Valorization of Anaerobic-Fermentation Liquid Digestates—Membrane-Based Process Development
Abstract
:1. Introduction—Scope
2. Methodology
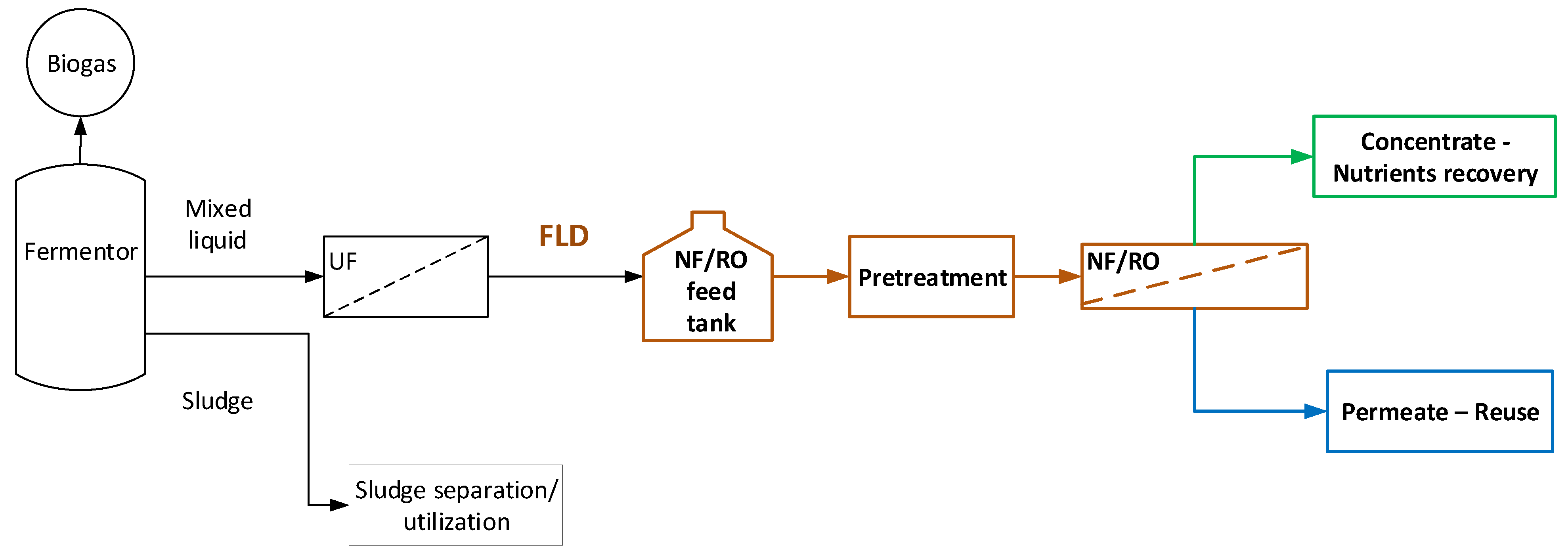
2.1. General Considerations—Effluent Treatment Scheme, Tasks
2.2. Outline of Methodology
- I.
- Bench scale testing—Dead-end membrane tests
- II.
- Laboratory membrane process performance assessment—Cross-flow equipment tests
- General membrane/process performance assessment and selection of best membrane type for further comprehensive testing.
- Comprehensive membrane process performance testing. Adequate data collection for key parameters and assessment of main effects, i.e., membrane-effective permeability, species rejection/separation, membrane fouling characteristics, and membrane cleaning.
- III.
- Process performance simulations—Pilot plant design
- Validation using comparison of simulation results with experimental process performance data from preceding step II.
- Identification of a (narrow) range of near-optimum design and operating process conditions.
- Basic design data for an appropriate industrial membrane pilot, based on the foregoing results.
- IV.
- Pilot plant construction and preliminary testing
- V.
- Industrial pilot testing
- Performance data collection, to validate the methodology and select key process parameters.
- Refinement of projections of a real plant performance, aiming to finalize process design.
- VI.
- Process optimization, techno-economic/sustainability assessment
3. Experimental Design—Equipment, Materials, Techniques
3.1. Materials, Techniques
- Mα+ and Ab− are the activities of the ionic species of a compound MnAm.
- Ksp is the thermodynamic solubility product of the solid forming compound.
- IAP is the respective ion activity product.
- Cw,I, Cb,i: Concentration of ions at the membrane surface and in bulk solution, respectively.
- Ri, J: Measured ions’ rejection and permeate flux, respectively.
- ki: Mass transfer coefficient at the membrane surface determined by a reliable correlation [52].
3.2. Bench—Scale Equipment, Procedures—Dead-End Cells
3.3. Cross-Flow Equipment, Procedures
4. Methodology Implementation—Results
4.1. Dead-End Tests
4.2. Cross-Flow Tests
4.2.1. Assessment of Membrane Fouling/Scaling Propensity and FLD Pretreatment
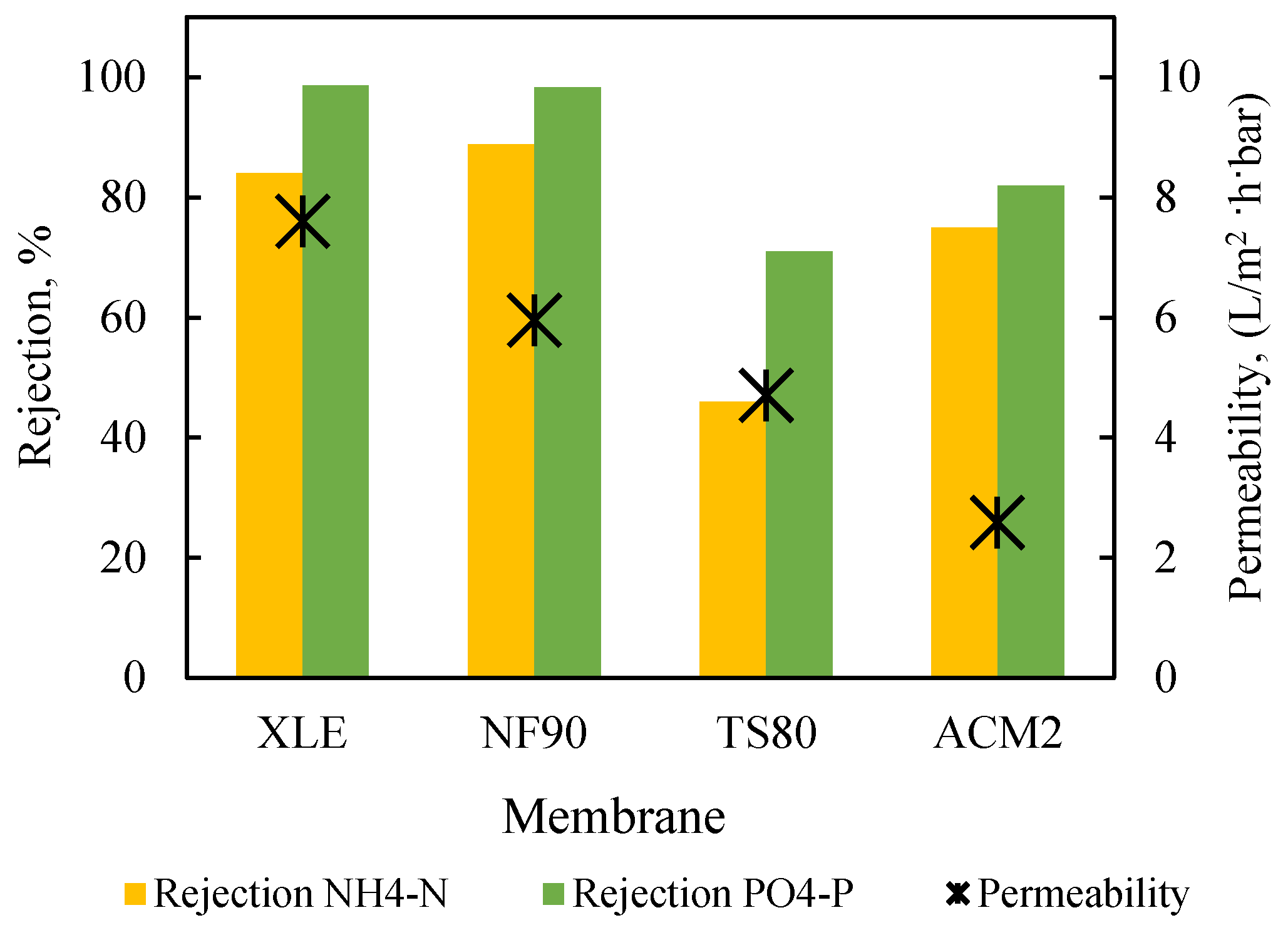
Permeability and Rejection Tests with Synthetic Solutions
Performance Tests with Untreated FLD
Performance Tests with Pretreated FLD
- (a)
- In tests 3P and 4P (Table A3), the feed pH was controlled at 6.5 throughout the filtration process with periodic addition of sulfuric acid (6N) that reached approximately 0.05% of the total feed volume.
- (b)
- In test 2P, part of the bicarbonate and ortho-phosphoric ions of FLD were precipitated with Ca(OH)2 addition. The supernatant was also acidified with H2SO4 due to its high pH; this is an alternative pretreatment process path for possible hydroxyapatite recovery [29] and membrane scaling mitigation.
4.2.2. Comprehensive Investigation of Membrane Process Performance
Selection of Experimental Conditions through Process Simulation
Comprehensive Membrane Performance Tests
- The flushing of the system with DI and/or with NF permeate resulted in slightly reduced membrane permeability, similar to that achieved after CIP.
- The filtration tests were repeatable in terms of the flux profiles.
- The NF retentate (ΝH4-Ν: ~250–450 mg/L and PO4-P: 110–160 mg/L at permeate recovery ~50%) is considered useful as a possible liquid fertilizer, compared to respective products of the literature with similar physicochemical properties [26,38], although dilution may be needed [21] to render it appropriate for soil fertilization [66]. Alternatively, the recovery of the retentate nutrients could be achieved, e.g., through the precipitation of phosphate salts such as struvite (Magnesium Ammonium Phosphate, MAP, MgNH4PO4·6H2O), which is a well-known slow-release fertilizer [24,67].
- NF permeate: The NF90 membrane exhibited high nutrient rejection (approx. 97% for NH4-N and 99% for PO4-P) in all tests, as shown in Figure S10, resulting in rather low concentrations in the collected composite permeate (NH4-N: 3–7 mg/L and PO4-P: 0.2–1.5 mg/L at permeate recovery 40–50%). Furthermore, pH and eC varied between 6.1–7.2 and 81.5–199.6 μS/cm, respectively. Generally, the NF permeate can be characterized as clean, transparent, and satisfying the regulations for water reuse in restricted irrigation [41]. However, the currently in-effect, rather stringent local regulations [59] regarding ΝH4-Ν (≤2 mg/L) do not allow permeate disposal.
4.3. Towards Process Scale-Up—Pilot Plant Design
4.3.1. Projections of Real/Pilot Plant Performance—Process Simulations
- 2 to 4 SWM modules (NF90- DuPont) in a pressure vessel, depending on the desired permeate % recovery.
- Active membrane surface area per SWM module 2.6 m2 (Commercial type NF90 ‘2540’—DuPont).
- Simulations for system/pilot operation under constant recovery of: (i) 20% (two membrane elements in series, Case No 1) and (ii) 40–44% (four membrane elements in series, Cases No 2–No 4).
- For an input inlet cross-flow velocity of 0.22 m/s, the feed flow rate is 13.3 L/min (i.e., ca. 798 L/h or 19,152 m3/d).
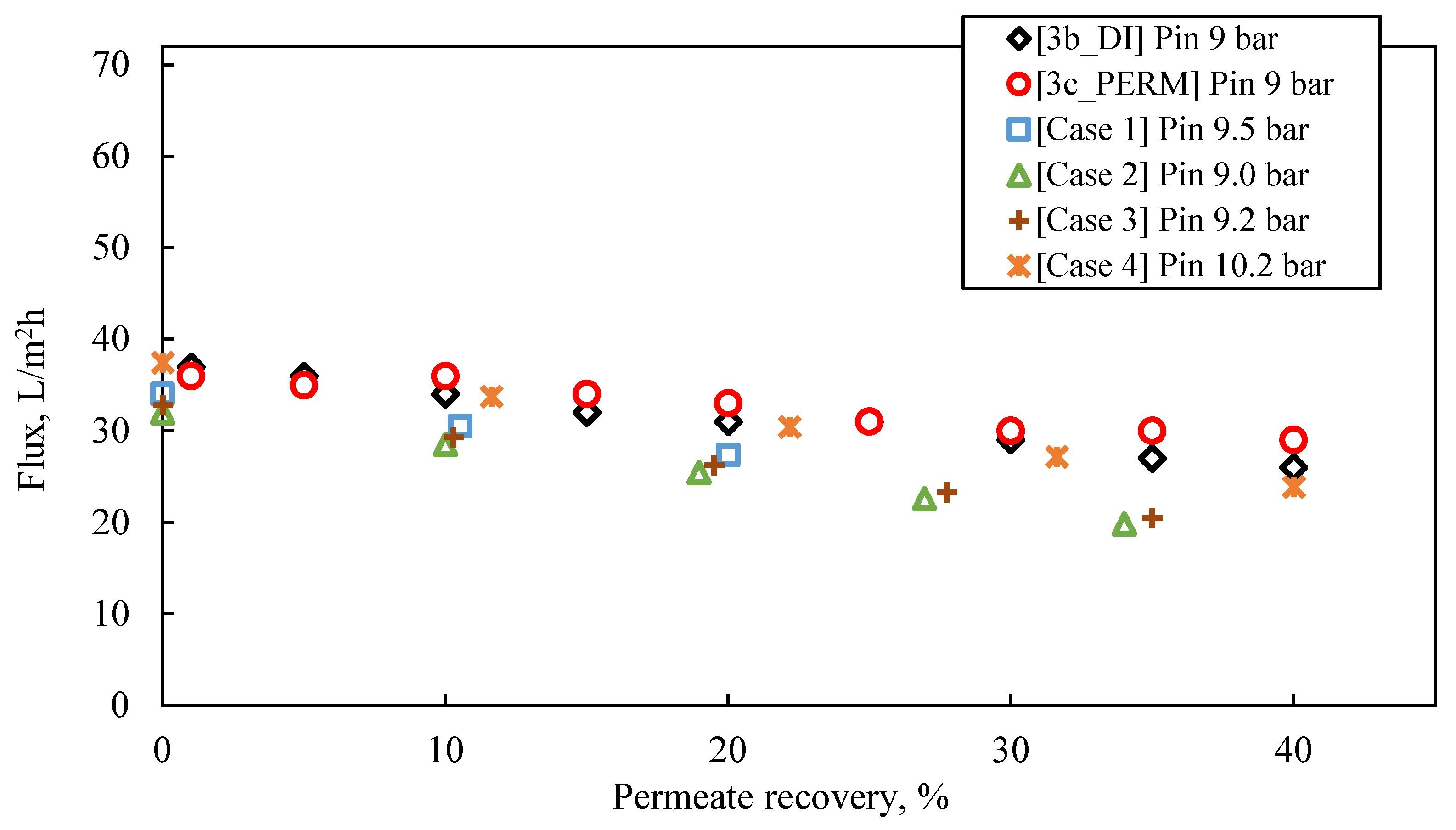
- For permeate recovery of 40% from the FLD membrane treatment (Figure 8), the prediction by the simulator inlet pressure (of ~9.0–10.0 bar), compared to the applied pressure in the laboratory cross-flow tests (i.e., 9.0 bar), is generally considered satisfactory, at this stage of process development.
- In the case of a pilot with two membrane modules (Case No 1) and permeate recovery of 20%, the simulation result regarding initial flux is 32 L/m2·h, with inlet pressure of 9.5 bar.
- Simulation Case No 4 (four SWM elements in series and permeate recovery 44.4%) generally provides the best fitting to the experimental data (as shown in Figure 8). The experimental values of initial flux (37 L/m2·h and 36 L/m2·h for the tests #3b_DI and #3c_PERM, respectively) are very close to those of the simulation. However, the predicted feed pressure from the simulation (10.2 bar) is somewhat greater (by ~10%) compared to the applied one in the laboratory cross-flow test (9 bar).
- The simulated flux variation (in case No 4 and others, where no fouling is considered) is very similar to that of experimental results, indicating mild/controllable fouling of the membrane surface.
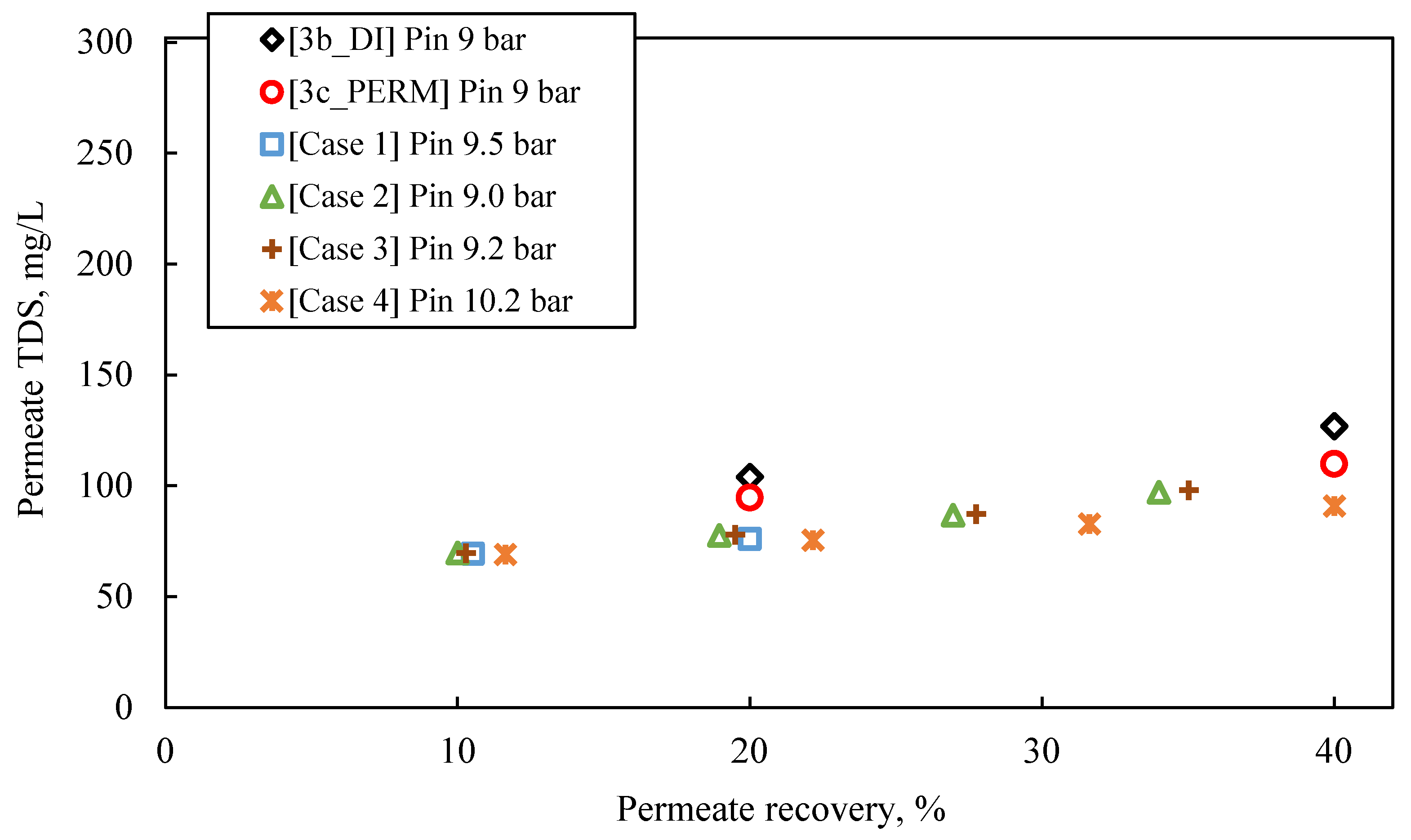
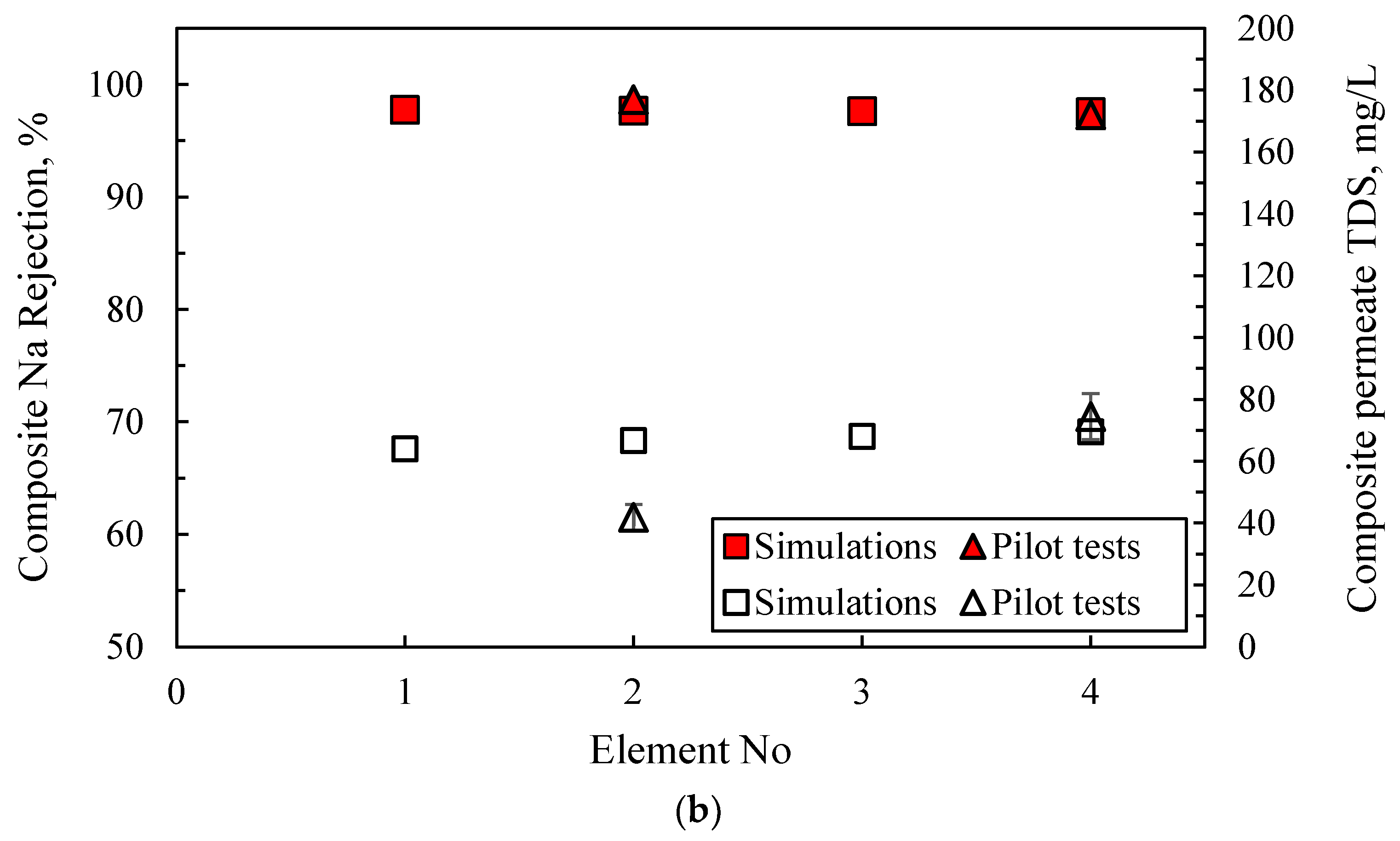
- The predicted quality of the composite permeate in terms of TDS (Figure 9) is comparable with that of test results, clearly satisfying the respective regulations for water reuse (i.e., <450 mg/L) [2,41]. Excellent agreement is observed between simulations and experimental data regarding retentate concentration variation with % recovery (Figure 10).
4.3.2. Pilot Plant Basic Design
- Membrane module type: NF90 2540 (DuPont).
- Main feed pump max. pressure: ~15 bar.
- Capacity of pilot unit: Feed flow rate ~15 L/min or ~22 m3/day for 24 h/day operation.
- Typical superficial cross-flow velocity at inlet of 1st pressure vessel: 20–25 cm/s.
- Targeted permeate recovery: 20% to ~60%, depending on operating mode.
- Feed pretreatment, i.e., controlled acidification of FLD and prefiltration with cartridge filter.
- Provision for membrane periodic cleaning: (i) Flushing with permeate and (ii) CIP facility.
4.4. Pilot Plant Preliminary Tests
4.4.1. Pilot Brief Description
4.4.2. Preliminary Pilot Tests
5. Comments
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample 1 (S1) | Sample 2 (S2) | Sample 3 (S3) | Sample 4 (S4) | Sample 5 (S5) | Limit Values for Reuse [41] | ||
|---|---|---|---|---|---|---|---|
| Months of plant operation | 1.5 | 4 | 7 | 8 | 9.5 | Unrestricted irrigation | Restricted irrigation |
| pH | 7.2 | 7.5 | 7.1 | 7.5 | 7.3 | 6.5–8.5 | |
| eC, μS/cm | 4170 | 5830 | 4580 | 4330 | 5860 | 700 | 3000 |
| Total dissolved solids (TDS), mg/L | 2670 | 3730 | 2930 | 2770 | 3750 | 450 | 2000 |
| Total organic carbon (TOC), mg/L | 37.6 | 29.7 | 19.7 | 69.6 | 32 | N/S | N/S |
| COD, mg/L | 85 | 115 | 65 | 161 | 60 | N/S | N/S |
| PO4-P, mg/L | 134 | 92.4 | 85.6 | 77.7 | 74 | 2 * | 2 * |
| NH4-N, mg/L | Ν/A | 168.4 | 169.4 | 173.7 | 211.7 | 2 ** | N/S |
| Cl−, mg/L | 437 | 515 | 366 | 363 | 431 | 140 | 350 |
| SO42−, mg/L | 72 | Ν/A | 125.3 | - | - | N/S | N/S |
| Alkalinity, mg CaCO3/L | 1515 | 2439 | 1521 | 1581 | 2037 | 90 | 500 |
| TSS, mg/L | 40 | 5 | 0.6 | 0.2 | 0.8 | 10 | 35 |
| Tested Membrane | XLE | NF90 | TS80 | ACM2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Operating pressure, bar | 3 | 5 | 5 | 5 | 5 | 7 | 3 | 5 | 5 | 5 | 5 | 7 | 5 | 5 |
| Test duration, min | 60 | 60 | 453 | 60 | 60 | 60 | 60 | 60 | 463 | 60 | 60 | 60 | 60 | 60 |
| pH | 4.7 | 4.7 | 4.9 | 5.4 | 8.5 | 4.9 | 4.8 | 4.9 | 4.8 | 5.4 | 8.5 | 5.1 | 4.9 | 4.9 |
| eC, μS/cm | 2060 | 1854 | 2270 | 1539 | 934 | 1837 | 2010 | 1822 | 1926 | 1496 | 872 | 1922 | 1921 | 1908 |
| Total dissolved solids (TDS), mg/L | 1320 | 1190 | 1450 | 985 | 598 | 1180 | 1290 | 1170 | 1230 | 957 | 558 | 1230 | 1230 | 1220 |
| Osmotic pressure, bar | 1.02 | 0.92 | 1.12 | 0.76 | 0.46 | 0.91 | 1.00 | 0.91 | 0.95 | 0.74 | 0.43 | 0.95 | 0.95 | 0.95 |
| PO4-P, mg/L | 163 | 162 | 129 | - | 125 | 146 | 145 | 158 | 168 | - | 130 | 148 | 159 | 157 |
| NH4-N, mg/L | 148 | 147 | 159 | 150 | - | 156 | 147 | 152 | 158 | 151 | - | 157 | 142 | 150 |
| Alkalinity, mg CaCO3/L | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Test | 0P | 1P | 2P | 3P | 4P |
|---|---|---|---|---|---|
| Membrane | XLE | NF90 | NF90 | NF90 | NF90 |
| Feed Solution | S1 * | S1 * | S2 | S2 | S3 |
| Pretreatment | None | Precipitation, separation of the supernatant and addition of acid | Acid addition | Acid addition | |
| pH | 7.4 | 6.6 | 6.6 | 6.4 | |
| TOC, mg/L | 16.5 | 21.8 | 15.4 | 22.9 | |
| NH4-N, mg/L | 104.1 | 118.6 | 126.6 | 151.7 | |
| PO4-P, mg/L | 165.1 | 11.9 | 74.8 | 98 | |
| Alkalinity, mg CaCO3/L | N/A | 1133 | 1937 | 1022 | |
| eC, μS/cm | 3380 | 4900 | 4930 | 3880 | |
| Osmotic Pressure, bar | 1.67 | 2.42 | 2.44 | 1.92 | |
| Supersaturation index (SI) of constituents having the tendency to precipitate in feed solution | |||||
| Aragonite | 0.19 | −1.62 | −0.58 | −1.09 | |
| Ca3(PO4)2(beta) | 2.63 | −4.32 | −0.28 | −0.78 | |
| Ca4H(PO4)3:3H2O | 1.92 | −7.19 | −1.55 | −2.08 | |
| CaHPO4 | 0.40 | −1.76 | −0.16 | −0.19 | |
| CaHPO4:2H2O | 0.12 | −2.04 | −0.44 | −0.47 | |
| Calcite | 0.37 | −1.44 | −0.4 | −0.91 | |
| Dolomite (ordered) | 0.51 | - | −0.82 | −1.7 | |
| Hydroxyapatite | 10.62 | −1.11 | 5.36 | 4.4 | |
| Test Conditions | |||||
| Applied pressure, bar | 5 | 5 | 5 | 5 | 5 |
| Permeate recovery, % | 41.5 | 40 | 55.5 | 53.7 | 69.5 |
| Initial flux, L/m2·h | 20 | 27 | 22 | 20 | 22 |
| Final flux, L/m2·h | 8 | 6 | 6 | 7 | 6 |
| Test duration, min | 702 | 600 | 610 | 590 | 975 |
| Permeate | |||||
| pH | 8.1 | 8.0 | 6.5 | 6.9 | 6.6 |
| NH4-N, mg/L (Rejection, %) | 14.8 (85.8) | 8.0 (92.3) | 11.5 (94.1) | 14.2 (92.4) | 21.7 (91.3) |
| PO4-P, mg/L (Rejection, %) | 2.3 (98.6) | 1.7 (99.0) | 0.5 (96.4) | 2.2 (98.5) | 4.9 (96.5) |
| SO42− | ND | ND | 56.3 | 30.2 | 75.5 |
| Cl− | 23.7 | 46.8 | 59.1 | 51.9 | 94.5 |
| eC, μS/cm (Rejection, %) | 277.3 (91.8) | 404.7 (88.0) | 489.1 (93.3) | 341.6 (94.4) | 610 (90.5) |
| Retentate | |||||
| NH4-N, mg/L (Cf) | 136 (1.3) | 155.4 (1.5) | 283.2 (2.3) | 272.8 (2.2) | 480 (3.2) |
| PO4-P, mg/L (Cf) | 95 (1.0) | 70 (0.9) | 24.8 (2.5) | 181 (2.4) | 193.3 (2.7) |
| eC, μS/cm | 5270 | 4830 | 11310 | 10680 | 11190 |
| Supersaturation index of constituents having the tendency to precipitate in retentate solution (recovery 40%) | |||||
| Aragonite | 1.34 | −1.58 | −0.38 | −1.58 | |
| Ca3(PO4)2(beta) | 4.71 | −2.76 | 1.97 | −2.76 | |
| Ca4H(PO4)3:3H2O | 4.07 | −5.13 | 1.28 | −5.13 | |
| CaHPO4 | 0.48 | −1.25 | 0.43 | −1.25 | |
| CaHPO4:2H2O | 0.19 | −1.54 | 0.14 | −1.54 | |
| Calcite | 1.52 | −1.4 | −0.2 | −1.4 | |
| Dolomite(disordered) | 2.08 | - | −1.01 | - | |
| Dolomite(ordered) | 2.63 | - | −0.46 | - | |
| Huntite | 0.49 | - | −5.33 | - | |
| Hydroxyapatite | 14.71 | 1.51 | 9.28 | 4.4 | |
| Series of Experiments | #1 | #2 a | #3 a | #4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | 1a | 1b | 1c | 2a | 2b | 2c | 3a | 3b | 3c | 4a | 4b | 4c |
| Membrane initial condition | FM | DI | CIP | FM | DI | CIP | FM | DI | PERM | FM | DI | CIP |
| Applied pressure, bar | 8 | 9 | 9 | 10 | ||||||||
| Clean water permeability, L/m2·h·bar b | 10.9 | 6.5 | 7.1 | 10.5 | 7.1 | 7.1 | 10.5 | 6.8 | - | 9.0 | 6.3 | 6.9 |
| Feed Solution | S3 | S5 | S5 | S4 | ||||||||
| Pretreatment | Acid addition | Acid addition | Acid addition | Acid addition | ||||||||
| pH | 6.7 | 6.8 | 6.9 | 6.5 | 6.6 | 6.6 | 6.6 | 6.6 | 6.7 | 6.5 | 6.5 | 6.5 |
| TOC, mg/L | 39.2 | 40.3 | 38.9 | 34.7 | 31.0 | 29.8 | 34.8 | 36.0 | 34.0 | 62.7 | 56.2 | 46.0 |
| NH4-N, mg/L | 157.4 | 164.8 | 158.4 | 196.4 | 202.8 | 177.1 | 204.1 | 194.4 | 195.4 | 154.8 | 157.8 | 150.3 |
| PO4-P, mg/L | 74.5 | 77 | 74 | 69.3 | 69.3 | 62.5 | 67.3 | 62.0 | 88.6 | 75.5 | 74.5 | 73.0 |
| Alkalinity, mg CaCO3/L | 1232 | 1305 | 1279 | 1640 | 1618 | 1477 | 1646 | 1647 | 1649 | 1235 | 1275 | 1055 |
| eC, μS/cm | 4010 | 4030 | 3960 | 4810 | 4910 | 4690 | 4830 | 4850 | 4900 | 3950 | 4000 | 3880 |
| TDS, mg/L | 2570 | 2580 | 2530 | 3080 | 3140 | 3000 | 3090 | 3100 | 3130 | 2530 | 2560 | 2480 |
| Osmotic pressure, bar | 1.65 | 1.71 | 1.66 | 1.93 | 2.12 | 2.00 | 2.00 | 2.05 | 2.07 | 1.66 | 1.61 | 1.59 |
| Test Conditions | ||||||||||||
| Test duration, min | 202 | 167 | 175 | 205 | 162 | 101 | 207 | 178 | 172 | 163 | 144 | 164 |
| Permeate recovery, % | 51.5 | 41.2 | 38.7 | 48.6 | 37.4 | 38.2 | 51.0 | 39.2 | 39.4 | 49.1 | 38.2 | 38.4 |
| ΔPap, bar | 8 | 8 | 8 | 9 | 9 | 9 | 9 | 9 | 9 | 10 | 10 | 10 |
| Initial flux, L/m2·h | 40 | 32 | 33 | 41 | 36 | 36 | 41 | 34 | 35 | 48 | 44 | 42 |
| Final flux, L/m2·h | 21 | 24 | 23 | 24 | 26 | 29 | 22 | 23 | 22 | 30 | 28 | 23 |
| Effective permeability, L/m2·h·bar | 6.7 | 5.4 | 5.7 | 6.3 | 5.4 | 5.7 | 6.3 | 5.4 | 5.5 | 6.1 | 5.6 | 5.6 |
| Permeate | ||||||||||||
| pH | 6.9 | 7.0 | 6.9 | 7.1 | 6.8 | 7.2 | 6.5 | 6.5 | 6.5 | 6.5 | 6.6 | 6.5 |
| eC, μS/cm (Rejection, %) | 124.8 (97.6) | 128.7 (97.2) | 107.7 (97.6) | 199.6 (96.8) | 165.4 (96.9) | 152.8 (97.0) | 160.9 (97.4) | 156.3 (97.0) | 144.7 (97.3) | 101.8 (98.2) | 88.9 (98.0) | 81.5 (98.1) |
| NH4-N, mg/L (Rejection, %) | 4.3 (97.8) | 5.0 (97.3) | 3.7 (97.9) | 7.0 (97.3) | 7.0 (97.1) | 5.8 (87.3) | 6.8 (97.5) | 6.6 (97.1) | 5.5 (97.5) | 3.6 (98.1) | 3.7 (97.9) | 2.9 (98.3) |
| PO4-P, mg/L (Rejection, %) | 0.7 (99.3) | 0.5 (99.4) | 0.4 (99.5) | 0.7 (99.2) | 0.6 (99.1) | 1.5 (97.7) | 0.8 (99.1) | 0.6 (99.1) | 0.7 (99.3) | 0.4 (99.6) | 0.3 (99.7) | 0.2 (99.8) |
| SO42−, mg/L | 6.0 | 5.1 | 3.7 | 9.0 | 7.7 | 9.8 | 12.0 | 6.5 | 9.7 | 4.6 | 3.4 | 2.8 |
| Cl−, mg/L | 14.3 | 12.8 | 9.8 | 19.8 | 16.6 | 13.4 | 18.7 | 16.1 | 14.5 | 10.0 | 6.0 | 8.0 |
| Retentate | ||||||||||||
| NH4-N, mg/L Concentration factor, Cf | 337.3 Cf: 2.1 | 254.2 Cf: 1.5 | 248.5 Cf: 1.6 | 432.3 Cf: 2.2 | 333.5 Cf: 1.6 | 289.4 Cf: 1.6 | 447.7 Cf: 2.2 | 376 Cf: 1.9 | 350.6 Cf: 1.8 | 297.7 Cf: 1.9 | 264.1 Cf: 1.7 | 257 Cf: 1.7 |
| PO4-P, mg/L Concentration factor, Cf | 160 Cf: 2.1 | 123.5 Cf: 1.6 | 118 Cf: 1.5 | 155.4 Cf: 2.2 | 108.1 Cf: 1.6 | 115.6 Cf: 1.8 | 150.5 Cf: 2.2 | 147.0 Cf: 2.4 | 147 Cf: 1.7 | 149.0 Cf: 2.0 | 127.0 Cf: 1.7 | 126.0 Cf: 1.7 |
| eC, μS/cm | 8350 | 6460 | 6390 | 10370 | 8060 | 7400 | 10790 | 8710 | 8530 | 7860 | 6620 | 6680 |
References
- European Commission. The European Green Deal Investment Plan and Just Transition Mechanism Explained, 2020, Brussels. Available online: https://ec.europa.eu/commission/presscorner/detail/en/qanda_20_24 (accessed on 4 March 2022).
- The European Parliament and the Council of the European Union. REGULATION (EU) 2020/741 on minimum requirements for water reuse. Off. J. Eur. Union 2020, 177, 32–55. [Google Scholar]
- Asunis, F.; De Gioannis, G.; Dessi, P.; Isipato, M.; Lens, P.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. The dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Environ. Manag. 2020, 276, 111240. [Google Scholar] [CrossRef]
- van der Wiel, B.; Weijma, J.; van Middelaar, C.; Kleinke, M.; Buisman, C.; Wichern, F. Restoring nutrient circularity: A review of nutrient stock and flow analyses of local agro-food-waste systems. Resour. Conserv. Recycl. 2020, 160, 104901. [Google Scholar] [CrossRef]
- Rekleitis, G.; Haralambous, K.-J.; Loizidou, M.; Aravossis, K. Utilization of Agricultural and Livestock Waste in Anaerobic Digestion (A.D): Applying the Biorefinery Concept in a Circular Economy. Energies 2020, 13, 4428. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Tracking phosphorus security: Indicators of phosphorus vulnerability in the global food system. Food Secur. 2015, 7, 337–350. [Google Scholar] [CrossRef]
- Cesaro, A. The valorization of the anaerobic digestate from the organic fractions of municipal solid waste: Challenges and perspectives. J. Environ. Manag. 2020, 280, 111742. [Google Scholar] [CrossRef]
- Amaya, O.; Barragán, M.; Tapia, F. Microbial Biomass in Batch and Continuous System. In Biomass Now; Darko, M.M., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Arnau, Á.; Pflüger, S.; Giacomazzi, M.; Decorte, M.; Genua, M. EBA Annual Report; European Biogas Association: Brussels, Belgium, 2021. [Google Scholar]
- Turley, D.; Hopwood, L.; Burns, C.; Maio, D. Assessment of Digestate Drying as an Eligible Heat Use in the Renewable Heat Incentive; NNFCC: York, UK, 2016. [Google Scholar]
- Gupta, V.; Ali, I. Chapter 5—Water Treatment by Membrane Filtration Techniques. In Environmental Water; Gupta, V., Ali, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–154. [Google Scholar]
- Chen, W.; Oldfield, T.; Patsios, S.; Holden, N. Hybrid life cycle assessment of agro-industrial wastewater valorisation. Water Res. 2020, 170, 115275. [Google Scholar] [CrossRef]
- Sheets, J.; Yang, L.; Ge, X.; Wang, Z.; Li, Y. Beyond land application: Emerging technologies for the treatment and reuse of anaerobically digested agricultural and food waste. Waste Manag. 2015, 44, 94–115. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Lu, F.; Hao, L.; Zhang, H.; Shao, L.; He, P. Digestate management for high-solid anaerobic digestion of organic wastes: A review. Bioresour. Technol. 2020, 297, 122485. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.; Tack, F.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valoriz. 2017, 8, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Xia, A.; Murphy, J. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Barampouti, E.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Exploring technological alternatives of nutrient recovery from digestate as a secondary resource. Renew. Sustain. Energy Rev. 2020, 134, 110379. [Google Scholar] [CrossRef]
- Zacharof, M.-P.; Mandale, S.; Oatley-Radcliffe, D.; Lovitt, R. Nutrient recovery and fractionation of anaerobic digester effluents employing pilot scale membrane technology. J. Water Process Eng. 2019, 31, 100846. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D. Valorization of anaerobic digestion digestate: A prospect review. Bioresour. Technol. 2021, 323, 124626. [Google Scholar] [CrossRef]
- Boehler, M.; Heisele, A.; Seyfried, A.; Grömping, M.; Siegrist, H. (NH4)2SO4 recovery from liquid side streams. Environ. Sci. Pollut. Res. 2015, 22, 7295–7305. [Google Scholar] [CrossRef]
- Bolzonella, D.; Fatone, F.; Gottardo, M.; Frison, N. Nutrients recovery from anaerobic digestate of agro-waste: Techno-economic assessment of full scale applications. J. Environ. Manag. 2018, 216, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Morales, N.; Boehler, M.; Buettner, S.; Liebi, C.; Siegrist, H. Recovery of N and P from Urine by Struvite Precipitation Followed by Combined Stripping with Digester Sludge Liquid at Full Scale. Water 2013, 5, 1262–1278. [Google Scholar] [CrossRef]
- Ledda, C.; Schievano, A.; Salati, S.; Adani, F. Nitrogen and water recovery from animal slurries by a new integrated ultrafiltration, reverse osmosis and cold stripping process: A case study. Water Res. 2013, 47, 6157–6166. [Google Scholar] [CrossRef]
- Lorick, D.; Macura, B.; Ahlström, M.; Grimvall, A.; Harder, R. Effectiveness of struvite precipitation and ammonia stripping for recovery of phosphorus and nitrogen from anaerobic digestate: A systematic review. Environ. Evid. 2020, 9, 27. [Google Scholar] [CrossRef]
- Adam, M.; Othman, M.; Samah, R.A.; Puteh, M.; Ismail, A.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Magalhães, N.; Silva, A.; Cunha, P.; Drewes, J.; Amaral, M. Role of nanofiltration or reverse osmosis integrated to ultrafiltration-anaerobic membrane bioreactor treating vinasse for the conservation of water and nutrients in the ethanol industry. J. Water Process Eng. 2020, 36, 101338. [Google Scholar] [CrossRef]
- Khan, E.; Nordberg, Å. Membrane distillation process for concentration of nutrients and water recovery from digestate reject water. Separ. Purif. Technol. 2018, 206, 90–98. [Google Scholar] [CrossRef]
- Aulenbach, D.; Clesceri, N. Treatment by Application Onto Land. In Biological Treatment Processes; Wang, L., Pereira, N., Hung, Y.-T., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 29–74. [Google Scholar]
- Cichy, B.; Kużdżał, E.; Krztoń, H. Phosphorus recovery from acidic wastewater by hydroxyapatite precipitation. J. Environ. Manag. 2019, 232, 421–427. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Meers, E.; Michels, E.; Buysse, J.; Tack, F. Ecological and economic benefits of the application of bio-based mineral fertilizers in modern agriculture. Biomass Bioenergy 2013, 49, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Simplicio, W.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life cycle assessment of biogas digestate processing technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Lu, F.; Wang, Z.; Zhang, H.; Shao, L.; He, P. Anaerobic digestion of organic waste: Recovery of value-added and inhibitory compounds from liquid fraction of digestate. Bioresour. Technol. 2021, 333, 125196. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.; Guo, W.; Chang, S.; Nguyen, D.; Zhang, X.; Zhang, J.; Liang, S. Nutrient recovery from wastewater: From technology to economy. Bioresour. Technol. Rep. 2020, 11, 100425. [Google Scholar] [CrossRef]
- Plakas, K.; Karabelas, A. Removal of pesticides from water by NF and RO membranes—A review. Desalination 2012, 287, 255–265. [Google Scholar] [CrossRef]
- Gerardo, M.; Aljohani, N.; Oatley-Radcliffe, D.; Lovitt, R. Moving to s sustainable resources: Recovery and fractionation of nutrients from dairy manure digestate using membranes. Water Res. 2015, 80, 80–89. [Google Scholar] [CrossRef]
- Adam, G.; Mottet, A.; Lemaigre, S.; Tsachidou, B.; Trouvé, E.; Delfosse, P. Fractionation of anaerobic digestates by dynamic nanofiltration and reverse osmosis: An industrial pilot case evaluation for nutrient recovery. J. Environ. Chem. Eng. 2018, 6, 6723–6732. [Google Scholar] [CrossRef]
- Fernandes, F.; Silkina, A.; Fuentes-Grunewald, C.; Wood, E.; Ndovela, V.; Oatley-Radcliffe, D.; Lovitt, R.; Llewellyn, C. Valorising nutrient-rich digestate: Dilution, settlement and membrane filtration processing for optimisation as a waste-based media for microalgal cultivation. Waste Manag. 2020, 118, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Karanasiou, A.; Karabelas, A.; Mitrouli, S. Incipient membrane scaling in the presence of polysaccharides during reverse osmosis desalination in spacer-filled channels. Desalination 2021, 500, 114821. [Google Scholar] [CrossRef]
- Greek Government. Determination of measures, conditions and procedures for the reuse of treated wastewater and other provisions. Gaz. Greek Repub. 2011, 354, 5229–5243. [Google Scholar]
- Veolia Water Technologies. Biothane Anaerobic Technology Memthane® 2.0; Biothane Anaerobic Technologies: Delft, The Netherlands, 2018. [Google Scholar]
- Rayshouni, H.; Wazne, M. Effects of Calcium on the Removal of Ammonium from Aged Landfill Leachate by Struvite Precipitation. Water 2022, 14, 1933. [Google Scholar] [CrossRef]
- Tian, X.; Wang, G.; Guan, D.; Li, J.; Wang, A.; Li, J.; Yu, Z.; Chen, Y.; Zhang, Z. Reverse osmosis brine for phosphorus recovery from source separated urine. Chemosphere 2016, 165, 202–210. [Google Scholar] [CrossRef] [PubMed]
- DuPont™. XLE Product Data Sheet. 2021. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/RO-FilmTec-XLE-Commercial-PDS-45-D01515-en.pdf (accessed on 8 May 2021).
- DuPont™. NF90 Product Data Sheet. 2021. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/NF-FilmTec-NF90-Commercial-Systems-PDS-45-D01520en.pdf (accessed on 8 June 2021).
- Microdyn-Nadir. PRODUCT SPECIFICATION—TRISEP® TS80. 2021. Available online: https://www.microdyn-nadir.com/wp-content/uploads/TS80.pdf (accessed on 8 June 2021).
- Microdyn-Nadir. PRODUCT SPECIFICATION—TRISEP® ACM2. 2021. Available online: https://www.microdyn-nadir.com/wp-content/uploads/ACM2.pdf (accessed on 8 July 2021).
- Song, Y.; Hahn, H.; Hoffmann, E. Effects of solution conditions on the precipitation of phosphate for recovery A thermodynamic evaluation. Chemosphere 2002, 48, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.; Appelo, C. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. U.S. Geol. Surv. Tech. Methods 2013, 6, 497. [Google Scholar]
- Todorov, T.; Rabadjieva, D.; Tepavitcharova, S. New thermodynamic database for more precise simulation of metal species in natural waters. J. Univ. Chem. Technol. Metall. 2006, 41, 97–102. [Google Scholar]
- Koutsou, C.; Yiantsios, S.; Karabelas, A. A numerical and experimental study of mass transfer in spacer-filled channels: Effects of spacer geometrical characteristics and Schmidt number. J. Membr. Sci. 2009, 326, 234–251. [Google Scholar] [CrossRef]
- Mitrouli, S.; Karabelas, A.; Isaias, N. Polyamide active layers of low pressure RO membranes: Data on spatial performance non-uniformity and degradation by hypochlorite solutions. Desalination 2010, 260, 91–100. [Google Scholar] [CrossRef]
- Koutsou, C.; Karabelas, A. Shear stresses and mass transfer at the base of a stirred filtration cell and corresponding conditions in narrow channels with spacers. J. Membr. Sci. 2012, 399, 60–72. [Google Scholar] [CrossRef]
- Karabelas, A.; Karanasiou, A.; Mitrouli, S. Incipient membrane scaling by calcium sulfate during desalination in narrow spacer-filled channels. Desalination 2014, 345, 146–157. [Google Scholar] [CrossRef]
- Ledda, C.; Villegas, G.R.; Adani, F.; Fernández, F.A.; Grima, E.M. Utilization of centrate from wastewater treatment for the outdoor production of Nannochloropsis gaditana biomass at pilot-scale. Algal Res. 2015, 12, 17–25. [Google Scholar]
- Escalante, H.; Castro, L.; Amaya, M.; Jaimes, L.; Jaimes-Estevez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, J.; Lindino, C.; de Azevedo, J.; de Souza, S.; Cremonez, P.; Rossi, E. Biogas production by the anaerobic digestion of whey. Rev. Ciências Agrárias 2016, 39, 463–468. [Google Scholar] [CrossRef]
- Greek Republic-Department of Environment & Spatial Affairs Planning. Amendment of Decision No. 588/37124/18-12-2017 Approval of Environmental Conditions (A.E.P.O.) of the Existing Cheese Production of “BIZIOS S.A.”, in the Municipality of Elassona, Larissa, Thessaly. 2018. Available online: https://yperdiavgeia.gr/pdfjs/web/viewer.html?file=/decisions/downloadPdf/30799130 (accessed on 5 October 2021).
- Karabelas, A.; Sioutopoulos, D. New insights into organic gel fouling of reverse osmosis desalination membranes. Desalination 2015, 368, 114–126. [Google Scholar] [CrossRef]
- Sioutopoulos, D.; Karabelas, A. Evolution of organic gel fouling resistance in constant pressure and constant flux dead-end ultrafiltration: Differences and similarities. J. Membr. Sci. 2016, 511, 265–277. [Google Scholar] [CrossRef]
- DuPont™. WAVE Water Treatment Design Software. 2022. Available online: https://www.dupont.com/water/resources/design-software.html#download-wave-software (accessed on 19 September 2022).
- Karabelas, A.; Koutsou, C.; Kostoglou, M. The effect of spiral wound membrane element design characteristics on its performance in steady state desalination—A parametric study. Desalination 2014, 332, 76–90. [Google Scholar] [CrossRef]
- Koutsou, C.; Karabelas, A.; Kostoglou, M. Membrane desalination under constant water recovery—The effect of module design parameters on system performance. Sep. Purif. Technol. 2015, 147, 90–113. [Google Scholar] [CrossRef]
- DuPont™. Cleaning Procedures for FilmTec™ FT30 Elements. 2020. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/RO-NF-FilmTec-Cleaning-Procedures-Manual-Exc-45-D01696-en.pdf (accessed on 9 November 2021).
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing No 2003/2003. Off. J. Eur. Union 2019, 62, 51–53. [Google Scholar]
- Wu, H.; Vaneeckhaute, C. Nutrient recovery from wastewater: A review on the integrated Physicochemical technologies of ammonia stripping, adsorption and struvite precipitation. Chem. Eng. J. 2022, 433, 133664. [Google Scholar] [CrossRef]
- Karabelas, A.; Mitrouli, S.; Koutsou, C.; Kostoglou, M. Prediction of spatial-temporal evolution of membrane scaling in spiral wound desalination modules by an advanced simulator. Desalination 2019, 458, 34–44. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Karanasiou, A.; Sioutopoulos, D.C. Experimental study on the effect of polysaccharides on incipient membrane scaling during desalination. Desalination 2017, 416, 106–121. [Google Scholar] [CrossRef]
- van Voorthuizen, E.M.; Zwijnenburg, A.; Wessling, M. Nutrient removal by NF and RO membranes in a decentralized sanitation system. Water Res. 2005, 39, 3657–3667. [Google Scholar] [CrossRef]


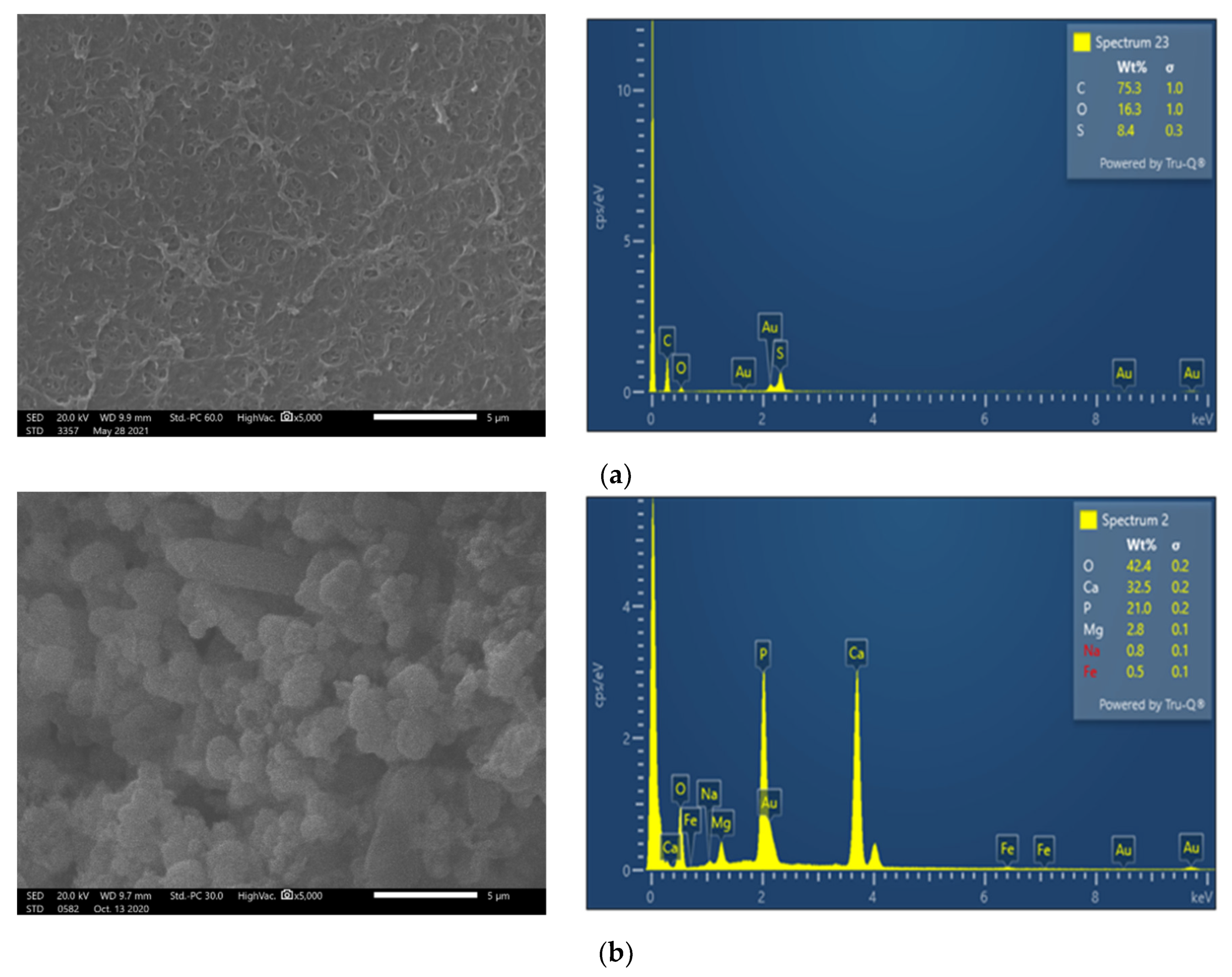
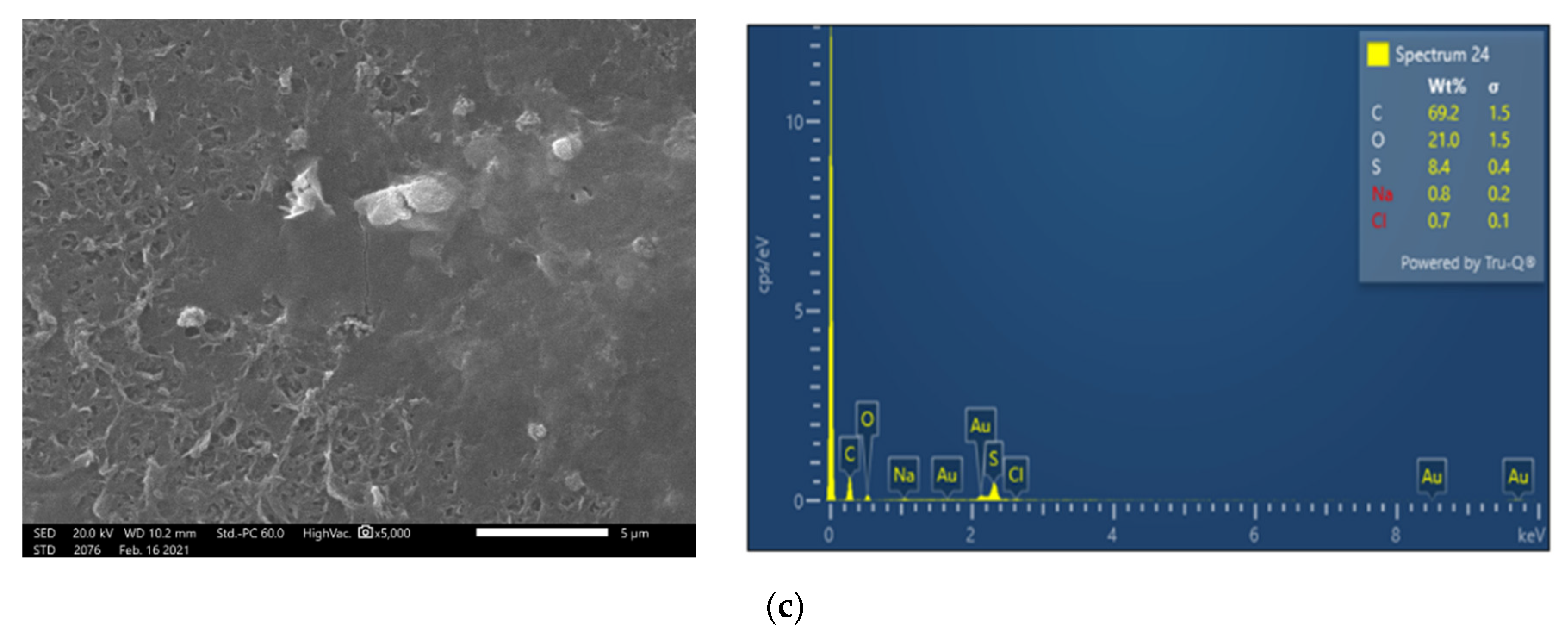





| SWM Element | Feed Pressure, bar | Feed Flow, L/min | Permeate Flow, L/min | Average Flux, L/m2·h | Recovery, % | Permeate TDS, mg/L | Feed TDS, mg/L |
|---|---|---|---|---|---|---|---|
| WAVE Simulator | |||||||
| 1 | 9.0 | 15.5 | 2.3 | 54.1 | 15.1 | 125.2 | 3069 |
| 2 | 8.6 | 13.2 | 2.0 | 47.8 | 28.0 | 163.9 | 3586 |
| 3 | 8.3 | 11.0 | 1.8 | 41.5 | 39.8 | 218.3 | 4217 |
| 4 | 8.1 | 9.3 | 1.5 | 35.3 | 49.5 | 296.3 | 4982 |
| SWM/NRRE Simulator | |||||||
| 1 | 9.4 | 12.1 | 1.8 | 39.0 | 15.1 | 69.1 | 2930 |
| 2 | 9.1 | 10.3 | 1.6 | 34.3 | 28.5 | 81.5 | 3440 |
| 3 | 8.9 | 8.6 | 1.4 | 29.3 | 40.2 | 97.1 | 4070 |
| 4 | 8.7 | 7.2 | 1.2 | 24.0 | 50.0 | 116.6 | 4846 |
| Test Series | #1_8 bar | #2_9 bar | #3_9 bar | #4_10 bar | ||||
|---|---|---|---|---|---|---|---|---|
| Test section | 1st * | 2nd ** | 1st * | 2nd ** | 1st * | 2nd ** | 1st * | 2nd ** |
| Species | Estimated species surface density on NF90 membrane, mg/m2 | |||||||
| TOC | 199.3 | 81.2 | 169.0 | 77.4 | 131.6 | 65.2 | 287.9 | 81.0 |
| P | 22.4 | ND | 145.8 | 40.9 | 13.2 | ND | 25.4 | ND |
| Ca | 51.9 | 50.2 | 278.7 | 83.8 | 300.4 | 2.7 | 20.3 | 7.1 |
| Mg | 8.0 | 1.4 | 11.0 | 3.4 | 56.0 | 8.4 | 10.2 | 1.1 |
| K | 44.7 | 14.7 | ND | ND | 33.1 | 14.5 | 47.0 | 3.9 |
| Na | 264.7 | 169.5 | 195.1 | 170.3 | 259.4 | 179.8 | 170.7 | 74.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsaridou, C.; Karanasiou, A.; Plakas, K.V.; Karabelas, A.J. Valorization of Anaerobic-Fermentation Liquid Digestates—Membrane-Based Process Development. Membranes 2023, 13, 297. https://doi.org/10.3390/membranes13030297
Tsaridou C, Karanasiou A, Plakas KV, Karabelas AJ. Valorization of Anaerobic-Fermentation Liquid Digestates—Membrane-Based Process Development. Membranes. 2023; 13(3):297. https://doi.org/10.3390/membranes13030297
Chicago/Turabian StyleTsaridou, Charikleia, Anthoula Karanasiou, Konstantinos V. Plakas, and Anastasios J. Karabelas. 2023. "Valorization of Anaerobic-Fermentation Liquid Digestates—Membrane-Based Process Development" Membranes 13, no. 3: 297. https://doi.org/10.3390/membranes13030297






