Novel Pyrrolidinium-Functionalized Styrene-b-ethylene-b-butylene-b-styrene Copolymer Based Anion Exchange Membrane with Flexible Spacers for Water Electrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Membrane Preparation
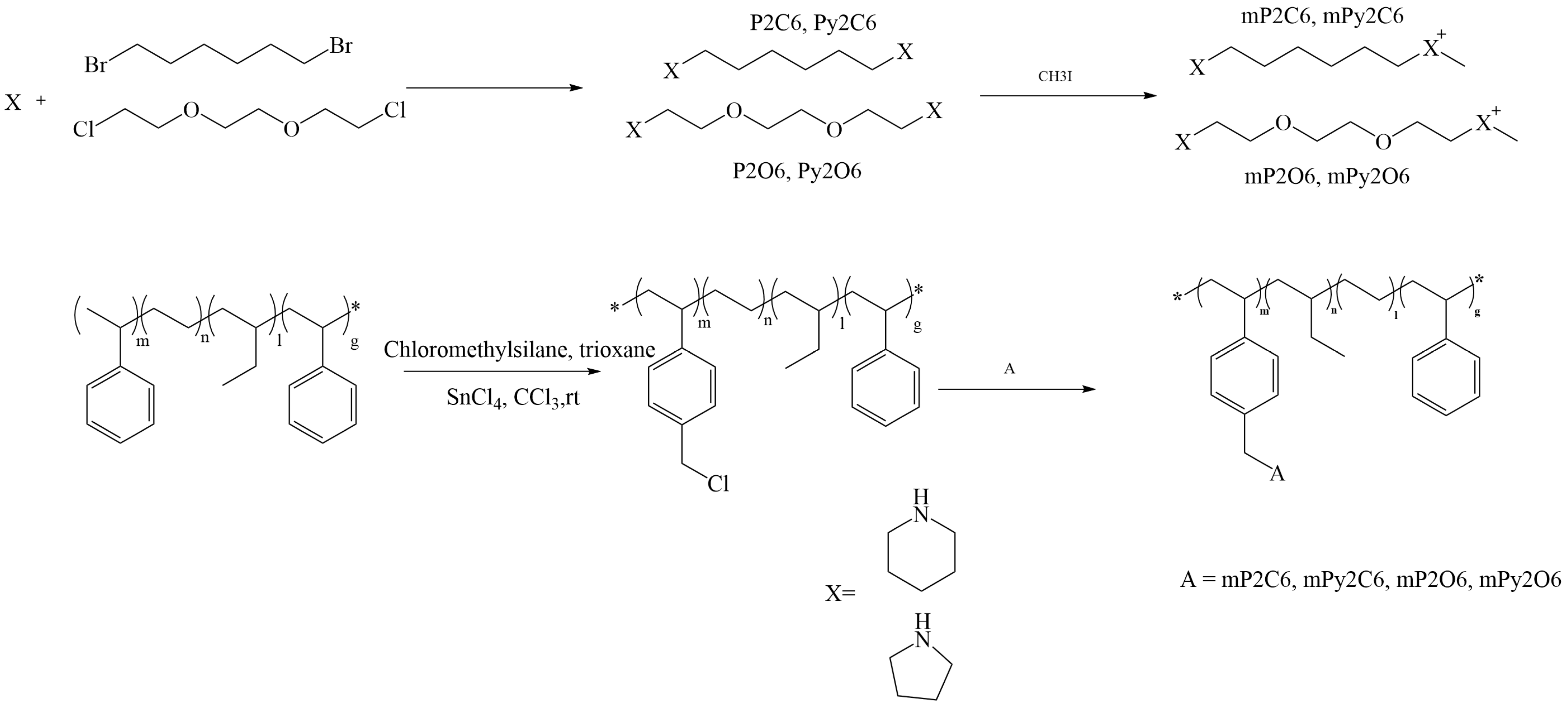
2.2.1. Synthesis of Chloromethylated SEBS (cmSEBS)
2.2.2. Synthesis of 1,6-Bis(piperidin-1-yl) Hexane (P2C6) and 1,6-Bis(pyrrolidin-1-yl) Hexane (Py2C6)
2.2.3. Synthesis of 1,2-bis(2-piperidinylethoxy) Ethane (P2O6) and 1,2-Bis(2-pyrrolidinylethoxy) Ethane (Py2O6)
2.2.4. Membrane Preparation
2.3. Instrumental
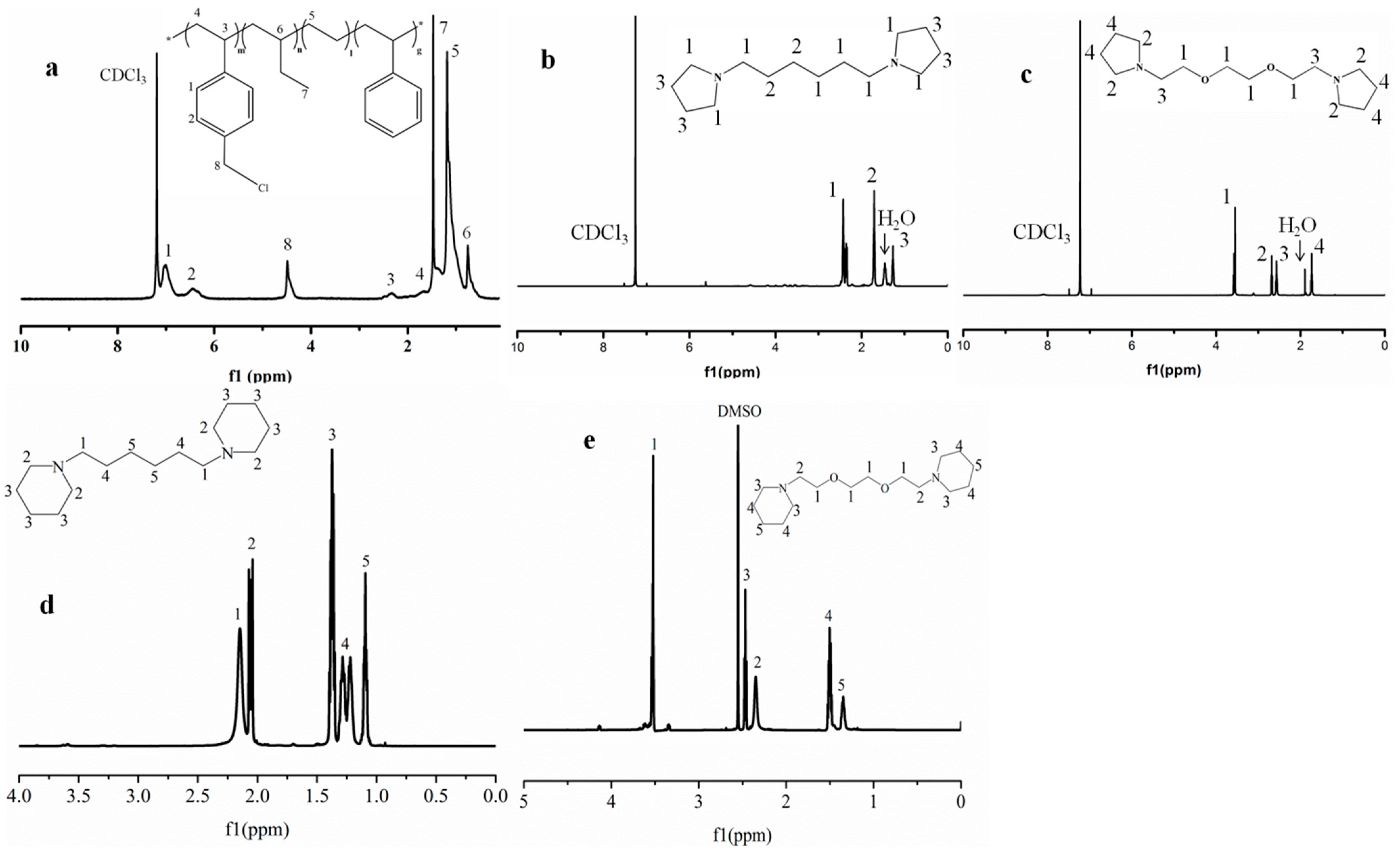
2.3.1. NMR Spectroscopy and Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3.2. Atomic Force Microscope (AFM)
2.3.3. Membrane Conductivity
2.3.4. Water Uptake and Swelling Ratio
2.3.5. Ion Exchange Capacity (IEC)
2.3.6. Chemical Stability of the Membranes
2.4. Fabrication of Membrane Electrode Assemblies (MEAs) for AEMWEs
2.5. AEMWE Cell Test
3. Results and Discussion
3.1. Synthesis and Preparation
3.2. Conductivities of Piperidinium/Pyrrolidinium Functionalized Membranes Based on SEBS
3.3. Membranes Morphology Study on SEBS-P2O6 and SEBS-Py2O6
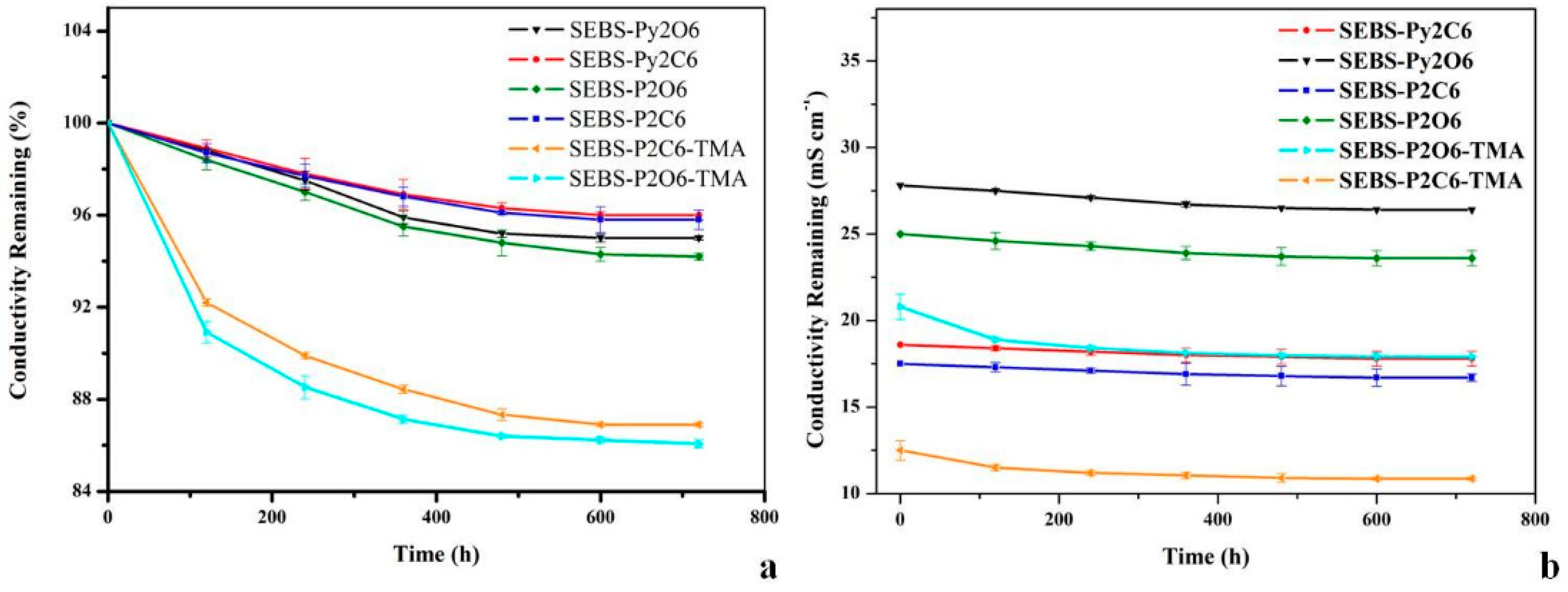
3.4. Chemical Stability of SEBS-Based Piperidinium/Pyrrolidinium-Functionalized Membranes
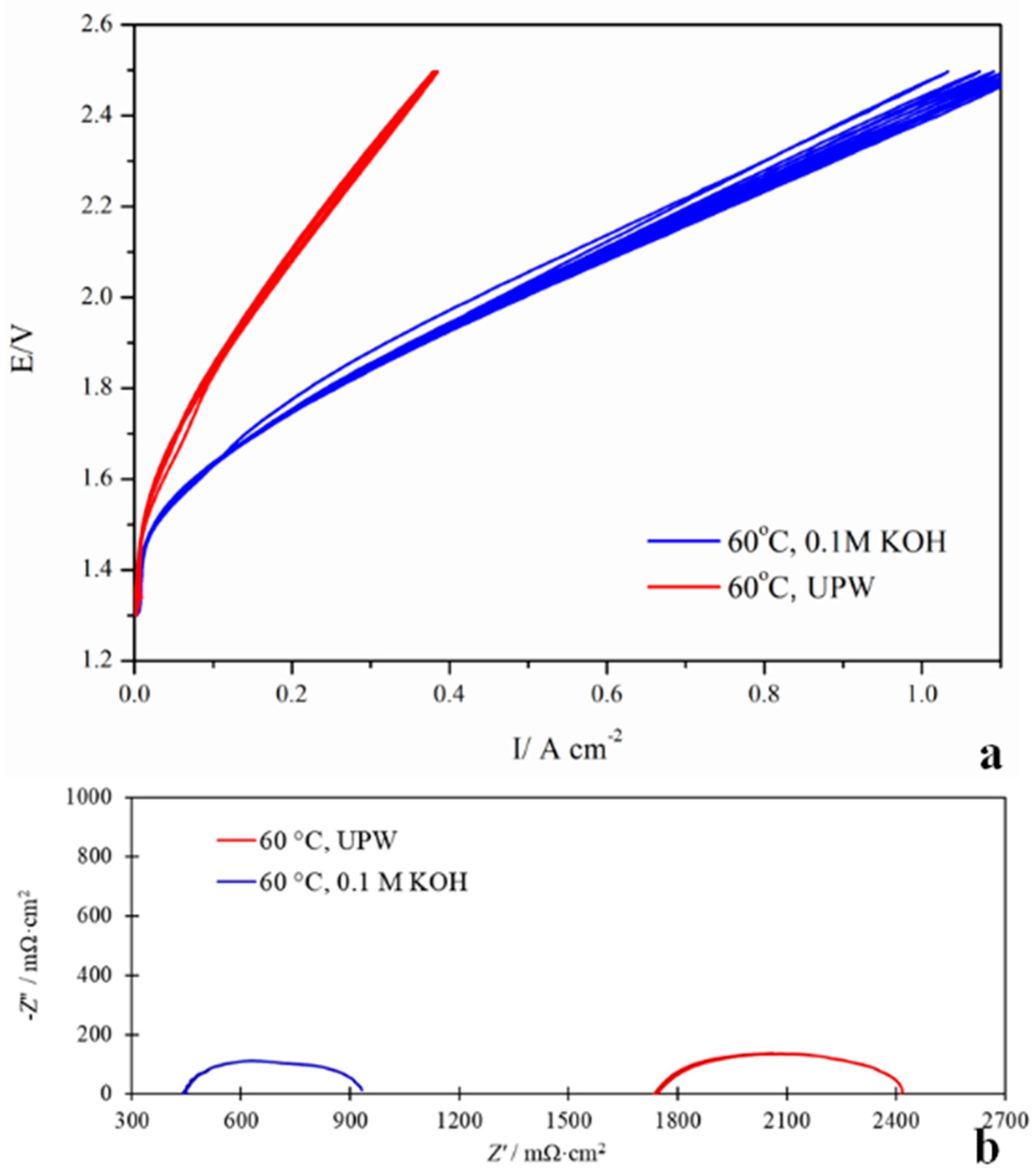
3.5. AEM Water Electrolysis Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.-Y. Solid-State Water Electrolysis with an Alkaline Membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; You, Y.; Zhang, S.; Zhao, Y. Polysulfone and zirconia composite separators for alkaline water electrolysis. Front. Chem. Sci. Eng. 2013, 7, 154–161. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Razmjooei, F.; Farooqui, A.; Reissner, R.; Gago, A.S.; Ansar, S.A.; Friedrich, K.A. Elucidating the Performance Limitations of Alkaline Electrolyte Membrane Electrolysis: Dominance of Anion Concentration in Membrane Electrode Assembly. ChemElectroChem 2020, 7, 3951–3960. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Burnat, D.; Schlupp, M.; Wichser, A.; Lothenbach, B.; Gorbar, M.; Züttel, A.; Vogt, U.F. Composite membranes for alkaline electrolysis based on polysulfone and mineral fillers. J. Power Sources 2015, 291, 163–172. [Google Scholar] [CrossRef]
- Lee, N.; Duong, D.T.; Kim, D. Cyclic ammonium grafted poly (arylene ether ketone) hydroxide ion exchange membranes for alkaline water electrolysis with high chemical stability and cell efficiency. Electrochim. Acta 2018, 271, 150–157. [Google Scholar] [CrossRef]
- Kaczur, J.J.; Yang, H.; Liu, Z.; Sajjad, S.D.; Masel, R.I. Carbon Dioxide and Water Electrolysis Using New Alkaline Stable Anion Membranes. Front. Chem. 2018, 6, 263. [Google Scholar] [CrossRef] [Green Version]
- Fortin, P.; Khoza, T.; Cao, X.; Martinsen, S.Y.; Oyarce Barnett, A.; Holdcroft, S. High-performance alkaline water electrolysis using Aemion™ anion exchange membranes. J. Power Sources 2020, 451, 227814. [Google Scholar] [CrossRef]
- Pushkareva, I.V.; Pushkarev, A.S.; Grigoriev, S.A.; Modisha, P.; Bessarabov, D.G. Comparative study of anion exchange membranes for low-cost water electrolysis. Int. J. Hydrogen Energy 2020, 45, 26070–26079. [Google Scholar] [CrossRef]
- Buggy, N.C.; Du, Y.; Kuo, M.-C.; Ahrens, K.A.; Wilkinson, J.S.; Seifert, S.; Coughlin, E.B.; Herring, A.M. A Polyethylene-Based Triblock Copolymer Anion Exchange Membrane with High Conductivity and Practical Mechanical Properties. ACS Appl. Polym. Mater. 2020, 2, 1294–1303. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.H.; Olsson, J.S.; Jannasch, P. Poly(arylene alkylene)s with pendant N-spirocyclic quaternary ammonium cations for anion exchange membranes. J. Mater. Chem. A 2018, 6, 16537–16547. [Google Scholar] [CrossRef] [Green Version]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Wang, S.; Ma, W.; Zhao, S.; Xu, Z.; Sun, G. Highly stable poly(ethylene glycol)-grafted alkaline anion exchange membranes. J. Mater. Chem. A 2016, 4, 3886–3892. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, X.; Shang, S.-L.; Kwasny, M.T.; Zimudzi, T.J.; Yu, X.; Saikia, N.; Pan, J.; Liu, Z.-K.; Tew, G.N.; et al. High Performance Anion Exchange Membrane Fuel Cells Enabled by Fluoropoly(olefin) Membranes. Adv. Funct. Mater. 2019, 29, 1902059. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Park, S.; Han, J.; Maurya, S.; Mohanty, A.D.; Tian, D.; Saikia, N.; Hickner, M.A.; Ryu, C.Y.; Tuckerman, M.E.; et al. Synthesis of Aromatic Anion Exchange Membranes by Friedel–Crafts Bromoalkylation and Cross-Linking of Polystyrene Block Copolymers. Macromolecules 2019, 52, 2139–2147. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Two-dimensional NMR spectroscopy reveals cation-triggered backbone degradation in polysulfone-based anion exchange membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490. [Google Scholar] [CrossRef] [Green Version]
- Edson, J.B.; Macomber, C.S.; Pivovar, B.S.; Boncella, J.M. Hydroxide based decomposition pathways of alkyltrimethylammonium cations. J. Membr. Sci. 2012, 399–400, 49–59. [Google Scholar] [CrossRef]
- Long, H.; Kim, K.; Pivovar, B.S. Hydroxide Degradation Pathways for Substituted Trimethylammonium Cations: A DFT Study. J. Phys. Chem. C 2012, 116, 9419–9426. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Tuning poly(arylene piperidinium) anion-exchange membranes by copolymerization, partial quaternization and crosslinking. J. Membr. Sci. 2019, 578, 183–195. [Google Scholar] [CrossRef]
- Pan, J.; Chen, C.; Li, Y.; Wang, L.; Tan, L.; Li, G.; Tang, X.; Xiao, L.; Lu, J.; Zhuang, L. Constructing ionic highway in alkaline polymer electrolytes. Energy Environ. Sci. 2014, 7, 354–360. [Google Scholar] [CrossRef]
- Li, N.; Yan, T.; Li, Z.; Thurn-Albrecht, T.; Binder, W.H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 2012, 5, 7888–7892. [Google Scholar] [CrossRef]
- Zhu, L.; Pan, J.; Wang, Y.; Han, J.; Zhuang, L.; Hickner, M.A. Multication Side Chain Anion Exchange Membranes. Macromolecules 2016, 49, 815–824. [Google Scholar] [CrossRef]
- Han, J.; Zhu, L.; Pan, J.; Zimudzi, T.J.; Wang, Y.; Peng, Y.; Hickner, M.A.; Zhuang, L. Elastic Long-Chain Multication Cross-Linked Anion Exchange Membranes. Macromolecules 2017, 50, 3323–3332. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, L.; Liang, X.; Shehzad, M.A.; Wang, L.; Ge, X.; He, Y.; Wu, L.; Varcoe, J.R.; Xu, T. Beneficial use of rotatable-spacer side-chains in alkaline anion exchange membranes for fuel cells. Energy Environ. Sci. 2018, 11, 3472–3479. [Google Scholar] [CrossRef]
- Liu, M.; Hu, X.; Hu, B.; Liu, L.; Li, N. Soluble poly(aryl piperidinium) with extended aromatic segments as anion exchange membranes for alkaline fuel cells and water electrolysis. J. Membr. Sci. 2022, 642, 119966. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, Y.; Shi, B.; Yang, C.; Kong, Y.; Liu, Y.; Wu, H.; Jiang, Z. Alkaline stable piperidinium-based biphenyl polymer for anion exchange membranes. Solid State Ion. 2022, 383, 115969. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, J.; Liu, X.; Huang, T.; Jiang, H.; Yin, Y.; Qin, Y.; Guiver, M.D. Toward alkaline-stable anion exchange membranes in fuel cells: Cycloaliphatic quaternary ammonium-based anion conductors. Electrochem. Energy Rev. 2022, 5, 348–400. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, W.; Liang, X.; Zhang, K.; Wang, H.; Ge, X.; Wei, C.; Song, W.; Ge, Z.; Wu, L.; et al. Flexible Bis-piperidinium Side Chains Construct Highly Conductive and Robust Anion-Exchange Membranes. ACS Appl. Energy Mater. 2021, 4, 9701–9711. [Google Scholar] [CrossRef]
- Gupta, G.; Scott, K.; Mamlouk, M. Performance of polyethylene based radiation grafted anion exchange membrane with polystyrene-b-poly (ethylene/butylene)-b-polystyrene based ionomer using NiCo2O4 catalyst for water electrolysis. J. Power Sources 2018, 375, 387–396. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef]
- Stiber, S.; Balzer, H.; Wierhake, A.; Wirkert, F.J.; Roth, J.; Rost, U.; Brodmann, M.; Lee, J.K.; Bazylak, A.; Waiblinger, W.; et al. Porous Transport Layers for Proton Exchange Membrane Electrolysis Under Extreme Conditions of Current Density, Temperature, and Pressure. Adv. Energy Mater. 2021, 11, 2100630. [Google Scholar] [CrossRef]
- Dai, P.; Mo, Z.-H.; Xu, R.-W.; Zhang, S.; Wu, Y.-X. Cross-Linked Quaternized Poly(styrene-b-(ethylene-co-butylene)-b-styrene) for Anion Exchange Membrane: Synthesis, Characterization and Properties. ACS Appl. Mater. Interfaces 2016, 8, 20329–20341. [Google Scholar] [CrossRef]
- Hao, J.; Gao, X.; Jiang, Y.; Zhang, H.; Luo, J.; Shao, Z.; Yi, B. Crosslinked high-performance anion exchange membranes based on poly(styrene-b-(ethylene-co-butylene)-b-styrene). J. Membr. Sci. 2018, 551, 66–75. [Google Scholar] [CrossRef]
- Yu, N.; Dong, J.; Li, H.; Wang, T.; Yang, J. Improving the performance of quaternized SEBS based anion exchange membranes by adjusting the functional group and side chain structure. Eur. Polym. J. 2021, 154, 110528. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Sang, J.; Cui, Y.; Zhu, H. Synthesis and characterization of a long side-chain double-cation crosslinked anion-exchange membrane based on poly(styrene-b-(ethylene-co-butylene)-b-styrene). Int. J. Hydrogen Energy 2021, 46, 36301–36313. [Google Scholar] [CrossRef]
- Al Munsur, A.Z.; Hossain, I.; Nam, S.Y.; Chae, J.E.; Kim, T.-H. Hydrophobic-hydrophilic comb-type quaternary ammonium-functionalized SEBS copolymers for high performance anion exchange membranes. J. Membr. Sci. 2020, 599, 117829. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Long, C.; Sang, J.; Tian, L.; Wang, F.; Wang, Z.; Zhu, H. Elastic and durable multi-cation-crosslinked anion exchange membrane based on poly(styrene-b-(ethylene-co-butylene)-b-styrene). J. Polym. Sci. 2020, 58, 2181–2196. [Google Scholar] [CrossRef]
- Sung, S.; Chae, J.E.; Min, K.; Kim, H.-J.; Nam, S.Y.; Kim, T.-H. Preparation of crosslinker-free anion exchange membranes with excellent physicochemical and electrochemical properties based on crosslinked PPO-SEBS. J. Mater. Chem. A 2021, 9, 1062–1079. [Google Scholar] [CrossRef]
- Xu, Z.; Wilke, V.; Chmielarz, J.J.; Tobias, M.; Atanasov, V.; Gago, A.S.; Friedrich, K.A. Novel piperidinium-functionalized crosslinked anion exchange membrane with flexible spacers for water electrolysis. J. Membr. Sci. 2022, 670, 121302. [Google Scholar] [CrossRef]
- Pandey, T.P.; Sarode, H.N.; Yang, Y.; Yang, Y.; Vezzù, K.; Noto, V.D.; Seifert, S.; Knauss, D.M.; Liberatore, M.W.; Herring, A.M. A Highly Hydroxide Conductive, Chemically Stable Anion Exchange Membrane, Poly(2,6 dimethyl 1,4 phenylene oxide)-b-Poly(vinyl benzyl trimethyl ammonium), for Electrochemical Applications. J. Electrochem. Soc. 2016, 163, H513–H520. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, C.; Liu, L.; Liao, J.; Chen, Q.; Zhu, M.; Wang, Y.; An, L.; Li, N. Facilitating Anion Transport in Polyolefin-Based Anion Exchange Membranes via Bulky Side Chains. ACS Appl. Mater. Interfaces 2016, 8, 23321–23330. [Google Scholar] [CrossRef]
- Shin, D.W.; Lee, S.Y.; Lee, C.H.; Lee, K.-S.; Park, C.H.; McGrath, J.E.; Zhang, M.; Moore, R.B.; Lingwood, M.D.; Madsen, L.A.; et al. Sulfonated Poly(arylene sulfide sulfone nitrile) Multiblock Copolymers with Ordered Morphology for Proton Exchange Membranes. Macromolecules 2013, 46, 7797–7804. [Google Scholar] [CrossRef]
- Su, X.; Gao, L.; Hu, L.; Qaisrani, N.A.; Yan, X.; Zhang, W.; Jiang, X.; Ruan, X.; He, G. Novel piperidinium functionalized anionic membrane for alkaline polymer electrolysis with excellent electrochemical properties. J. Membr. Sci. 2019, 581, 283–292. [Google Scholar] [CrossRef]
- Omasta, T.J.; Park, A.M.; LaManna, J.M.; Zhang, Y.; Peng, X.; Wang, L.; Jacobson, D.L.; Varcoe, J.R.; Hussey, D.S.; Pivovar, B.S.; et al. Beyond catalysis and membranes: Visualizing and solving the challenge of electrode water accumulation and flooding in AEMFCs. Energy Environ. Sci. 2018, 11, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Willdorf-Cohen, S.; Schibli, E.M.; Paula, Z.; Li, W.; Skalski, T.J.G.; Sergeenko, A.T.; Hohenadel, A.; Frisken, B.J.; Magliocca, E.; et al. Poly(bis-arylimidazoliums) possessing high hydroxide ion exchange capacity and high alkaline stability. Nat. Commun. 2019, 10, 2306. [Google Scholar] [CrossRef] [Green Version]
- Movil, O.; Frank, L.; Staser, J.A. Graphene Oxide–Polymer Nanocomposite Anion-Exchange Membranes. J. Electrochem. Soc. 2015, 162, F419. [Google Scholar] [CrossRef]




| IEC (mmol g−1) | Conductivity (Cl−) (mS cm−1) | Conductivity (OH−) (mS cm−1) | Swelling Ratio (%) | Water Uptake (Cl−) (%) | Thickness (µm) | |
|---|---|---|---|---|---|---|
| SEBS-P2C6-TMA | 1.10 ± 0.01 | 6.45 ± 0.30 | 12.5 ± 0.42 | 6.5 ± 2.79 | 20.1 ± 8.43 | 70 ± 2.8 |
| SEBS-P2O6-TMA | 1.05 ± 0.04 | 11.7 ± 0.54 | 20.8 ± 0.74 | 9.2 ± 4.81 | 36.8 ± 7.35 | 68 ± 4.3 |
| SEBS-P2C6 | 1.35 ± 0.22 | 9.85 ± 0.30 | 17.5 ± 0.72 | 10.5 ± 4.67 | 32.1 ± 9.13 | 65 ± 3.3 |
| SEBS-P2O6 | 1.38 ± 0.18 | 13.6 ± 0.86 | 25.0 ± 1.32 | 14.3 ± 6.72 | 50.6 ± 10.30 | 68 ± 2.8 |
| SEBS-Py2C6 | 1.42 ± 0.21 | 10.4 ± 0.60 | 18.6 ± 0.38 | 8.5 ± 2.79 | 30.1 ± 10.41 | 72 ± 4.8 |
| SEBS-Py2O6 | 1.40 ± 0.25 | 14.1 ± 0.67 | 27.8 ± 0.73 | 12.2 ± 3.79 | 47.8 ± 9.35 | 70 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Delgado, S.; Atanasov, V.; Morawietz, T.; Gago, A.S.; Friedrich, K.A. Novel Pyrrolidinium-Functionalized Styrene-b-ethylene-b-butylene-b-styrene Copolymer Based Anion Exchange Membrane with Flexible Spacers for Water Electrolysis. Membranes 2023, 13, 328. https://doi.org/10.3390/membranes13030328
Xu Z, Delgado S, Atanasov V, Morawietz T, Gago AS, Friedrich KA. Novel Pyrrolidinium-Functionalized Styrene-b-ethylene-b-butylene-b-styrene Copolymer Based Anion Exchange Membrane with Flexible Spacers for Water Electrolysis. Membranes. 2023; 13(3):328. https://doi.org/10.3390/membranes13030328
Chicago/Turabian StyleXu, Ziqi, Sofia Delgado, Vladimir Atanasov, Tobias Morawietz, Aldo Saul Gago, and Kaspar Andreas Friedrich. 2023. "Novel Pyrrolidinium-Functionalized Styrene-b-ethylene-b-butylene-b-styrene Copolymer Based Anion Exchange Membrane with Flexible Spacers for Water Electrolysis" Membranes 13, no. 3: 328. https://doi.org/10.3390/membranes13030328
APA StyleXu, Z., Delgado, S., Atanasov, V., Morawietz, T., Gago, A. S., & Friedrich, K. A. (2023). Novel Pyrrolidinium-Functionalized Styrene-b-ethylene-b-butylene-b-styrene Copolymer Based Anion Exchange Membrane with Flexible Spacers for Water Electrolysis. Membranes, 13(3), 328. https://doi.org/10.3390/membranes13030328






