Nanoparticle-Enhanced PVDF Flat-Sheet Membranes for Seawater Desalination in Direct Contact Membrane Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Synthesis and Functionalisation of Silica Nanoparticles (SiO2NPs)
2.3. Functionalisation of CNTs
2.4. Membrane Preparation
2.5. Characterisation of the NPs and Membranes
2.6. Membrane Distillation
3. Results and Discussion
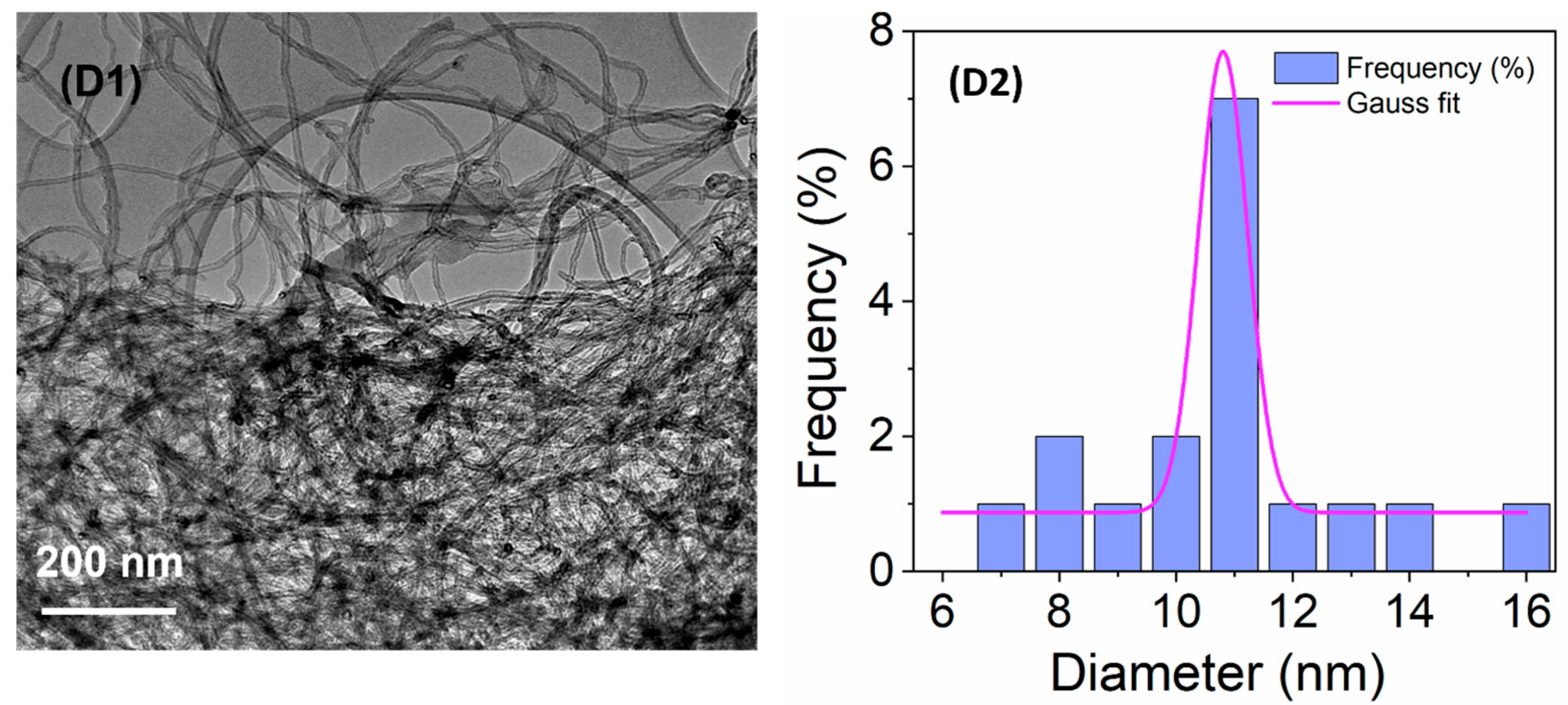
3.1. TEM Analysis of the SiO2NPs and CNTs
3.2. Membrane Porosity, Pore Size, WCA, and LEP
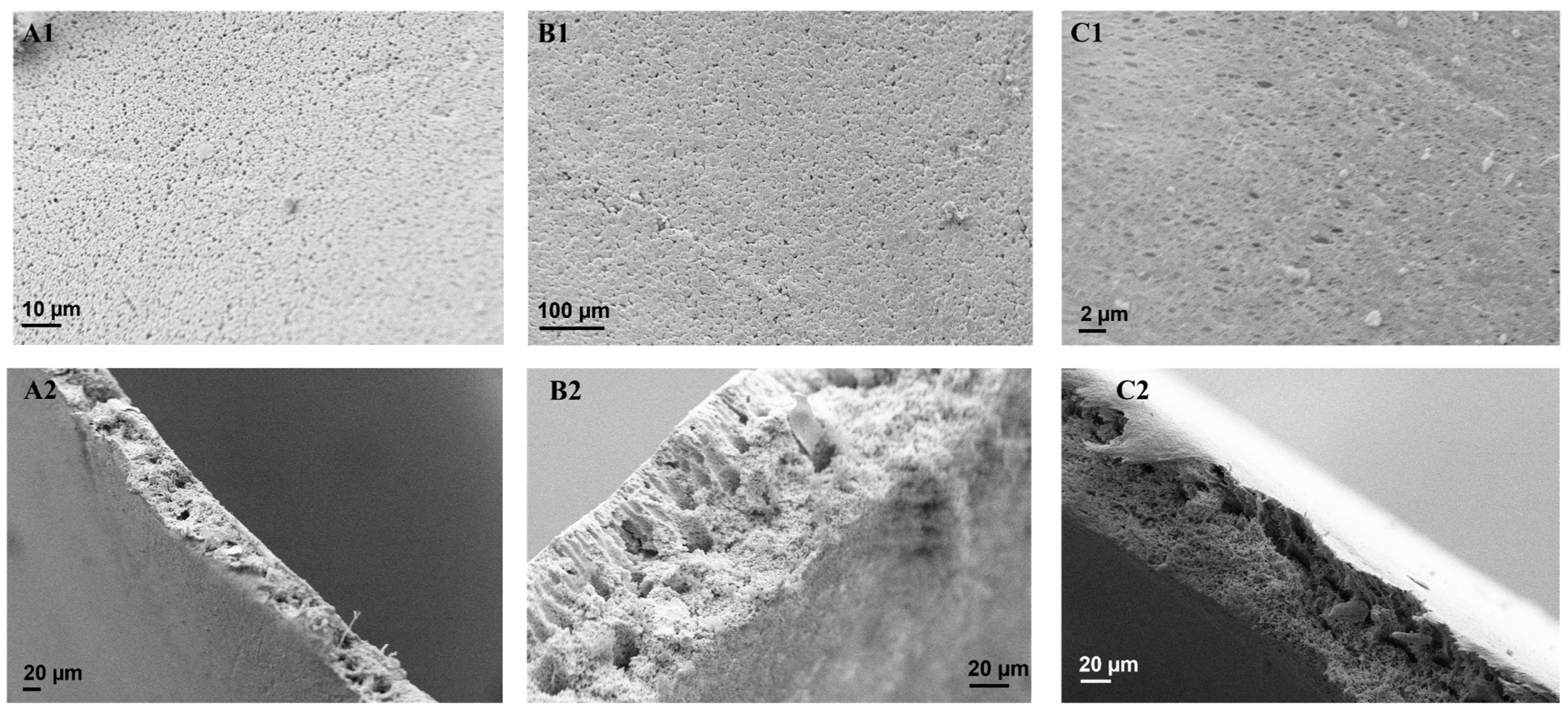
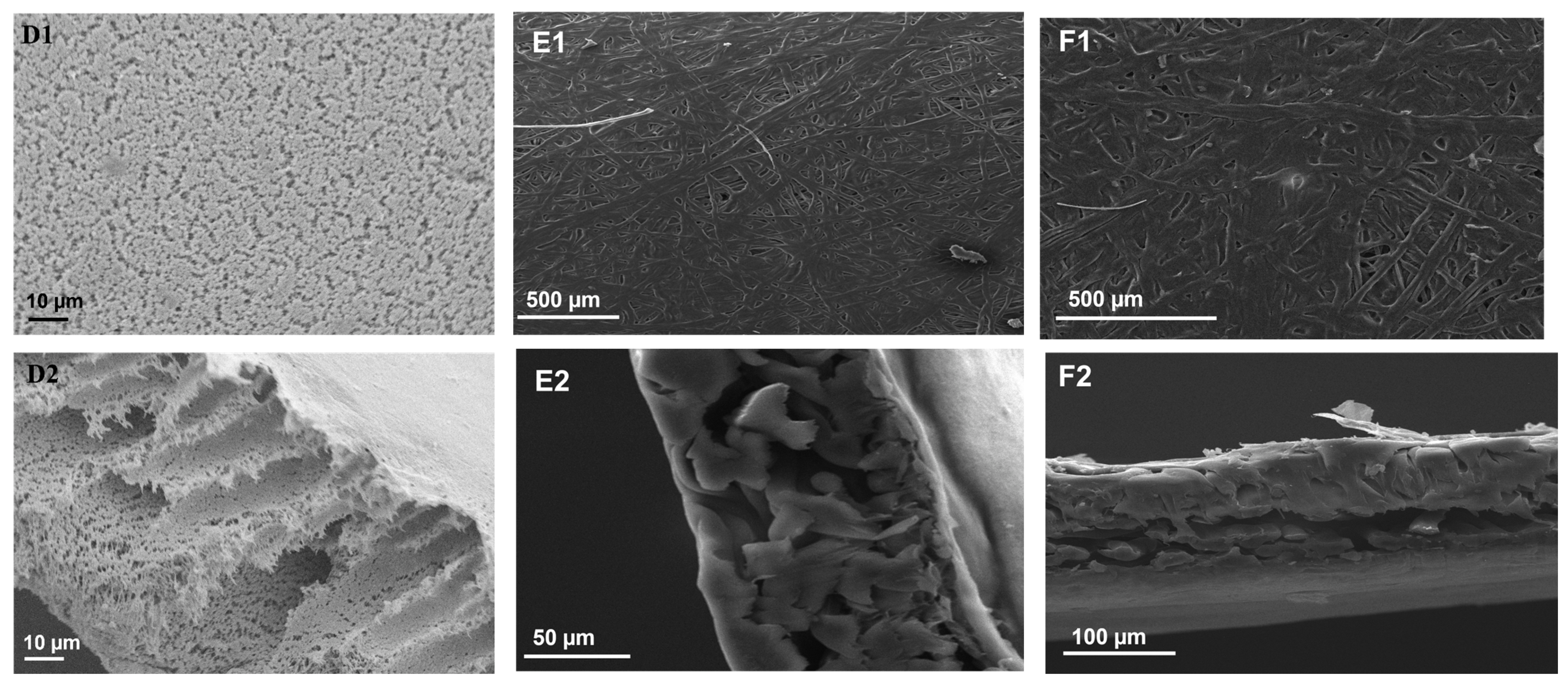
3.3. SEM Analysis of the Membranes
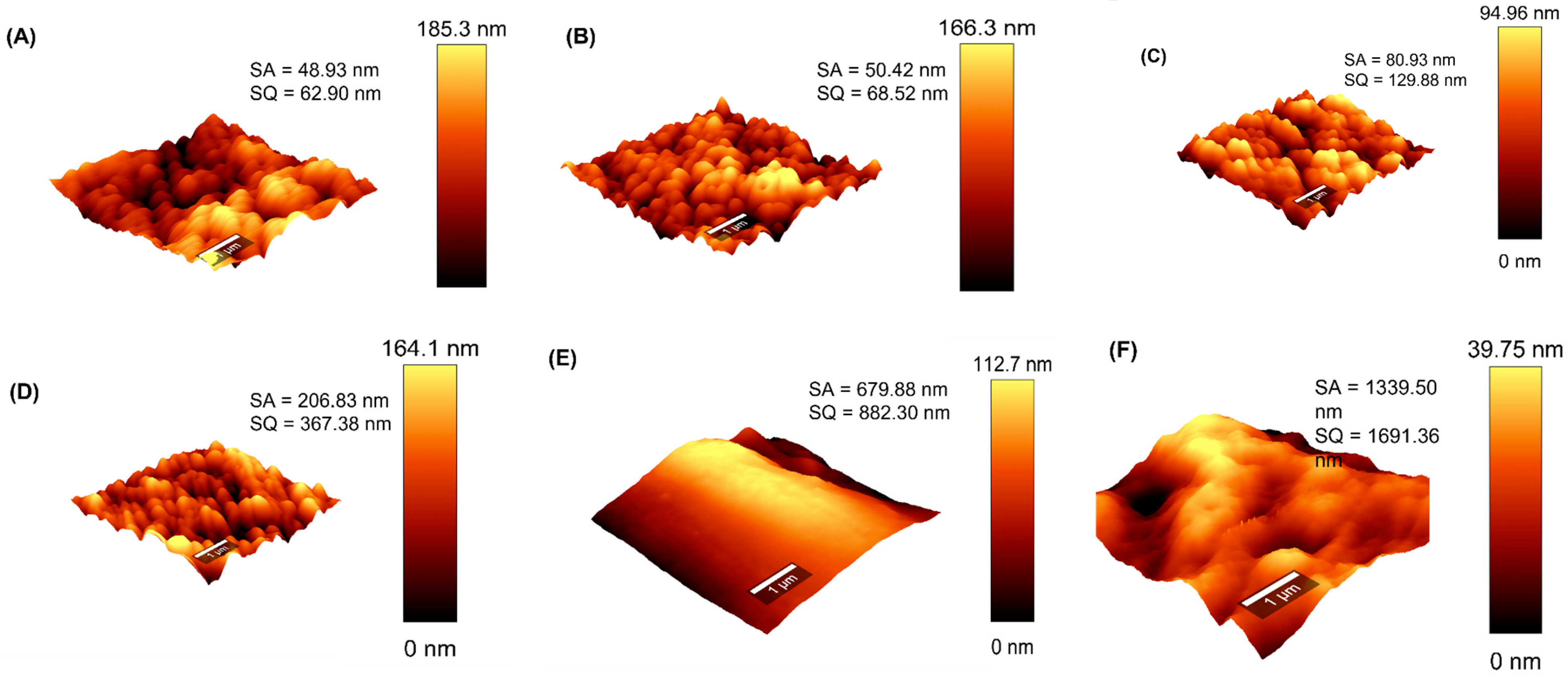
3.4. AFM Analysis of the Membranes
3.5. Mechanical Properties of the Membranes
3.6. Flux and Salt Rejection Evaluation in DCMD Using Synthetic Salt Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UNICEF. Progress on Drinking Water, Sanitation and Hygiene: 2000–2017. 2019. Available online: https://data.unicef.org/resources/progress-drinking-water-sanitation-hygiene-2019/ (accessed on 4 July 2022).
- Vineis, P.; Chan, Q.; Khan, A. Climate change impacts on water salinity and health. J. Epidemiol. Glob. Health 2011, 1, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Masheane, M.L.; George, M.; Kime, M.-B.; Mhlanga, S.D. Removal of Fe and Mn from polluted water sources in Lesotho using modified clays. J. Water Chem. Technol. 2019, 41, 81–86. [Google Scholar] [CrossRef]
- Loubser, C.; Chimbanga, B.M.; Jacobs, H. Intermittent water supply: A South African perspective. Water SA 2021, 47, 1–9. [Google Scholar] [CrossRef]
- Sershen, S.; Rodda, N.; Stenström, T.A.; Schmidt, S.; Dent, M.; Bux, F.; Hanke, N.; Buckley, C.A.; Fennemore, C. Water security in South Africa: Perceptions on public expectations and municipal obligations, governance and water re-use. Water SA 2016, 42, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Farmani, R.; Dalton, J.; Charalambous, B.; Lawson, E.; Bunney, S.; Cotterill, S. Intermittent water supply systems and their resilience to COVID-19: IWA IWS SG survey. Aqua Water Infrastruct. Ecosyst. Soc. 2021, 70, 507–520. [Google Scholar] [CrossRef]
- Calvo, G.; Valero, A. Strategic mineral resources: Availability and future estimations for the renewable energy sector. Environ. Dev. 2022, 41, 100640. [Google Scholar] [CrossRef]
- Makgabutlane, B.; Nthunya, L.N.; Nxumalo, E.N.; Musyoka, N.M.; Mhlanga, S.D. Microwave Irradiation-Assisted Synthesis of Zeolites from Coal Fly Ash: An Optimization Study for a Sustainable and Efficient Production Process. ACS Omega 2020, 5, 25000–25008. [Google Scholar] [CrossRef]
- Schmidt, M. Scarcity and environmental impact of mineral resources—An old and never-ending discussion. Resources 2019, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, M.; Sharaky, A.M.; Al-Sherbini, A.S.; Bassyouni, M.; Rezakazemi, M.; Elhenawy, Y. Water Desalination Using Solar Thermal Collectors Enhanced by Nanofluids. Chem. Eng. Technol. 2022, 45, 15–25. [Google Scholar] [CrossRef]
- Mericq, J.-P.; Laborie, S.; Cabassud, C. Vacuum membrane distillation of seawater reverse osmosis brines. Water Res. 2010, 44, 5260–5273. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.A.; Kattan Readi, O.M. An industrial perspective on membrane distillation processes. J. Chem. Technol. Biotechnol. 2018, 93, 2047–2055. [Google Scholar] [CrossRef]
- Ashoor, B.B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S.W. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Bopape, M.F.; Mahlangu, O.T.; Mamba, B.B.; Van der Bruggen, B.; Quist-Jensen, C.A.; Richards, H. Fouling, performance and cost analysis of membrane-based water desalination technologies: A critical review. J. Environ. Manag. 2022, 301, 113922. [Google Scholar] [CrossRef] [PubMed]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van Der Bruggen, B. How to optimize the membrane properties for membrane distillation: A review. Ind. Eng. Chem. Res. 2016, 55, 9333–9343. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Horbez, D.; Drioli, E. Reclamation of sodium sulfate from industrial wastewater by using membrane distillation and membrane crystallization. Desalination 2017, 401, 112–119. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Edward, N.; Verliefde, A.R.; Mamba, B.; Mhlanga, S.D. A review of nanoparticle-enhanced membrane distillation membranes: Membrane synthesis and applications in water treatment. Chem. Technol. Biotechnol. 2019, 94, 2757–2771. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Fane, A.G.; Pasquier, A.C.S.; Bing, W. A novel membrane bioreactor based on membrane distillation. Desalination 2008, 223, 386–395. [Google Scholar] [CrossRef]
- Goh, S.; Zhang, J.; Liu, Y.; Fane, A.G. Membrane Distillation Bioreactor (MDBR)—A lower Green-House-Gas (GHG) option for industrial wastewater reclamation. Chemosphere 2015, 140, 129–142. [Google Scholar] [CrossRef]
- Ali, A.; Hvid Jacobsen, J.; Casper Jensen, H.; Lykkegaard Christensen, M.; Quist-Jensen, C. Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization. Appl. Sci. 2019, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Macedonio, F.; Ali, A.; Poerio, T.; El-Sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct contact membrane distillation for treatment of oilfield produced water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Xu, L.; Shang, X.; Chen, K. Water regeneration from human urine by vacuum membrane distillation and analysis of membrane fouling characteristics. Sep. Purif. Technol. 2013, 118, 369–376. [Google Scholar] [CrossRef]
- Lu, D.; Li, P.; Xiao, W.; He, G.; Jiang, X. Simultaneous Recovery and Crystallization Control of Saline Organic Wastewater by Membrane Distillation Crystallization. AIChE J. 2017, 63, 2187–2197. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Hong, S. Recovery of water and minerals from shale gas produced water by membrane distillation crystallization. Water Res. 2018, 129, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwon, H.; Lee, S.; Lee, S.; Hong, S. Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 2017, 403, 172–178. [Google Scholar] [CrossRef]
- Elhenawy, Y.; Moustafa, G.H.; Attia, A.M.; Mansi, A.E.; Majozi, T.; Bassyouni, M. Performance enhancement of a hybrid multi effect evaporation/membrane distillation system driven by solar energy for desalination. J. Environ. Chem. Eng. 2022, 10, 108855. [Google Scholar] [CrossRef]
- El Haj Assad, M.; Bani-Hani, E.; Al-Sawafta, I.; Issa, S.; Hmida, A.; Gupta, M.; Atiqurea, R.S.; Hidouric, K. Applications of nanotechnology in membrane distillation: A review study. Desalin. Water Treat. 2020, 192, 61–77. [Google Scholar] [CrossRef]
- Chimanlal, I.; Nthunya, L.N.; Quist-Jensen, C.; Richards, H. Membrane distillation crystallization for water and mineral recovery: The occurrence of fouling and its control during wastewater treatment. Front. Chem. Eng. 2022, 4, 1066027. [Google Scholar] [CrossRef]
- Alanezi, A.A.; Bassyouni, M.; Abdel-hamid, S.M.S.; Ahmed, H.S.; Abdel-aziz, M.H.; Zoromba, M.S.; Elhenawy, Y. Theoretical Investigation of Vapor Transport Mechanism Using Tubular Membrane Distillation Module. Membranes 2021, 11, 560. [Google Scholar] [CrossRef]
- Toh, M.J.; Oh, P.C.; Chew, T.L.; Ahmad, A.L. Antiwettability enhancement of PVDF-HFP membrane via superhydrophobic modification by SiO2 nanoparticles. Comptes Rendus Chim. 2019, 22, 369–372. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, W.; Rajabzadeh, S.; Jia, Y.; Shen, Q.; Fang, C.; Kato, N.; Matsuyama, H. Modification of PVDF hollow fiber membrane by co-deposition of PDA/MPC-co-AEMA for membrane distillation application with anti-fouling and anti-scaling properties. J. Membr. Sci. 2021, 636, 119596. [Google Scholar] [CrossRef]
- Afsari, M.; Shon, H.K.; Tijing, L.D. Janus membranes for membrane distillation: Recent advances and challenges. Adv. Colloid Interface Sci. 2021, 289, 102362. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Guo, H.; He, H.; Liu, Y.; Li, X.; Zhang, Y.; Yin, H.; Volkov, A.V.; He, T. Unprecedented scaling/fouling resistance of omniphobic polyvinylidene fluoride membrane with silica nanoparticle coated micropillars in direct contact membrane distillation. J. Membr. Sci. 2020, 599, 117819. [Google Scholar] [CrossRef]
- Kharraz, J.A.; An, A.K. Patterned superhydrophobic polyvinylidene fluoride (PVDF) membranes for membrane distillation: Enhanced flux with improved fouling and wetting resistance. J. Membr. Sci. 2020, 595, 117596. [Google Scholar] [CrossRef]
- Yue, D.; Wang, Y.; Zhang, H.; Sun, D.; Li, B.; Ye, X.; Fang, W.; Liu, M. A novel silver/activated- Polyvinylidene fluoride-Polydimethyl siloxane hydrophilic-hydrophobic Janus membrane for vacuum membrane distillation and its anti-oil-fouling ability. J. Membr. Sci. 2021, 638, 119718. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Silva, T.L.S.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M.T. Multi-walled carbon nanotube/PVDF blended membranes with sponge- and finger-like pores for direct contact membrane distillation. Desalination 2015, 357, 233–245. [Google Scholar] [CrossRef]
- Nitodas, S.F.; Das, M.; Shah, R. Applications of Polymeric Membranes with Carbon Nanotubes: A Review. Membranes 2022, 12, 454. [Google Scholar] [CrossRef]
- Gao, C.; Deng, W.; Pan, F.; Feng, X.; Li, Y. Superhydrophobic Electrospun PVDF Membranes with Silanization and Fluorosilanization Co-Functionalized CNTs for Improved Direct Contact Membrane Distillation. Eng. Sci. 2020, 9, 35–43. [Google Scholar] [CrossRef]
- Khalid, A.; Ibrahim, A.; Al-Hamouz, O.C.S.; Laoui, T.; Benamor, A.; Atieh, M.A. Fabrication of polysulfone nanocomposite membranes with silver-doped carbon nanotubes and their antifouling performance. J. Appl. Polym. Sci. 2017, 134, 44688. [Google Scholar] [CrossRef]
- Pan, J.; Chen, M.; Xu, X.; Sun, S.-P.; Wang, Z.; Cui, Z.; Xing, W.; Tavajohi, N. Enhanced anti-wetted PVDF membrane for pulping RO brine treatment by vacuum membrane distillation. Desalination 2022, 526, 115533. [Google Scholar] [CrossRef]
- Kong, X.; Shu, G.; Lu, X.; Wu, C.; Gai, Y. Manipulating membrane surface porosity via deep insight into surfactants during nonsolvent induced phase separation. J. Membr. Sci. 2020, 611, 118358. [Google Scholar] [CrossRef]
- Tan, H.F.; Tan, W.L.; Ooi, B.S.; Leo, C.P. Superhydrophobic PVDF/micro fibrillated cellulose membrane for membrane distillation crystallization of struvite. Chem. Eng. Res. Des. 2021, 170, 54–68. [Google Scholar] [CrossRef]
- Fernandes, C.S.; Md Nordin, N.A.H.; Bilad, M.R.; Matsuura, T.; Putra, Z.A.; Wirzal, M.D.H.; Jaafar, J. Explication of hydrophobic silica as effective pore former for membrane fabrication. Appl. Surf. Sci. Adv. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Alibakhshi, S.; Youssefi, M.; Hosseini, S.S.; Zadhoush, A. Tuning morphology and transport in ultrafiltration membranes derived from polyethersulfone through exploration of dope formulation and characteristics. Mater. Res. Express 2019, 6, 125326. [Google Scholar] [CrossRef]
- Dong, Z.-Q.; Ma, X.-H.; Xu, Z.-L.; You, W.-T.; Li, F.-B. Superhydrophobic PVDF-PTFE electrospun nanofibrous membranes for desalination by vacuum membrane distillation. Desalination 2014, 347, 175–183. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Esmaeilbeig, M.A. Chapter 6—Membrane Wetting in Membrane Distillation. In Current Trends and Future Developments on (Bio-) Membranes Elsevier; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 143–174. Available online: https://www.sciencedirect.com/science/article/pii/B9780128135518000061 (accessed on 20 November 2022).
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Hernández-Aguirre, O.A.; Núñez-Pineda, A.; Tapia-Tapia, M.; Espinosa, R.M.G. Surface Modification of Polypropylene Membrane Using Biopolymers with Potential Applications for Metal Ion Removal. J. Chem. 2016, 2016, 2741023. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Liu, Z.; Xiao, C. Excellent performance of novel superhydrophobic composite hollow membrane in the vacuum membrane distillation. Sep. Purif. Technol. 2021, 268, 118603. [Google Scholar] [CrossRef]
- Hamzah, N.; Leo, C.P.; Ooi, B.S. Superhydrophobic PVDF/TiO2-SiO2 Membrane with Hierarchical Roughness in Membrane Distillation for Water Recovery from Phenolic Rich Solution Containing Surfactant. Chin. J. Polym. Sci. 2019, 37, 609–616. (In English) [Google Scholar] [CrossRef]
- Pramono, E.; Simamora, A.L.; Radiman, C.L.; Wahyuningrum, D. Effects of PVDF concentration on the properties of PVDF membranes. IOP Conf. Ser. Earth Environ. Sci. 2017, 75, 012027. [Google Scholar] [CrossRef] [Green Version]
- Manawi, Y.M.; Wang, K.; Kochkodan, V.; Johnson, D.J.; Atieh, M.A.; Khraisheh, M.K. Engineering the surface and mechanical properties of water desalination membranes using ultralong carbon nanotubes. Membranes 2018, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Makgabutlane, B.; Nthunya, L.N.; Maubane-Nkadimeng, M.S.; Mhlanga, S.D. Green synthesis of carbon nanotubes to address the water-energy-food nexus: A critical review. J. Environ. Chem. Eng. 2021, 9, 104736. [Google Scholar] [CrossRef]
- Loh, C.H.; Wang, R.; Shi, L.; Fane, A.G. Fabrication of high performance polyethersulfone UF hollow fiber membranes using amphiphilic Pluronic block copolymers as pore-forming additives. J. Membr. Sci. 2011, 380, 114–123. [Google Scholar] [CrossRef]
- Guo, J.L.; Li, Y.; Xu, Z.L.; Zhang, P.Y.; Yang, H. Investigation of polyvinylidene fluoride membranes prepared by using surfactant OP-10 alone or with a second component, as additives, via the Non-Solvent-Induced Phase Separation (NIPS) process. J. Macromol. Sci. Part B Phys. 2014, 53, 1319–1334. [Google Scholar] [CrossRef]
- Attia, H.; Osman, M.S.; Johnson, D.J.; Wright, C.; Hilal, N. Modelling of air gap membrane distillation and its application in heavy metals removal. Desalination 2017, 424, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Tan, G.; Lei, D.; Yang, Q.; Tan, X.; Liang, N.; Ma, D. Omniphobic membrane with process optimization for advancing flux and durability toward concentrating reverse-osmosis concentrated seawater with membrane distillation. J. Membr. Sci. 2021, 639, 119763. [Google Scholar] [CrossRef]
- Edwie, F.; Chung, T.-S. Development of simultaneous membrane distillation-crystallization (SMDC) technology for treatment of saturated brine. Chem. Eng. Sci. 2013, 98, 160–172. [Google Scholar] [CrossRef]
- Ardeshiri, F.; Salehi, S.; Peyravi, M.; Jahanshahi, M.; Amiri, A.; Rad, A.S. PVDF membrane assisted by modified hydrophobic ZnO nanoparticle for membrane distillation. Asia-Pac. J. Chem. Eng. 2018, 13, e2196. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Mamba, B.B. Adsorption of phenolic compounds by polyacrylonitrile nano fibre membranes: A pretreatment for the removal of hydrophobic bearing compounds from water. J. Environ. Chem. Eng. 2019, 7, 103254. [Google Scholar] [CrossRef]
- Wae AbdulKadir, W.A.F.; Ahmad, A.L.; Ooi, B.S. Hydrophobic PVDF-HNT membrane for oxytetracycline removal via DCMD: The influence of fabrication parameters on permeability, selectivity and antifouling properties. J. Water Process. Eng. 2022, 49, 102960. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Yi, L.; Yan, X.; Li, J. Applied Surface Science Fabricating superhydrophobic and oleophobic surface with silica nanoparticles modified by silanes and environment-friendly fluorinated chemicals. Appl Surf Sci. 2018, 450, 102–111. [Google Scholar] [CrossRef]
- Puspitasari, V.; Granville, A.; Le-Clech, P.; Chen, V. Cleaning and ageing effect of sodium hypochlorite on polyvinylidene fluoride (PVDF) membrane. Sep. Purif. Technol. 2010, 72, 301–308. [Google Scholar] [CrossRef]
- Wang, H.; Wen, Y.; Peng, H.; Zheng, C.; Li, Y.; Wang, S.; Sun, S.; Xie, X.; Zhou, X. Grafting Polytetrafluoroethylene Micropowder via in Situ Electron Beam Irradiation-Induced Polymerization. Polymers 2018, 10, 503. [Google Scholar] [CrossRef] [Green Version]
- Gryta, M. Separation of saline oily wastewater by membrane distillation. Chem. Pap. 2020, 74, 2277–2286. [Google Scholar] [CrossRef] [Green Version]
- Dumée, L.F.; Gray, S.; Duke, M.; Sears, K.; Schutz, J.; Finn, N. The role of membrane surface energy on direct contact membrane distillation performance. Desalination 2013, 323, 22–30. [Google Scholar] [CrossRef]

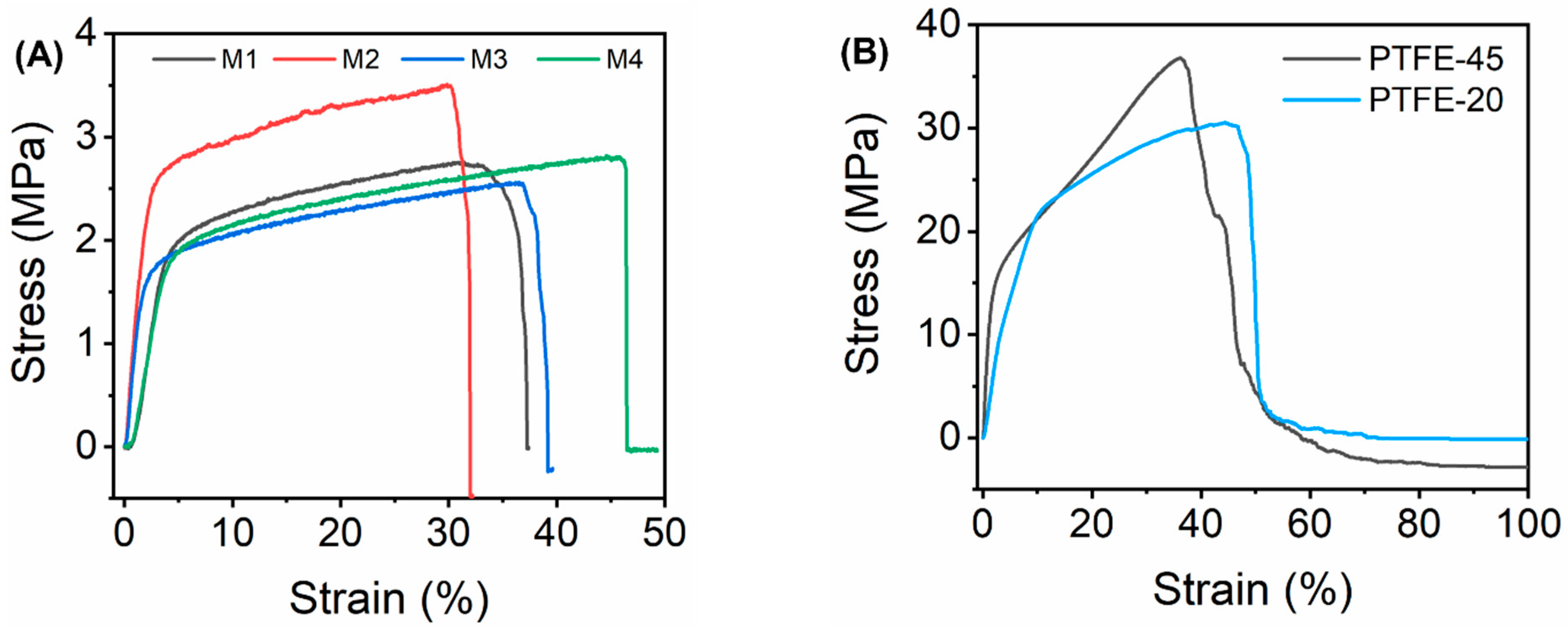
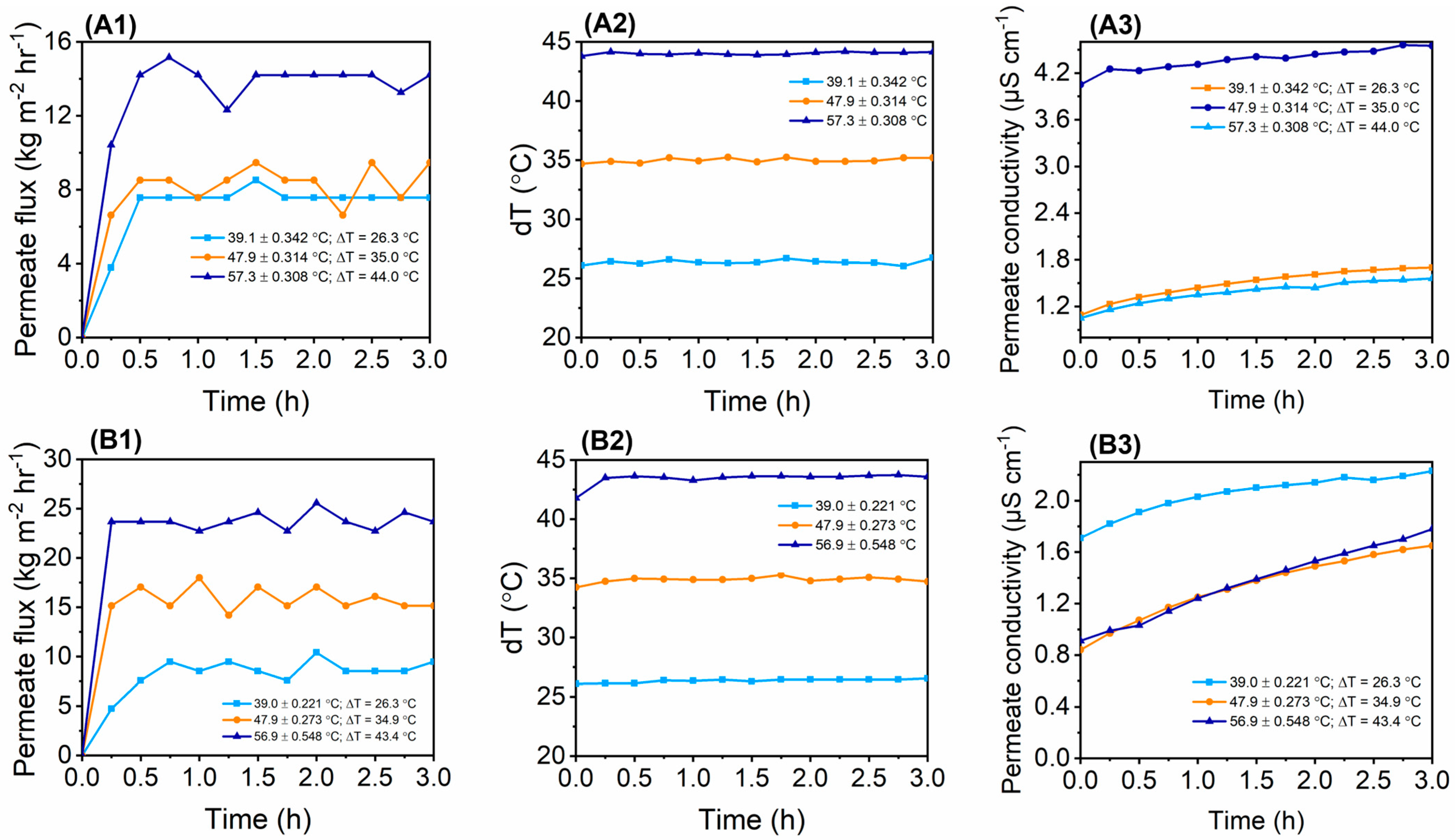

| Membrane | DMF | DMAc | PVDF (wt%) | PVP (wt%) | fCNTs (wt%) | fSiO2NPS (wt%) |
|---|---|---|---|---|---|---|
| M1 | 51.0 | 34.0 | 15.0 | 0.0 | 0.0 | 0.0 |
| M2 | 50.9 | 34.0 | 15.0 | 0.1 | 0.0 | 0.0 |
| M3 | 50.8 | 33.9 | 15.0 | 0.1 | 0.2 | 0.0 |
| M4 | 48.7 | 32.5 | 15.0 | 0.1 | 0.2 | 3.5 |
| Membrane | Porosity (%) | LEP (kPa) | Pore Size (µm) | |
|---|---|---|---|---|
| M1 | 81.68 ± 3.00 | 116.52 | 393 ± 4.00 | 0.27 |
| M2 | 73.00 ± 5.14 | 84.69 | 273 ± 37.7 | 0.21 |
| M3 | 64.34 ± 0.13 | 101.60 | 500 ± 97.9 | 0.19 |
| M4 | 63.61 ± 0.95 | 103.81 | 590 ± 90.0 | 0.22 |
| PTFE-20 | 54.13 ± 1.94 | 97.35 | 603 ± 20.5 | 0.20 |
| PTFE-45 | 51.03 ± 1.48 | 101.57 | 200 ± 90.0 | 0.45 |
| Membrane | Young’s Modulus |
|---|---|
| M1 | 0.45 ± 0.24 |
| M2 | 1.06 ± 0.42 |
| M3 | 1.07 ± 0.25 |
| M4 | 0.39 ± 0.30 |
| PTFE-20 | 2.90 ± 0.88 |
| PTFE-45 | 6.96 ± 4.63 |
| Membranes | Modifying NPs | Operating Conditions | Process Performance | Ref. | |||
|---|---|---|---|---|---|---|---|
| Feed Temperature (°C) | Permeate Temperature (°C) | Flow Rate (mL min−1) | Flux (kg m−2 h−1) | Rejection (%) | |||
| PVDF | ZnO | 86 | 22 | 400.00 | 25.00 | 99 | [64] |
| PVDF | Halloysite Nanotubes | 60 | 20 | 252.36 | 5.52 | 95 | [66] |
| PVDF nanofiber | SiO2NPs | 20–80 | 20 | 750.00 | 34.2 | 99 | [65] |
| PVDF | TiO2-SiO2 | 40 | 20 | 300.00 | 11.00 | 99 | [55] |
| PVDF | CNTs | 82 | 20 | 48.00 | 34.20 | 100 | [39] |
| PVDF | fSiO2NPs/fCNTs | 60 | 10 | 601.00 | 39.77 | 99 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimanlal, I.; Nthunya, L.N.; Mahlangu, O.T.; Kirkebæk, B.; Ali, A.; Quist-Jensen, C.A.; Richards, H. Nanoparticle-Enhanced PVDF Flat-Sheet Membranes for Seawater Desalination in Direct Contact Membrane Distillation. Membranes 2023, 13, 317. https://doi.org/10.3390/membranes13030317
Chimanlal I, Nthunya LN, Mahlangu OT, Kirkebæk B, Ali A, Quist-Jensen CA, Richards H. Nanoparticle-Enhanced PVDF Flat-Sheet Membranes for Seawater Desalination in Direct Contact Membrane Distillation. Membranes. 2023; 13(3):317. https://doi.org/10.3390/membranes13030317
Chicago/Turabian StyleChimanlal, Indira, Lebea N. Nthunya, Oranso T. Mahlangu, Bastian Kirkebæk, Aamer Ali, Cejna A. Quist-Jensen, and Heidi Richards. 2023. "Nanoparticle-Enhanced PVDF Flat-Sheet Membranes for Seawater Desalination in Direct Contact Membrane Distillation" Membranes 13, no. 3: 317. https://doi.org/10.3390/membranes13030317
APA StyleChimanlal, I., Nthunya, L. N., Mahlangu, O. T., Kirkebæk, B., Ali, A., Quist-Jensen, C. A., & Richards, H. (2023). Nanoparticle-Enhanced PVDF Flat-Sheet Membranes for Seawater Desalination in Direct Contact Membrane Distillation. Membranes, 13(3), 317. https://doi.org/10.3390/membranes13030317








