-flop and membrane- protein interactions | [68] |
| LUV | 0.44 9 | eSM | POPE | 1H-NMR, 31P-NMR | mβCD-induced lipid exchange (hd-strategy), studies of bending fluctuations via neutron spin-echo spectroscopy | [69] |
| LUV | 7 mol% 10, 3 mol% 5 | POPC-d31 | POPS | GC-MS, F Annexin V assay (Annexin V-568) | mβCD-mediated exchange (hd-strategy), influence of PS asymmetry on the membrane interaction of pHLIP | [70] |
| LUV | ≤100% 8 | POePC DOTAP POPS POPG POPA | POPC POePC/POPC DOTAP/POPC POPS/POPC POPG/POPC POPA/POPC ± chol | HPTLC, F (DPH, TMA-DPH) | mαCD-induced lipid exchange (ha-strategy), entrapment properties of aLUVs containing one cationic and/or anionic leaflet | [83] |
| LUV | 41–96% 11 | POPE/TOCL | POPC | TNBS-labeling F (TTAPE-Me) | mβCD-mediated exchange with donor-SUVs instead of MLVs, effect of lipid asymmetry on MOM permeabilization by apoptotic proteins (tBid/Bax) | [89] |
| LUV | 73% 4 | POPC | POPE/POPS/chol | TLC, F (TMA-DPH) | mαCD-induced lipid exchange with CsCl entrapped in aLUVs | [84] |
| LUV | n.a. | POPE | POPG POPG-d31 | UPLC-MS, GC | mβCD-mediated exchange (hd-strategy), interactions between aLUVs and frog peptides (L18W-PGLa, MG2a) or lactoferricin derivative LF11-215 | [71] |
| LUV | 55–70% 4 | MSPC SMPC PMPC MSM POPC SOPC | DPPC | GC, SANS | mβCD-induced exchange (hd-strategy), transleaflet coupling of aLUVs in the fluid phase | [72] |
3. Testing Asymmetry
4. Cholesterol
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric lipid membranes: Towards more realistic model systems. Membranes 2015, 5, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Hossain, K.R.; Cao, K. Physiological roles of transverse lipid asymmetry of animal membranes. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183382. [Google Scholar] [CrossRef]
- Dimova, R.; Aranda, S.; Bezlyepkina, N.; Nikolov, V.; Riske, K.A.; Lipowsky, R. A practical guide to giant vesicles. Probing the membrane nanoregime via optical microscopy. J. Phys. Condens. Matter 2006, 18, S1151–S1176. [Google Scholar] [CrossRef]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant Vesicles: Preparations and Applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef]
- Kamiya, K.; Takeuchi, S. Giant liposome formation toward the synthesis of well-defined artificial cells. J. Mater. Chem. B 2017, 5, 5911–5923. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K. Development of artificial cell models using microfluidic technology and synthetic biology. Micromachines 2020, 11, 559. [Google Scholar] [CrossRef]
- Hu, P.C.; Malmstadt, N. Asymmetric Giant Lipid Vesicle Fabrication. Methods Membr. Lipids Second Ed. 2014, 1232, 1–327. [Google Scholar] [CrossRef]
- Huang, Y.; Kim, S.H.; Arriaga, L.R. Emulsion templated vesicles with symmetric or asymmetric membranes. Adv. Colloid Interface Sci. 2017, 247, 413–425. [Google Scholar] [CrossRef]
- Ai, Y.; Xie, R.; Xiong, J.; Liang, Q. Microfluidics for Biosynthesizing: From Droplets and Vesicles to Artificial Cells. Small 2020, 16, e1903940. [Google Scholar] [CrossRef]
- Céspedes, P.F.; Beckers, D.; Dustin, M.L.; Sezgin, E. Model membrane systems to reconstitute immune cell signaling. FEBS J. 2021, 288, 1070–1090. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, S.; Li, B.; London, E. Preparation and Utility of Asymmetric Lipid Vesicles for Studies of Perfringolysin O-Lipid Interactions, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 649, ISBN 9780128238585. [Google Scholar] [CrossRef]
- London, E. Ordered Domain (Raft) Formation in Asymmetric Vesicles and Its Induction upon Loss of Lipid Asymmetry in Artificial and Natural Membranes. Membranes 2022, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.L.; Kennison, K.B.; Enoki, T.A.; Doktorova, M.; Kinnun, J.J.; Heberle, F.A.; Katsaras, J. Model Membrane Systems Used to Study Plasma Membrane Lipid Asymmetry. Symmetry 2021, 13, 1356. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, R.; Kurosaki, H.; Nakao, H.; Ikeda, K.; Nakano, M. Formation of asymmetric vesicles via phospholipase D-mediated transphosphatidylation. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 245–249. [Google Scholar] [CrossRef]
- Drechsler, C.; Markones, M.; Choi, J.Y.; Frieling, N.; Fiedler, S.; Voelker, D.R.; Schubert, R.; Heerklotz, H. Preparation of Asymmetric Liposomes Using a Phosphatidylserine Decarboxylase. Biophys. J. 2018, 115, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Denkins, Y.M.; Schroit, A.J. Phosphatidylserine decarboxylase: Generation of asymmetric vesicles and determination of the transbilayer distribution of fluorescent phosphatidylserine in model membrane systems. BBA-Biomembr. 1986, 862, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Klotz, K.H.; Bartoldus, I.; Stegmann, T. Membrane asymmetry is maintained during influenza-induced fusion. J. Biol. Chem. 1996, 271, 2383–2386. [Google Scholar] [CrossRef]
- Madden, T.D.; Harrigan, P.R.; Tai, L.C.L.; Bally, M.B.; Mayer, L.D.; Redelmeier, T.E.; Loughrey, H.C.; Tilcock, C.P.S.; Reinish, L.W.; Cullis, P.R. The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: A survey. Chem. Phys. Lipids 1990, 53, 37–46. [Google Scholar] [CrossRef]
- Eastman, S.J.; Hope, M.J.; Wong, K.F.; Cullis, P.R. Influence of Phospholipid Asymmetry on Fusion Between Large Unilamellar Esicles. Biochemistry 1992, 31, 4262–4268. [Google Scholar] [CrossRef]
- Bailey, A.L.; Cullis, P.R. Modulation of Membrane Fusion by Asymmetric Transbilayer Distributions of Amino Lipids. Biochemistry 1994, 33, 12573–12580. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.J.; Redelmeier, T.E.; Wong, K.F.; Rodrigueza, W.; Cullis, P.R. Phospholipid Asymmetry in Large Unilamellar Vesicles Induced by Transmembrane pH Gradients. Biochemistry 1989, 28, 4181–4187. [Google Scholar] [CrossRef] [PubMed]
- Redelmeier, T.E.; Hope, M.J.; Cullis, P.R. On the Mechanism of Transbilayer Transport of Phosphatidylglycerol in Response to Transmembrane pH Gradients. Biochemistry 1990, 29, 3046–3053. [Google Scholar] [CrossRef]
- Tilcock, C.; Eastman, S.; Fisher, D. Induction of lipid asymmetry and exchange in model membrane systems. J. Dispers. Sci. Technol. 1991, 12, 129–144. [Google Scholar] [CrossRef]
- Farge, E.; Devaux, P.F. Shape changes of giant liposomes induced by an asymmetric transmembrane distribution of phospholipids. Biophys. J. 1992, 61, 347–357. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tachibana, K. NMR observation on transbilayer distribution of N-[13C]methylated chlorpromazine in asymmetric lipid bilayer of unilamellar vesicles. Chem. Lett. 2000, 29, 302–303. [Google Scholar] [CrossRef]
- Sun, H.Y.; Deng, G.; Jiang, Y.W.; Zhou, Y.; Xu, J.; Wu, F.G.; Yu, Z.W. Controllable engineering of asymmetric phosphatidylserine-containing lipid vesicles using calcium cations. Chem. Commun. 2017, 53, 12762–12765. [Google Scholar] [CrossRef]
- Guo, H.Y.; Sun, H.Y.; Deng, G.; Xu, J.; Wu, F.G.; Yu, Z.W. Fabrication of asymmetric phosphatidylserine-containing lipid vesicles: A study on the effects of size, temperature, and lipid composition. Langmuir 2020, 36, 12684–12691. [Google Scholar] [CrossRef] [PubMed]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Engineering Asymmetric Vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 10718–10721. [Google Scholar] [CrossRef] [PubMed]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Production of unilamellar vesicles using an inverted emulsion. Langmuir 2003, 19, 2870–2879. [Google Scholar] [CrossRef]
- Hu, P.C.; Li, S.; Malmstadt, N. Microfluidic fabrication of asymmetric giant lipid vesicles. ACS Appl. Mater. Interfaces 2011, 3, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S.; Paegel, B.M. Layer-by-layer cell membrane assembly. Nat. Chem. 2013, 5, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Schertzer, J.W.; Chiarot, P.R. Continuous microfluidic fabrication of synthetic asymmetric vesicles. Lab Chip 2015, 15, 3591–3599. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, L.R.; Huang, Y.; Kim, S.H.; Aragones, J.L.; Ziblat, R.; Koehler, S.A.; Weitz, D.A. Single-step assembly of asymmetric vesicles. Lab Chip 2019, 19, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Romanov, V.; McCullough, J.; Gale, B.K.; Frost, A. A Tunable Microfluidic Device Enables Cargo Encapsulation by Cell- or Organelle-Sized Lipid Vesicles Comprising Asymmetric Lipid Bilayers. Adv. Biosyst. 2019, 3, 1900010. [Google Scholar] [CrossRef]
- Maktabi, S.; Malmstadt, N.; Schertzer, J.W.; Chiarot, P.R. An integrated microfluidic platform to fabricate single-micrometer asymmetric giant unilamellar vesicles (GUVs) using dielectrophoretic separation of microemulsions. Biomicrofluidics 2021, 15, 024112. [Google Scholar] [CrossRef] [PubMed]
- Richmond, D.L.; Schmid, E.M.; Martens, S.; Stachowiak, J.C.; Liska, N.; Fletcher, D.A. Forming giant vesicles with controlled membrane composition, asymmetry, and contents. Proc. Natl. Acad. Sci. USA 2011, 108, 9431–9436. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Kawano, R.; Osaki, T.; Akiyoshi, K.; Takeuchi, S. Cell-sized asymmetric lipid vesicles facilitate the investigation of asymmetric membranes. Nat. Chem. 2016, 8, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Gotanda, M.; Kamiya, K.; Osaki, T.; Fujii, S.; Misawa, N.; Miki, N.; Takeuchi, S. Sequential generation of asymmetric lipid vesicles using a pulsed-jetting method in rotational wells. Sensors Actuators B Chem. 2018, 261, 392–397. [Google Scholar] [CrossRef]
- Kamiya, K.; Osaki, T.; Takeuchi, S. Formation of nano-sized lipid vesicles with asymmetric lipid components using a pulsed-jet flow method. Sensors Actuators B Chem. 2021, 327, 128917. [Google Scholar] [CrossRef]
- Kamiya, K.; Arisaka, C.; Suzuki, M. Investigation of fusion between nanosized lipid vesicles and a lipid monolayer toward formation of giant lipid vesicles with various kinds of biomolecules. Micromachines 2021, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Miura, Y.; Komatsu, Y.; Kishimoto, Y.; Vestergaard, M.; Takagi, M. Construction of asymmetric cell-sized lipid vesicles from lipid-coated water-in-oil microdroplets. J. Phys. Chem. B 2008, 112, 14678–14681. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Doak, W.J.; Schertzer, J.W.; Chiarot, P.R. Membrane mechanical properties of synthetic asymmetric phospholipid vesicles. Soft Matter 2016, 12, 7521–7528. [Google Scholar] [CrossRef] [PubMed]
- Karamdad, K.; Law, R.V.; Seddon, J.M.; Brooks, N.J.; Ces, O. Studying the effects of asymmetry on the bending rigidity of lipid membranes formed by microfluidics. Chem. Commun. 2016, 52, 5277–5280. [Google Scholar] [CrossRef] [PubMed]
- Elani, Y.; Purushothaman, S.; Booth, P.J.; Seddon, J.M.; Brooks, N.J.; Law, R.V.; Ces, O. Measurements of the effect of membrane asymmetry on the mechanical properties of lipid bilayers. Chem. Commun. 2015, 51, 6976–6979. [Google Scholar] [CrossRef]
- Enoki, T.A.; Feigenson, G.W. Asymmetric Bilayers by Hemifusion: Method and Leaflet Behaviors. Biophys. J. 2019, 117, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.A.; Wu, J.; Heberle, F.A.; Feigenson, G.W. Investigation of the domain line tension in asymmetric vesicles prepared via hemifusion. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183586. [Google Scholar] [CrossRef]
- Enoki, T.A.; Feigenson, G.W. Improving our picture of the plasma membrane: Rafts induce ordered domains in a simplified model cytoplasmic leaflet. Biochim. Biophys. Acta-Biomembr. 2022, 1864, 183995. [Google Scholar] [CrossRef] [PubMed]
- Markones, M.; Drechsler, C.; Kaiser, M.; Kalie, L.; Heerklotz, H.; Fiedler, S. Engineering Asymmetric Lipid Vesicles: Accurate and Convenient Control of the Outer Leaflet Lipid Composition. Langmuir 2018, 34, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Markones, M.; Fippel, A.; Kaiser, M.; Drechsler, C.; Hunte, C.; Heerklotz, H. Stairway to Asymmetry: Five Steps to Lipid-Asymmetric Proteoliposomes. Biophys. J. 2020, 118, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Petroff, J.T.; Dietzen, N.M.; Santiago-McRae, E.; Deng, B.; Washington, M.S.; Chen, L.J.; Trent Moreland, K.; Deng, Z.; Rau, M.; Fitzpatrick, J.A.J.; et al. Open-channel structure of a pentameric ligand-gated ion channel reveals a mechanism of leaflet-specific phospholipid modulation. Nat. Commun. 2022, 13, 7017. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.A.P.; Schleiff, E.; Röhring, C.; Loidl-Stahlhofen, A.; Vergères, G. Interactions of myristoylated alanine-rich C kinase substrate (MARCKS)- related protein with a novel solid-supported lipid membrane system (TRANSIL). Anal. Biochem. 1999, 268, 343–353. [Google Scholar] [CrossRef]
- Reinl, H.M.; Bayerl, T.M. Lipid Transfer between Small Unilamellar Vesicles and Single Bilayers on a Solid Support: Self-Assembly of Supported Bilayers with Asymmetric Lipid Distribution. Biochemistry 1994, 33, 14091–14099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kelley, E.G.; Batchu, K.C.; Porcar, L.; Perez-Salas, U. Creating Asymmetric Phospholipid Vesicles via Exchange with Lipid-Coated Silica Nanoparticles. Langmuir 2020, 36, 8865–8873. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Iwamoto, M.; Kato, A.; Yoshikawa, K.; Oiki, S. Oriented reconstitution of a membrane protein in a giant unilamellar vesicle: Experimental verification with the potassium channel KcsA. J. Am. Chem. Soc. 2011, 133, 11774–11779. [Google Scholar] [CrossRef] [PubMed]
- Perillo, V.L.; Peñalva, D.A.; Vitale, A.J.; Barrantes, F.J.; Antollini, S.S. Transbilayer asymmetry and sphingomyelin composition modulate the preferential membrane partitioning of the nicotinic acetylcholine receptor in Lo domains. Arch. Biochem. Biophys. 2016, 591, 76–86. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, B.; Wirtz, K.W.A. Induction of a relatively fast transbilayer movement of phosphatidylcholine in vesicles. A 13C NMR study. BBA-Biomembr. 1977, 468, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Everett, J.; Zlotnick, A.; Tennyson, J.; Holloway, P.W. Fluorescence quenching of cytochrome b5 in vesicles with an asymmetric transbilayer distribution of brominated phosphatidylcholine. J. Biol. Chem. 1986, 261, 6725–6729. [Google Scholar] [CrossRef] [PubMed]
- Sandra, A.; Pagano, R.E. Liposome-cell interactions. Studies of lipid transfer using isotopically asymmetric vesicles. J. Biol. Chem. 1979, 254, 2244–2249. [Google Scholar] [CrossRef]
- Holzer, M.; Momm, J.; Schubert, R. Lipid transfer mediated by a recombinant pro-sterol carrier protein 2 for the accurate preparation of asymmetrical membrane vesicles requires a narrow vesicle size distribution: A free-flow electrophoresis study. Langmuir 2010, 26, 4142–4151. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Heberle, F.A.; Miti, T.; Eicher, B.; London, E.; Katsaras, J.; Pabst, G. 1H NMR Shows Slow Phospholipid Flip-Flop in Gel and Fluid Bilayers. Langmuir 2017, 33, 3731–3741. [Google Scholar] [CrossRef]
- Cheng, H.T.; Megha; London, E. Preparation and properties of asymmetric vesicles that mimic cell membranes. Effect upon lipid raft formation and transmembrane helix orientation. J. Biol. Chem. 2009, 284, 6079–6092. [Google Scholar] [CrossRef]
- Heberle, F.A.; Marquardt, D.; Doktorova, M.; Geier, B.; Standaert, R.F.; Heftberger, P.; Kollmitzer, B.; Nickels, J.D.; Dick, R.A.; Feigenson, G.W.; et al. Subnanometer Structure of an Asymmetric Model Membrane: Interleaflet Coupling Influences Domain Properties. Langmuir 2016, 32, 5195–5200. [Google Scholar] [CrossRef] [PubMed]
- Doktorova, M.; Heberle, F.A.; Eicher, B.; Standaert, R.F.; Katsaras, J.; London, E.; Pabst, G.; Marquardt, D. Preparation of asymmetric phospholipid vesicles for use as cell membrane models. Nat. Protoc. 2018, 13, 2086–2101. [Google Scholar] [CrossRef]
- Sugiura, T.; Ikeda, K.; Nakano, M. Kinetic Analysis of the Methyl-β-cyclodextrin-Mediated Intervesicular Transfer of Pyrene-Labeled Phospholipids. Langmuir 2016, 32, 13697–13705. [Google Scholar] [CrossRef] [PubMed]
- Eicher, B.; Heberle, F.A.; Marquardt, D.; Rechberger, G.N.; Katsaras, J.; Pabst, G. Joint small-angle X-ray and neutron scattering data analysis of asymmetric lipid vesicles. J. Appl. Crystallogr. 2017, 50, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Eicher, B.; Marquardt, D.; Heberle, F.A.; Letofsky-Papst, I.; Rechberger, G.N.; Appavou, M.S.; Katsaras, J.; Pabst, G. Intrinsic Curvature-Mediated Transbilayer Coupling in Asymmetric Lipid Vesicles. Biophys. J. 2018, 114, 146–157. [Google Scholar] [CrossRef]
- Courtney, K.C.; Pezeshkian, W.; Raghupathy, R.; Zhang, C.; Darbyson, A.; Ipsen, J.H.; Ford, D.A.; Khandelia, H.; Presley, J.F.; Zha, X. C24 Sphingolipids Govern the Transbilayer Asymmetry of Cholesterol and Lateral Organization of Model and Live-Cell Plasma Membranes. Cell Rep. 2018, 24, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Doktorova, M.; Heberle, F.A.; Marquardt, D.; Rusinova, R.; Sanford, R.L.; Peyear, T.A.; Katsaras, J.; Feigenson, G.W.; Weinstein, H.; Andersen, O.S. Gramicidin Increases Lipid Flip-Flop in Symmetric and Asymmetric Lipid Vesicles. Biophys. J. 2019, 116, 860–873. [Google Scholar] [CrossRef]
- Rickeard, B.W.; Nguyen, M.H.L.; Dipasquale, M.; Yip, C.G.; Baker, H.; Heberle, F.A.; Zuo, X.; Kelley, E.G.; Nagao, M.; Marquardt, D. Transverse lipid organization dictates bending fluctuations in model plasma membranes. Nanoscale 2020, 12, 1438–1447. [Google Scholar] [CrossRef]
- Scott, H.L.; Heberle, F.A.; Katsaras, J.; Barrera, F.N. Phosphatidylserine Asymmetry Promotes the Membrane Insertion of a Transmembrane Helix. Biophys. J. 2019, 116, 1495–1506. [Google Scholar] [CrossRef]
- Marx, L.; Frewein, M.P.K.; Semeraro, E.F.; Rechberger, G.N.; Lohner, K.; Porcar, L.; Pabst, G. Antimicrobial peptide activity in asymmetric bacterial membrane mimics. Faraday Discuss. 2021, 232, 435–447. [Google Scholar] [CrossRef]
- Frewein, M.P.K.; Piller, P.; Semeraro, E.F.; Batchu, K.C.; Heberle, F.A.; Scott, H.L.; Gerelli, Y.; Porcar, L.; Pabst, G. Interdigitation-Induced Order and Disorder in Asymmetric Membranes. J. Membr. Biol. 2022, 255, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Chiantia, S.; Schwille, P.; Klymchenko, A.S.; London, E. Asymmetric GUVs prepared by MβCD-mediated lipid exchange: An FCS study. Biophys. J. 2011, 100, L1–L3. [Google Scholar] [CrossRef]
- Cheng, H.T.; London, E. Preparation and properties of asymmetric large unilamellar vesicles: Interleaflet coupling in asymmetric vesicles is dependent on temperature but not curvature. Biophys. J. 2011, 100, 2671–2678. [Google Scholar] [CrossRef]
- Chiantia, S.; London, E. Acyl Chain length and saturation modulate interleaflet coupling in asymmetric bilayers: Effects on dynamics and structural order. Biophys. J. 2012, 103, 2311–2319. [Google Scholar] [CrossRef]
- Son, M.; London, E. The dependence of lipid asymmetry upon phosphatidylcholine acyl chain structure. J. Lipid Res. 2013, 54, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Petazzi, R.A.; Gramatica, A.; Herrmann, A.; Chiantia, S. Time-controlled phagocytosis of asymmetric liposomes: Application to phosphatidylserine immunoliposomes binding HIV-1 virus-like particles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1985–1992. [Google Scholar] [CrossRef]
- Lin, Q.; London, E. Preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry. PLoS ONE 2014, 9, e87903. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; London, E. The influence of natural lipid asymmetry upon the conformation of a membrane-inserted protein (perfringolysin O). J. Biol. Chem. 2014, 289, 5467–5478. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; London, E. Ordered raft domains induced by outer leaflet sphingomyelin in cholesterol-rich asymmetric vesicles. Biophys. J. 2015, 108, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; London, E. Lipid Structure and Composition Control Consequences of Interleaflet Coupling in Asymmetric Vesicles. Biophys. J. 2018, 115, 664–678. [Google Scholar] [CrossRef] [PubMed]
- St. Clair, J.W.; London, E. Effect of sterol structure on ordered membrane domain (raft) stability in symmetric and asymmetric vesicles. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 1112–1122. [Google Scholar] [CrossRef]
- Li, B.; London, E. Preparation and drug entrapment properties of asymmetric liposomes containing cationic and anionic lipids. Langmuir 2020, 36, 12521–12531. [Google Scholar] [CrossRef]
- Li, M.H.; Raleigh, D.P.; London, E. Preparation of Asymmetric Vesicles with Trapped CsCl Avoids Osmotic Imbalance, Non-Physiological External Solutions, and Minimizes Leakage. Langmuir 2021, 37, 11611–11617. [Google Scholar] [CrossRef]
- Epand, R.F.; Martinou, J.C.; Montessuit, S.; Epand, R.M. Transbilayer Lipid Diffusion Promoted by Bax: Implications for Apoptosis. Biochemistry 2003, 42, 14576–14582. [Google Scholar] [CrossRef]
- Konetski, D.; Zhang, D.; Schwartz, D.K.; Bowman, C.N. Photoinduced Pinocytosis for Artificial Cell and Protocell Systems. Chem. Mater. 2018, 30, 8757–8763. [Google Scholar] [CrossRef]
- Howe, A.; Sofou, S. Daptomycin-Induced Lipid Phases on Model Lipid Bilayers: Effect of Lipid Type and of Lipid Leaflet Order on Membrane Permeability. J. Phys. Chem. B 2021, 125, 5775–5785. [Google Scholar] [CrossRef] [PubMed]
- Miwa, A.; Kamiya, K. Control of Enzyme Reaction Initiation inside Giant Unilamellar Vesicles by the Cell-Penetrating Peptide-Mediated Translocation of Cargo Proteins. ACS Synth. Biol. 2022, 11, 3836–3846. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C.; Hou, Y.H.; Schreier, S.; Epand, R.M. Lipid asymmetry of a model mitochondrial outer membrane affects Bax-dependent permeabilization. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183241. [Google Scholar] [CrossRef]
- Varma, M.; Deserno, M. Distribution of cholesterol in asymmetric membranes driven by composition and differential stress. Biophys. J. 2022, 121, 4001–4018. [Google Scholar] [CrossRef] [PubMed]
- Doktorova, M.; Levental, I. Cholesterol’s balancing act: Defying the status quo. Biophys. J. 2022, 121, 3771–3773. [Google Scholar] [CrossRef] [PubMed]
- Tsamaloukas, A.; Szadkowska, H.; Slotte, P.J.; Heerklotz, H. Interactions of cholesterol with lipid membranes and cyclodextrin characterized by calorimetry. Biophys. J. 2005, 89, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.G.; Tan, A.; Ganz, P.; Seelig, J. Calorimetric Measurement of Phospholipid Interaction with Methyl-β-Cyclodextrin. Biochemistry 2004, 43, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krompers, M.; Heerklotz, H. A Guide to Your Desired Lipid-Asymmetric Vesicles. Membranes 2023, 13, 267. https://doi.org/10.3390/membranes13030267
Krompers M, Heerklotz H. A Guide to Your Desired Lipid-Asymmetric Vesicles. Membranes. 2023; 13(3):267. https://doi.org/10.3390/membranes13030267
Chicago/Turabian StyleKrompers, Mona, and Heiko Heerklotz. 2023. "A Guide to Your Desired Lipid-Asymmetric Vesicles" Membranes 13, no. 3: 267. https://doi.org/10.3390/membranes13030267
APA StyleKrompers, M., & Heerklotz, H. (2023). A Guide to Your Desired Lipid-Asymmetric Vesicles. Membranes, 13(3), 267. https://doi.org/10.3390/membranes13030267






