Cardiolipin Strongly Inhibits the Leakage Activity of the Short Antimicrobial Peptide ATRA-1 in Comparison to LL-37, in Model Membranes Mimicking the Lipid Composition of Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Lipid Systems and Peptide Structure Prediction
2.2. Fluorescence Spectroscopy
2.3. Fourier Transform Infrared Spectroscopy
3. Results
3.1. Prediction of the 3D Structure of LL-37 and ATRA-1
3.2. Pore Forming Activity of LL-37 and ATRA-1 in Model Membrane Systems
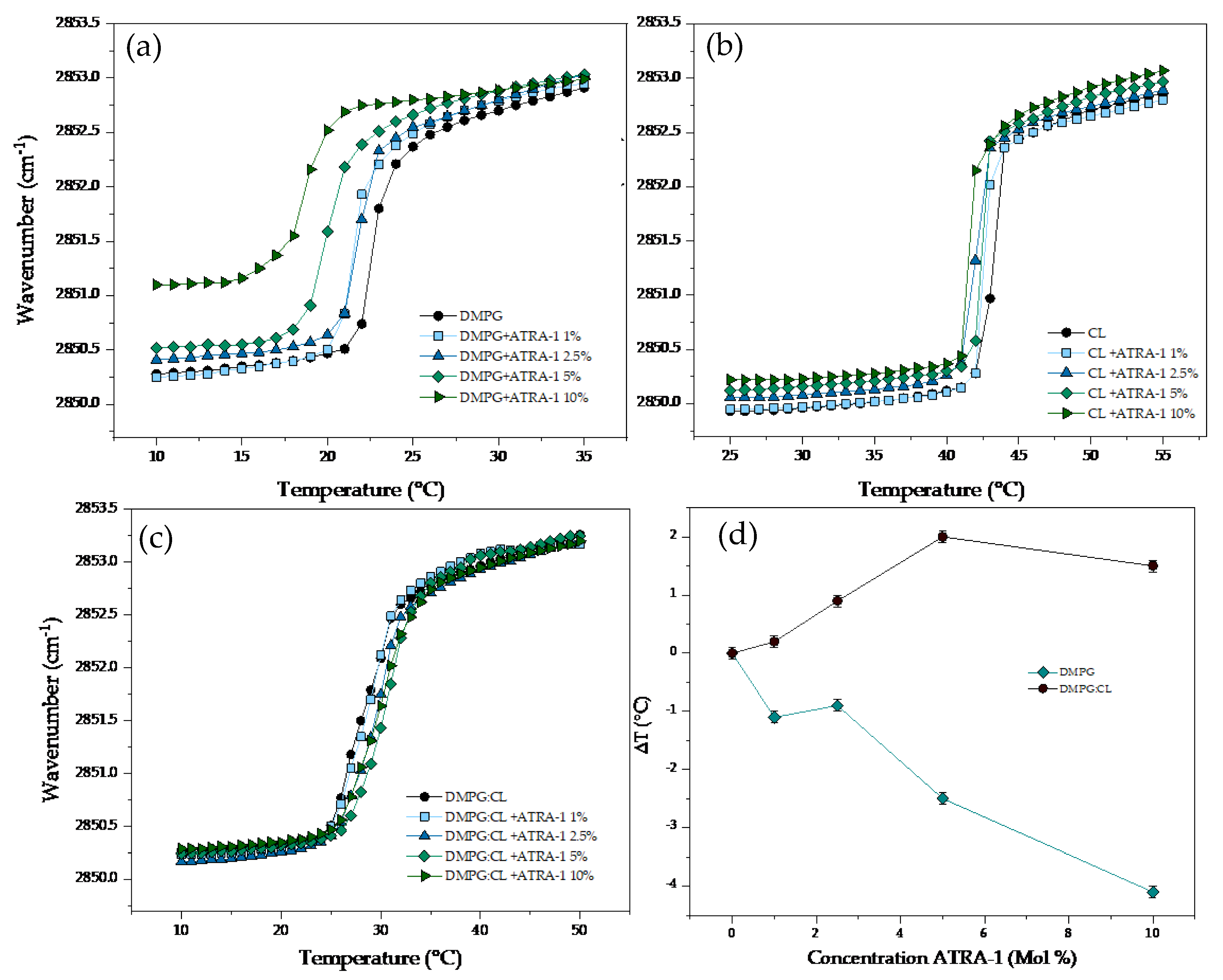
3.3. FTIR Experiments on the Interaction of Antimicrobial Peptides with Model Membranes
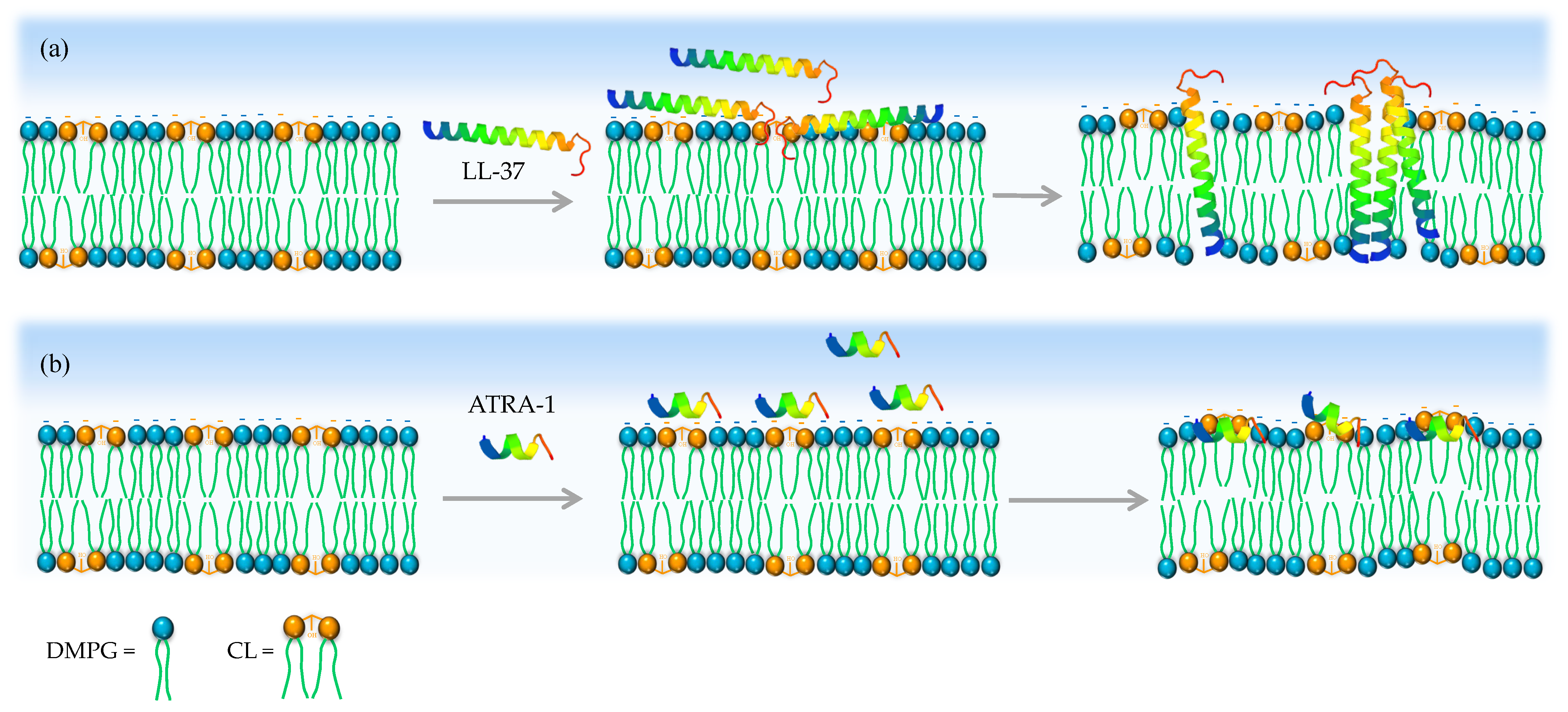
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hancock, R.E.W.; Lehrer, R. Cationic peptides: A new source of antibiotics. Trends Biotechnol. 1998, 16, 82–88. [Google Scholar] [CrossRef]
- Hancock, R.E.W. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-P.; Deber, C.M. Anionic Phospholipids Modulate Peptide Insertion into Membranes. Biochemistry. 1997, 36, 5476–5482. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Chen, F.-Y.; Lee, M.-T. Molecular mechanism of peptide-induced pores in membranes. Phys. Rev. Lett. 2004, 92, 198304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [Green Version]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2015, 40, 133–159. [Google Scholar] [CrossRef] [Green Version]
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta 2009, 1788, 2092–2100. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Villa, L.; Manrique-Moreno, M.; Leidy, C.; Jemioła-Rzemińska, M.; Ortíz, C.; Strzałka, K. Biophysical evaluation of cardiolipin content as a regulator of the membrane lytic effect of antimicrobial peptides. Biophys. Chem. 2018, 238, 8–15. [Google Scholar] [CrossRef]
- Jackson, H.; Ocampo Ariza, P.J.; Escobar, C.; Becerra, J.L.R.; Vives, M.J.; Leidy, C. Staphylococcus aureus Enriched in Ordered Lipids Present Resistance Towards the Antibacterial Agent sPLA2-IIA: An Unusual Mechanism to Survive. Biophys. J. 2010, 98 (Suppl. S1), 285a. [Google Scholar] [CrossRef] [Green Version]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Maupetit, J.; Derreumaux, P.; Tuffery, P. Improved PEP-FOLD approach for peptide and miniprotein structure prediction. J. Chem. Theory Comput. 2014, 10, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
- Thévenet, P.; Shen, Y.; Maupetit, J.; Guyon, F.; Derreumaux, P.; Tuffery, P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012, 40, W288–W293. [Google Scholar] [CrossRef] [Green Version]
- Burton, M.F.; Steel, P.G. The chemistry and biology of LL-37. Nat. Prod. Rep. 2009, 26, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Chennupati, S.K.; Chiu, A.G.; Tamashiro, E.; Banks, C.A.; Cohen, M.B.; Bleier, B.S.; Kofonow, J.M.; Tam, E.; Cohen, N.A. Effects of an LL-37-derived antimicrobial peptide in an animal model of biofilm Pseudomonas sinusitis. Am. J. Rhinol. Allergy 2009, 23, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Dietz, M.J.; Li, B. Antimicrobial peptide LL-37 is bactericidal against Staphylococcus aureus biofilms. PLoS ONE 2019, 14, e0216676. [Google Scholar] [CrossRef] [Green Version]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [Green Version]
- Dürr, U.H.; Sudheendra, U.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [Green Version]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [Green Version]
- Henzler Wildman, K.A.; Lee, D.-K.; Ramamoorthy, A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003, 42, 6545–6558. [Google Scholar] [CrossRef]
- Amer, L.S.; Bishop, B.M.; van Hoek, M.L. Antimicrobial and antibiofilm activity of cathelicidins and short, synthetic peptides against Francisella. Biochem. Biophys. Res. Commun. 2010, 396, 246–251. [Google Scholar] [CrossRef]
- de Latour, F.A.; Amer, L.S.; Papanstasiou, E.A.; Bishop, B.M.; van Hoek, M.L. Antimicrobial activity of the Naja atra cathelicidin and related small peptides. Biochem. Biophys. Res. Commun. 2010, 396, 825–830. [Google Scholar] [CrossRef]
- Tsai, M.; Ohniwa, R.L.; Kato, Y.; Takeshita, S.L.; Ohta, T.; Saito, S.; Hayashi, H.; Morikawa, K. Staphylococcus aureus requires cardiolipin for survival under conditions of high salinity. BMC Microbiol. 2011, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestro, L.; Axelsen, P.H. Infrared spectroscopy of supported lipid monolayer, bilayer, and multibilayer membranes. Chem. Phys. Lipids 1998, 96, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Seydel, U. Infrared spectroscopy of glycolipids. Chem. Phys. Lipids 1998, 96, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Wennerstrom, H.; Sparr, E. Thermodynamics of membrane lipid hydration. Pure Appl. Chem. 2003, 75, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Manrique-Moreno, M.; Suwalsky, M.; Villena, F.; Garidel, P. Effects of the nonsteroidal anti-inflammatory drug naproxen on human erythrocytes and on cell membrane molecular models. Biophys. Chem. 2010, 147, 53–58. [Google Scholar] [CrossRef]
- Lamy-Freund, M.T.; Riske, K.A. The peculiar thermo-structural behavior of the anionic lipid DMPG. Chem. Phys. Lipids 2003, 122, 19–32. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2009, 1788, 2069–2079. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.N.; Zweytick, D.; Pabst, G.; Lohner, K.; McElhaney, R.N. Calorimetric, X-ray diffraction, and spectroscopic studies of the thermotropic phase behavior and organization of tetramyristoyl cardiolipin membranes. Biophys. J. 2007, 92, 3166–3177. [Google Scholar] [CrossRef] [Green Version]
- Ohniwa, R.L.; Kitabayashi, K.; Morikawa, K. Alternative cardiolipin synthase Cls1 compensates for stalled Cls2 function in Staphylococcus aureus under conditions of acute acid stress. FEMS Microbiol. Lett. 2013, 338, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Short, S.A.; White, D.C. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J. Bacteriol. 1971, 108, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, S.A.; White, D.C. Biosynthesis of cardiolipin from phosphatidylglycerol in Staphylococcus aureus. J. Bacteriol. 1972, 109, 820–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koprivnjak, T.; Zhang, D.; Ernst, C.; Peschel, A.; Nauseef, W.; Weiss, J. Characterization of Staphylococcus aureus cardiolipin synthases 1 and 2 and their contribution to accumulation of cardiolipin in stationary phase and within phagocytes. J. Bacteriol. 2011, 193, 4134–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, J.; Panesso, D.; Tran, T.T.; Mishra, N.N.; Cruz, M.R.; Munita, J.M.; Singh, K.V.; Yeaman, M.R.; Murray, B.E.; Shamoo, Y.; et al. A liaR Deletion Restores Susceptibility to Daptomycin and Antimicrobial Peptides in Multidrug-Resistant Enterococcus faecalis. J. Infect. Dis. 2014, 211, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Roa, C.; Orjuela, J.D.; Leidy, C.; Cossio, P.; Aponte-Santamaría, C. Cardiolipin prevents pore formation in phosphatidylglycerol bacterial membrane models. FEBS Lett. 2021, 595, 2701–2714. [Google Scholar] [CrossRef]
- Poger, D.; Pöyry, S.; Mark, A.E. Could cardiolipin protect membranes against the action of certain antimicrobial peptides? Aurein 1.2, a Case Study. ACS Omega 2018, 3, 16453–16464. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Kopiasz, R.J.; Rukasz, A.; Chreptowicz, K.; Podgórski, R.; Kuźmińska, A.; Mierzejewska, J.; Tomaszewski, W.; Ciach, T.; Jańczewski, D. Influence of lipid bilayer composition on the activity of antimicrobial quaternary ammonium ionenes, the interplay of intrinsic lipid curvature and polymer hydrophobicity, the role of cardiolipin. Colloids Surf. B 2021, 207, 112016. [Google Scholar] [CrossRef]
- Marín-Medina, N.; Ramírez, D.A.; Trier, S.; Leidy, C. Mechanical properties that influence antimicrobial peptide activity in lipid membranes. Appl. Microbiol. Biotechnol. 2016, 100, 10251–10263. [Google Scholar] [CrossRef]
- Prenner, E.J.; Lewis, R.N.A.H.; Kondejewski, L.H.; Hodges, R.S.; McElhaney, R.N. Differential scanning calorimetric study of the effect of the antimicrobial peptide gramicidin S on the thermotropic phase behavior of phosphatidylcholine, phosphatidylethanolamine and phosphatidylglycerol lipid bilayer membranes. Biochim. Biophys. Acta 1999, 1417, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Seto, G.W.J.; Marwaha, S.; Kobewka, D.M.; Lewis, R.N.A.H.; Separovic, F.; McElhaney, R.N. Interactions of the Australian tree frog antimicrobial peptides aurein 1.2, citropin 1.1 and maculatin 1.1 with lipid model membranes: Differential scanning calorimetric and Fourier transform infrared spectroscopic studies. Biochim. Biophys. Acta 2007, 1768, 2787–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrushchenko, V.V.; Vogel, H.J.; Prenner, E.J. Interactions of tryptophan-rich cathelicidin antimicrobial peptides with model membranes studied by differential scanning calorimetry. Biochim. Biophys. Acta 2007, 1768, 2447–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
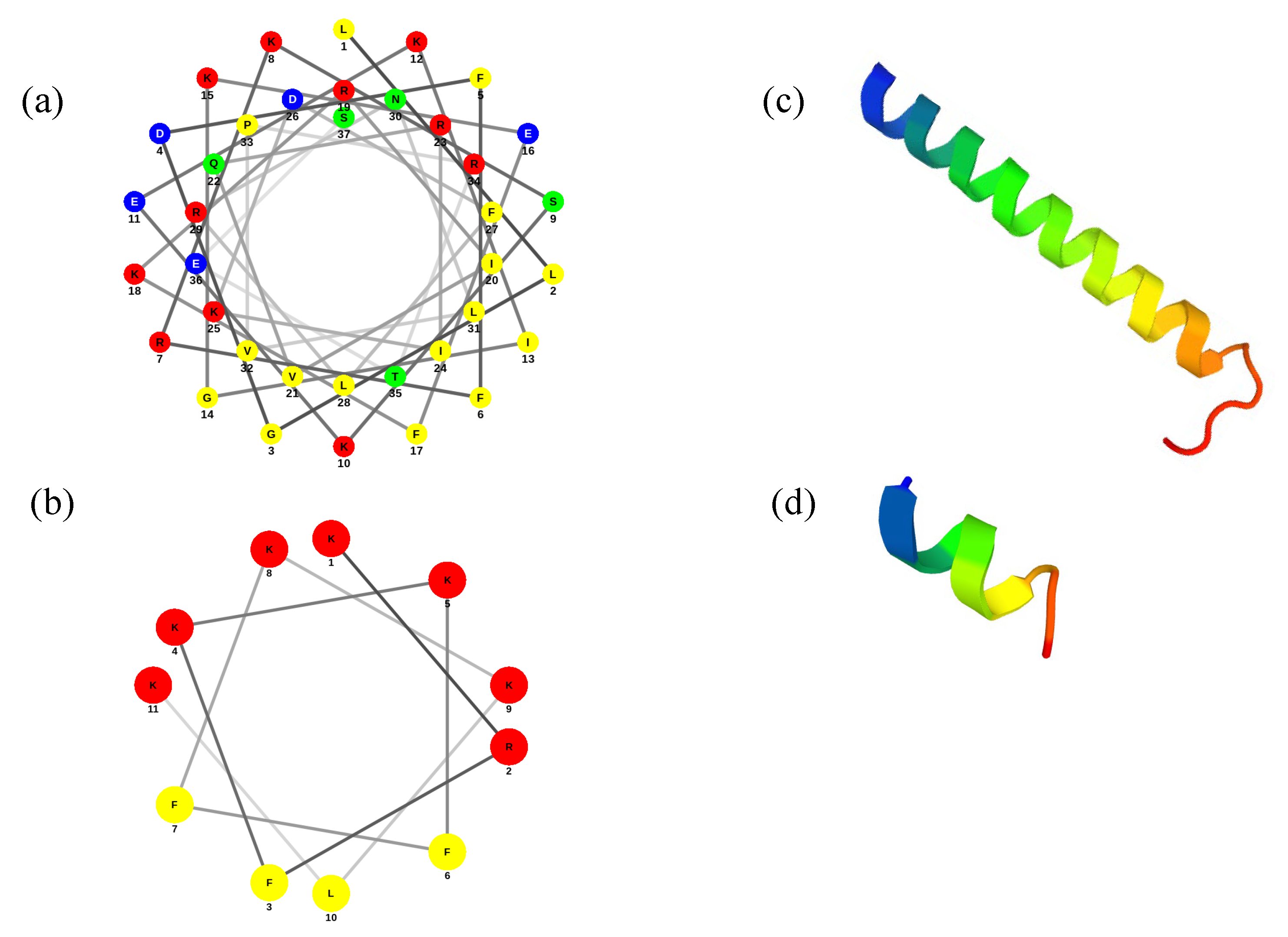
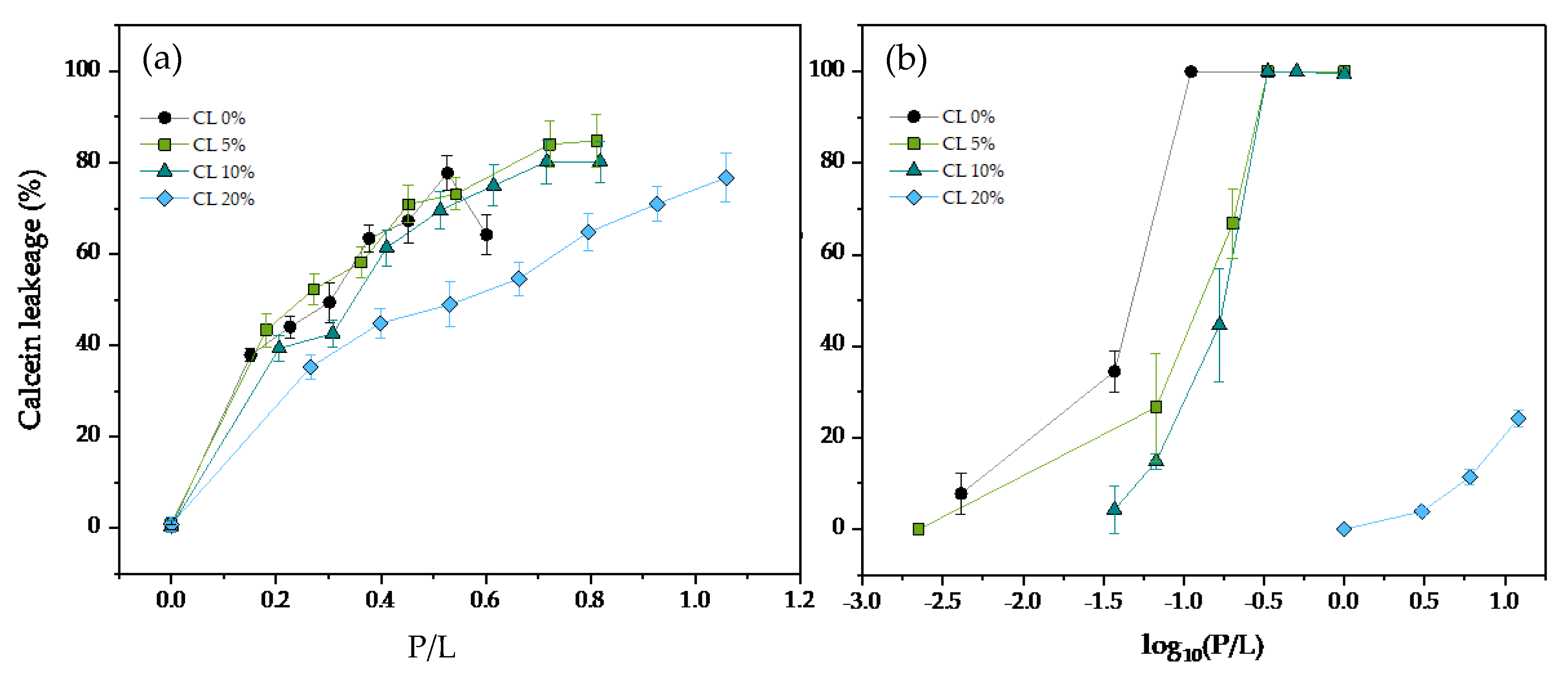
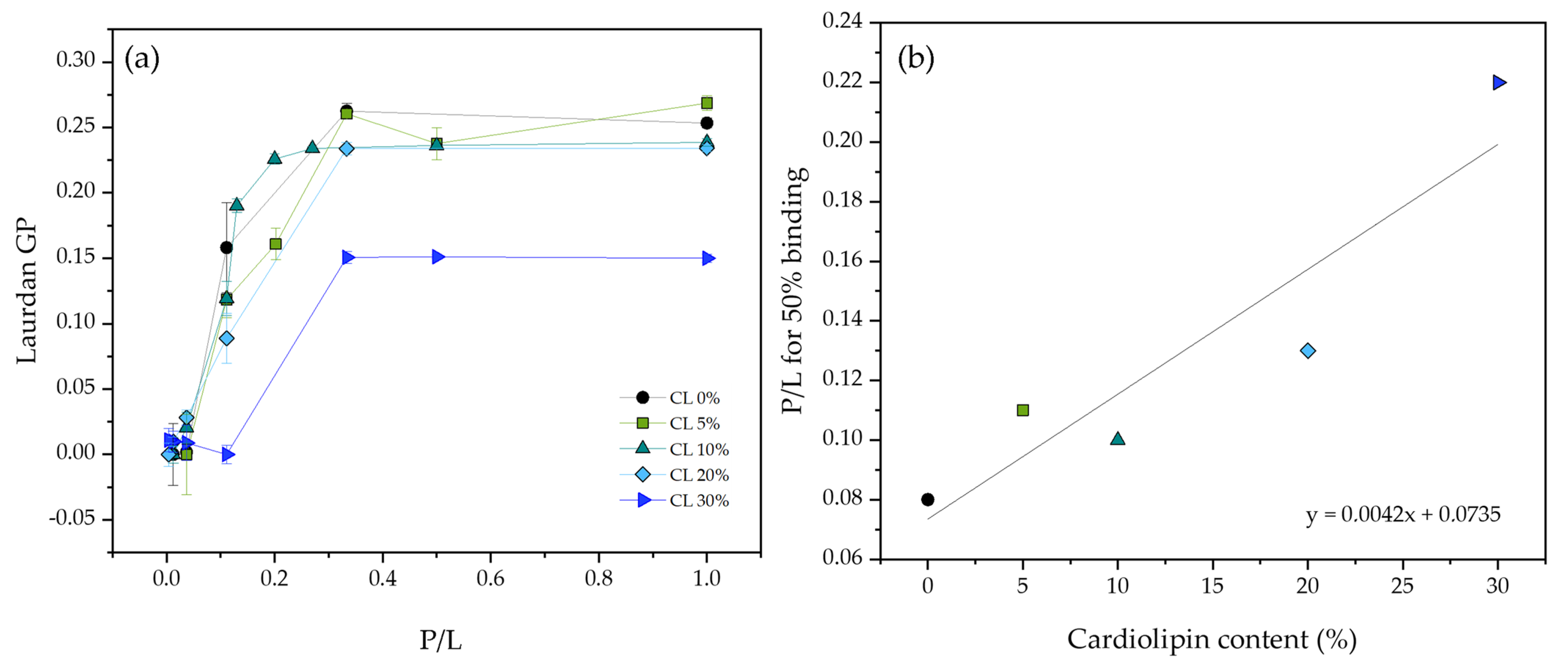
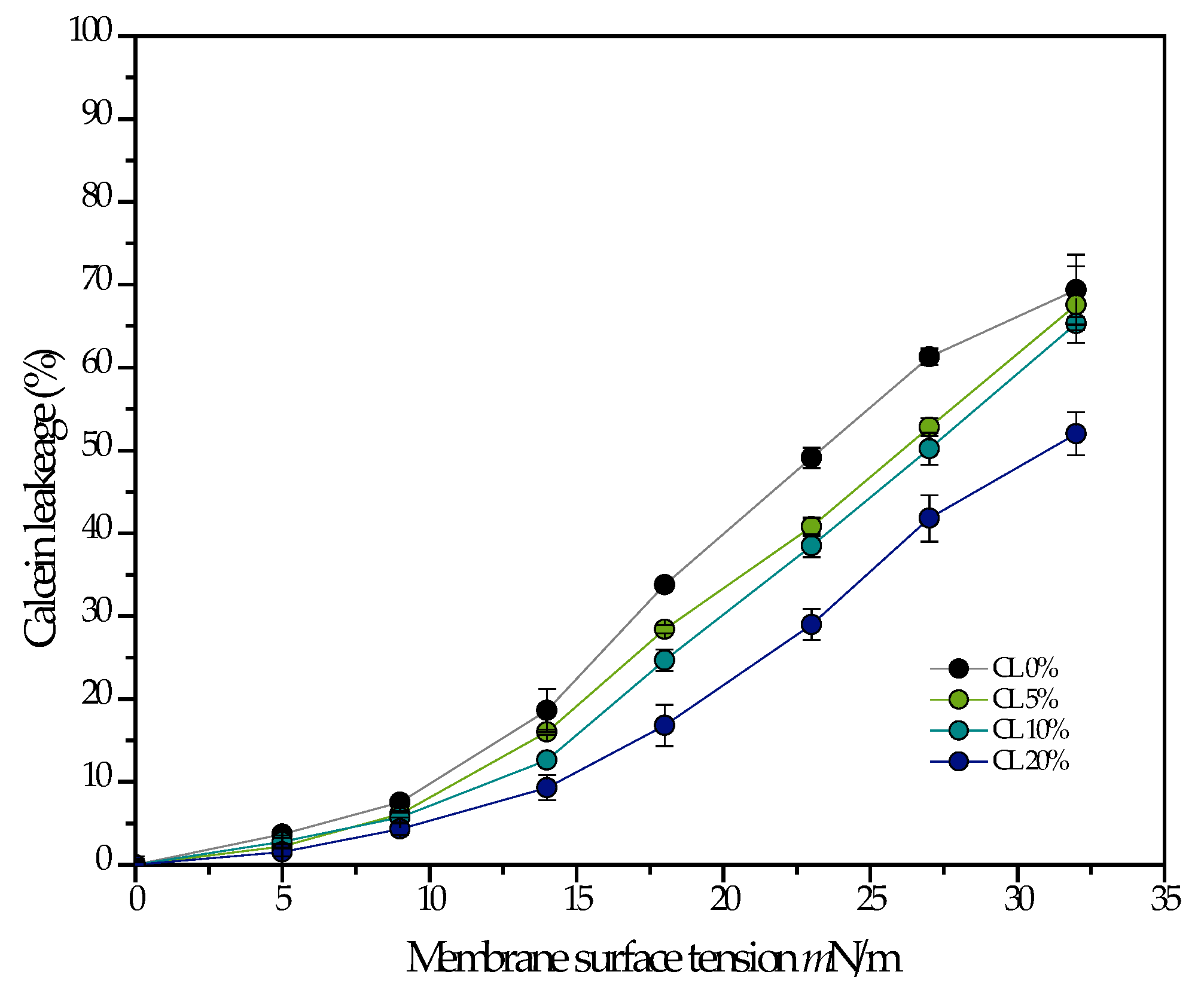

| Peptide | Sequence | Charge | Hydrophobicity (%) |
|---|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | +6 | 37.8 |
| ATRA-1 | KRFKKFFKKLK-NH2 | +8 | 36.4 |
| LL-37 (mol%) | Tm (°C) | ||
|---|---|---|---|
| DMPG | CL | DMPG:CL | |
| 0 | 22.9 | 43.2 | 28.7 |
| 1 | 23.1 | 43.2 | 30.3 |
| 2.5 | 23.5 | 42.1 | 31.1 |
| 5 | 21.0 | 42.8 | 29.8 |
| 10 | 20.8 | 45.1 | 26.2 |
| ATRA-1 (mol%) | Tm (°C) | ||
|---|---|---|---|
| DMPG | CL | DMPG:CL | |
| 0 | 22.9 | 43.2 | 28.7 |
| 1 | 21.8 | 42.6 | 28.9 |
| 2.5 | 22.0 | 42.0 | 29.6 |
| 5 | 20.4 | 42.4 | 30.7 |
| 10 | 18.8 | 41.7 | 30.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Rivera, N.; Múnera-Jaramillo, J.; Jaramillo-Berrio, S.; Suesca, E.; Manrique-Moreno, M.; Leidy, C. Cardiolipin Strongly Inhibits the Leakage Activity of the Short Antimicrobial Peptide ATRA-1 in Comparison to LL-37, in Model Membranes Mimicking the Lipid Composition of Staphylococcus aureus. Membranes 2023, 13, 304. https://doi.org/10.3390/membranes13030304
Calderón-Rivera N, Múnera-Jaramillo J, Jaramillo-Berrio S, Suesca E, Manrique-Moreno M, Leidy C. Cardiolipin Strongly Inhibits the Leakage Activity of the Short Antimicrobial Peptide ATRA-1 in Comparison to LL-37, in Model Membranes Mimicking the Lipid Composition of Staphylococcus aureus. Membranes. 2023; 13(3):304. https://doi.org/10.3390/membranes13030304
Chicago/Turabian StyleCalderón-Rivera, Nathalia, Jessica Múnera-Jaramillo, Sara Jaramillo-Berrio, Elizabeth Suesca, Marcela Manrique-Moreno, and Chad Leidy. 2023. "Cardiolipin Strongly Inhibits the Leakage Activity of the Short Antimicrobial Peptide ATRA-1 in Comparison to LL-37, in Model Membranes Mimicking the Lipid Composition of Staphylococcus aureus" Membranes 13, no. 3: 304. https://doi.org/10.3390/membranes13030304
APA StyleCalderón-Rivera, N., Múnera-Jaramillo, J., Jaramillo-Berrio, S., Suesca, E., Manrique-Moreno, M., & Leidy, C. (2023). Cardiolipin Strongly Inhibits the Leakage Activity of the Short Antimicrobial Peptide ATRA-1 in Comparison to LL-37, in Model Membranes Mimicking the Lipid Composition of Staphylococcus aureus. Membranes, 13(3), 304. https://doi.org/10.3390/membranes13030304







