Treatment of Semiconductor Wastewater Containing Tetramethylammonium Hydroxide (TMAH) Using Nanofiltration, Reverse Osmosis, and Membrane Capacitive Deionization
Abstract
1. Introduction
2. Materials and Methods
2.1. Membranes, Electrode, and Chemicals
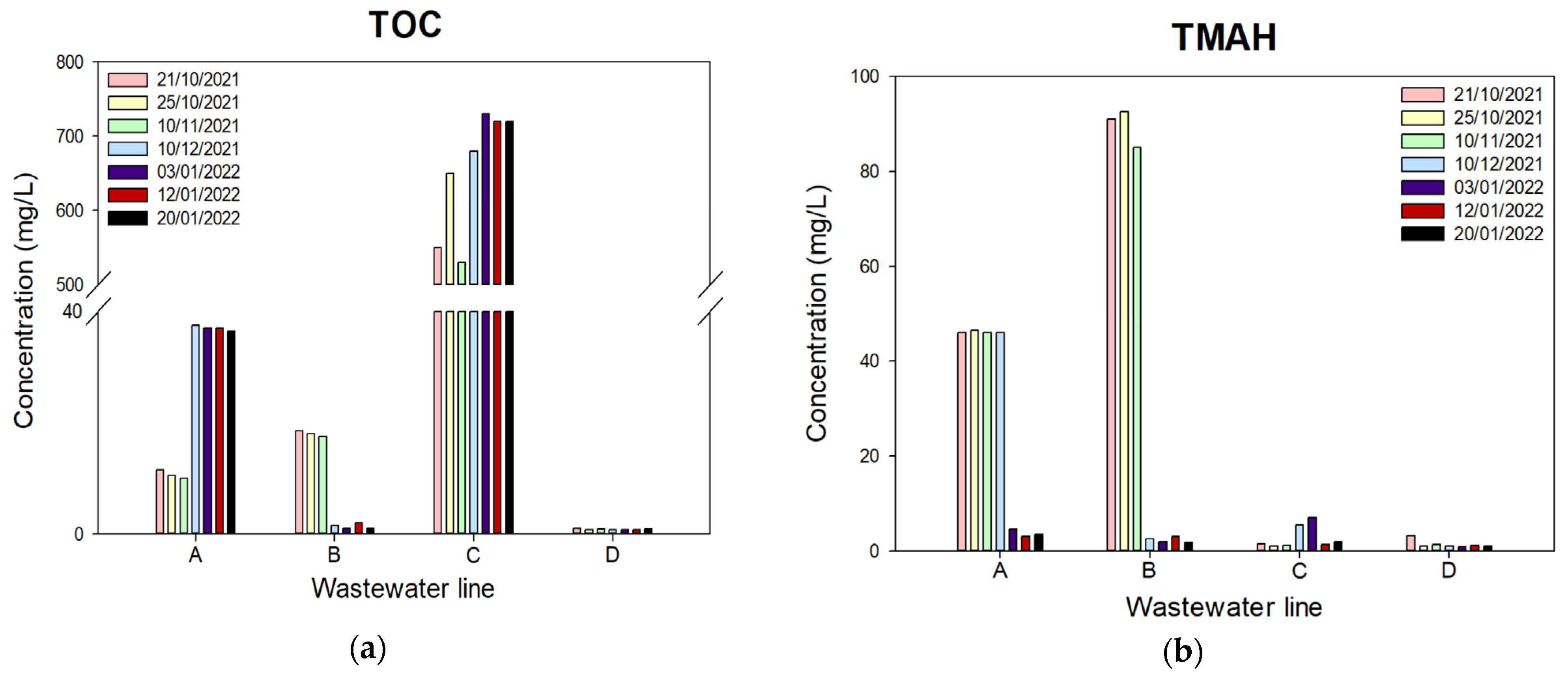
2.2. Semiconductor Wastewater
2.3. Lab-Scale Membrane Capacitive Deionization Device
2.4. Experimental Procedure
3. Results and Discussion
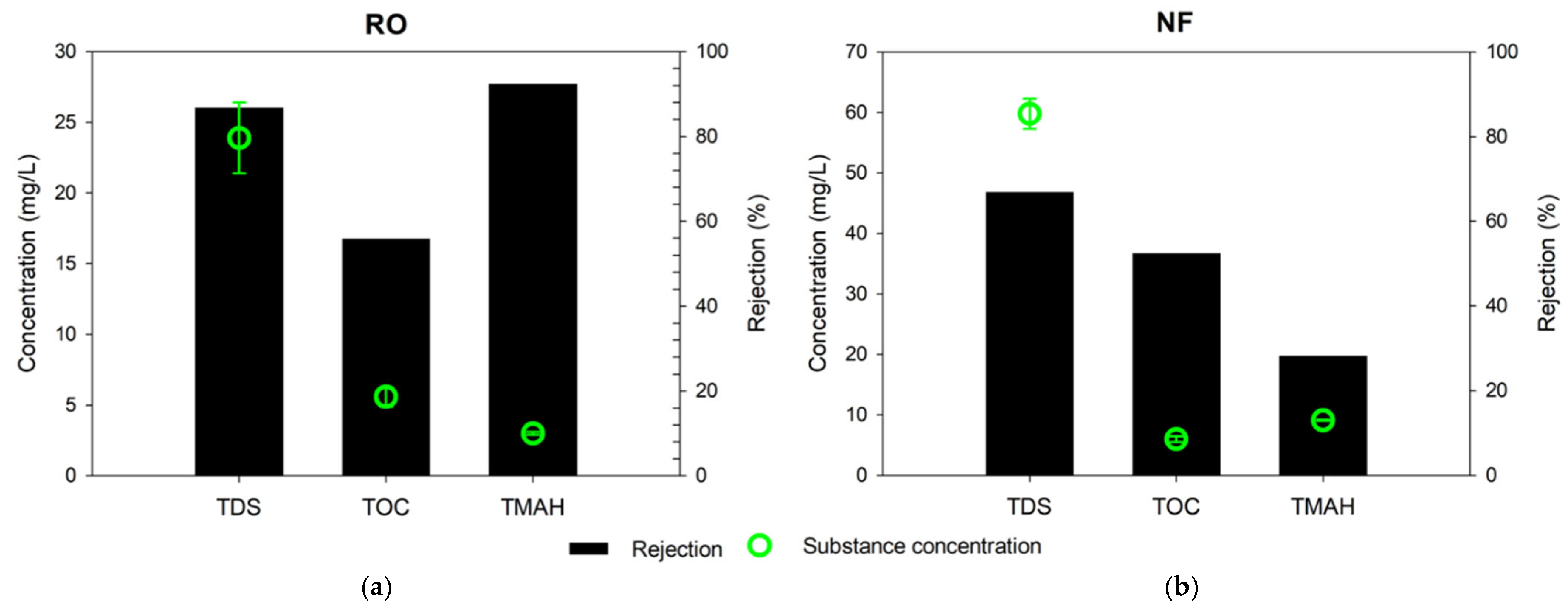
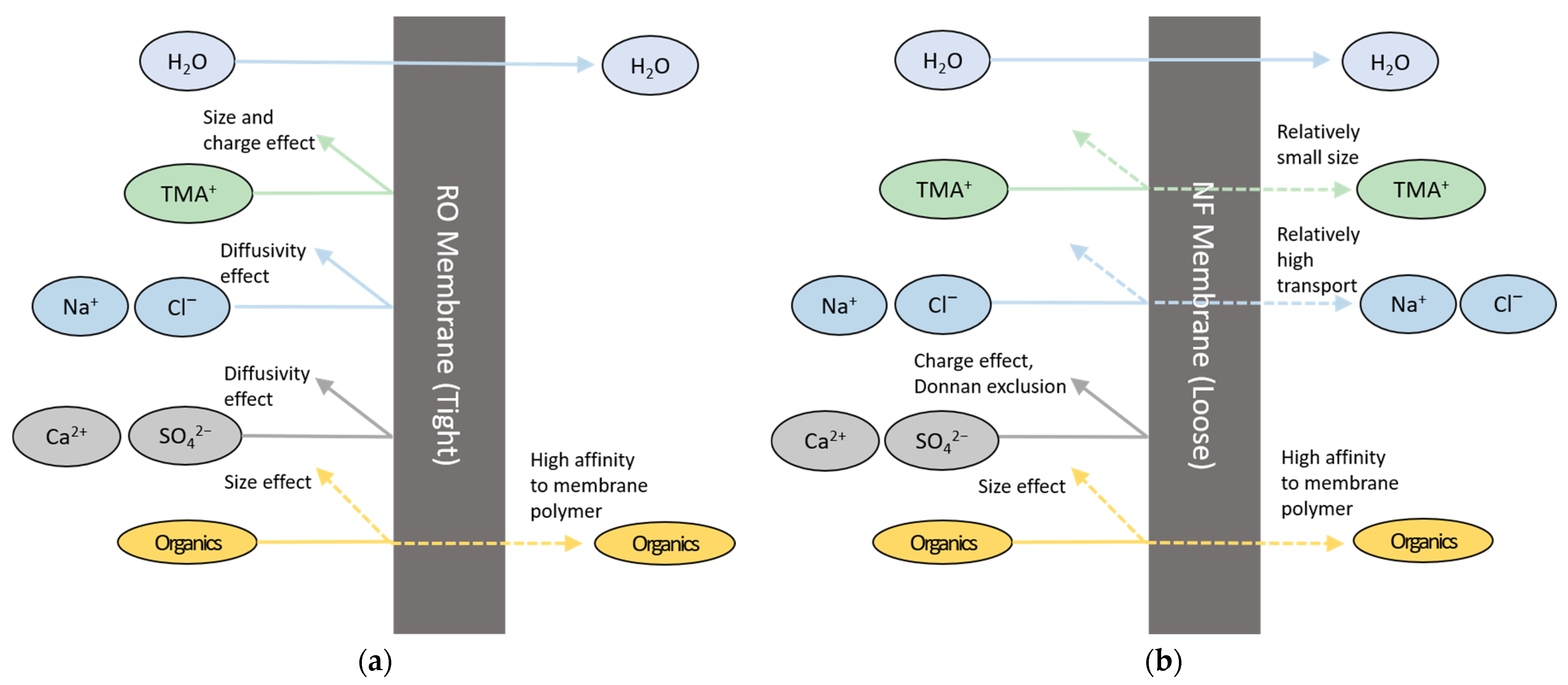
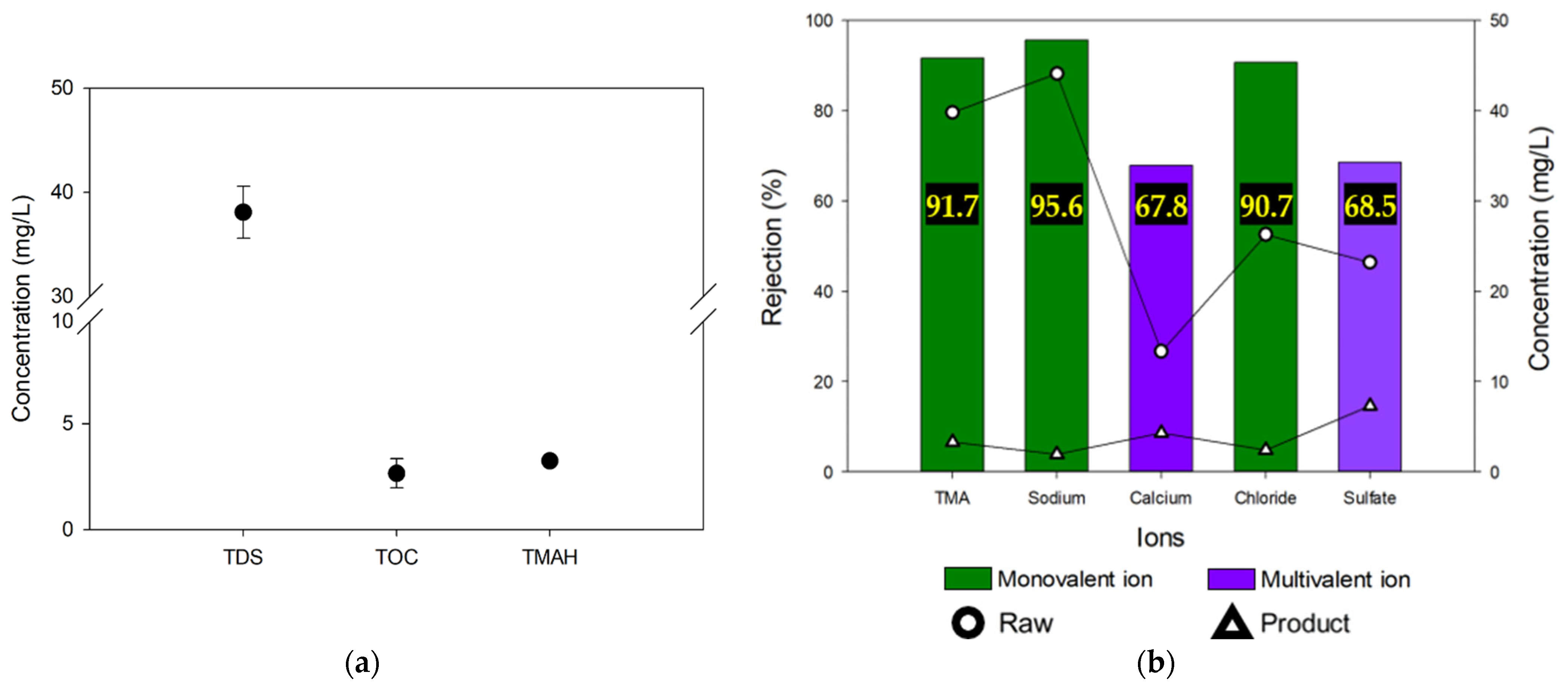
3.1. RO and NF Tests: Comparison of Contaminant Removals
3.2. MCDI Test #1: Contaminant Removal with Low Recovery (48%)
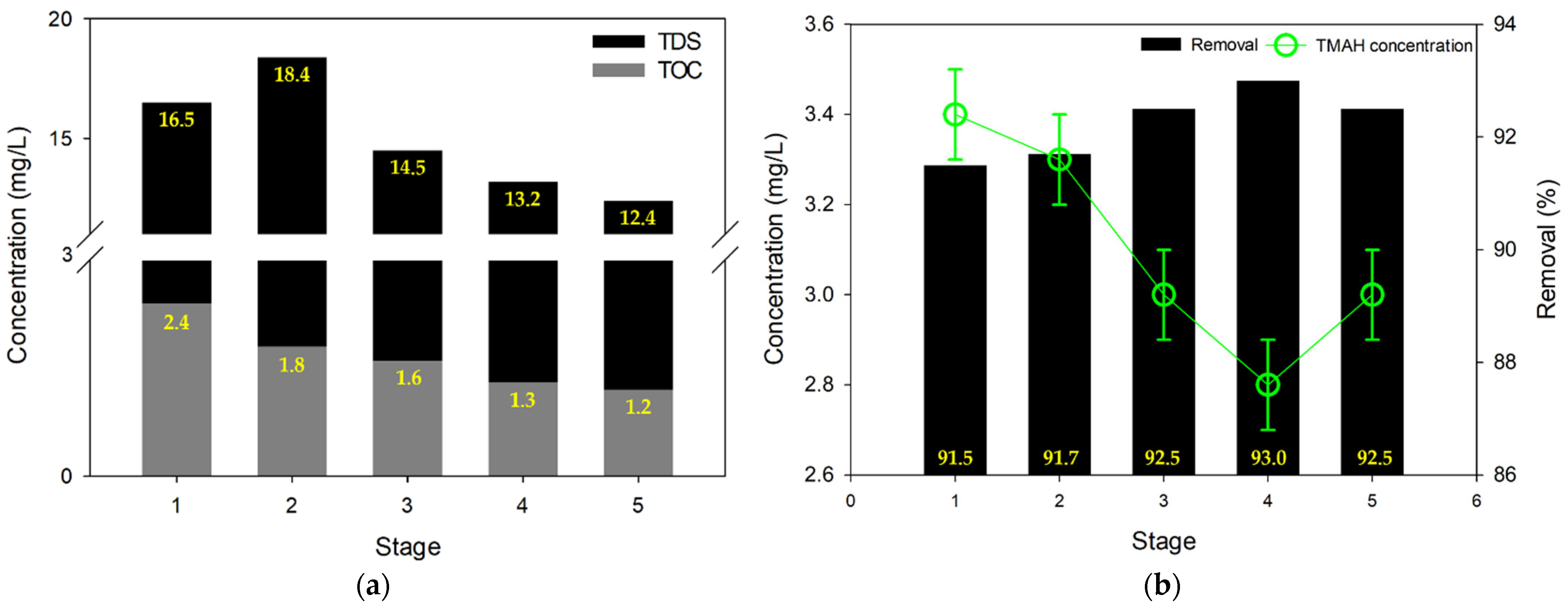
3.3. MCDI Test #2: Contaminant Removal with High Recovery (90%)
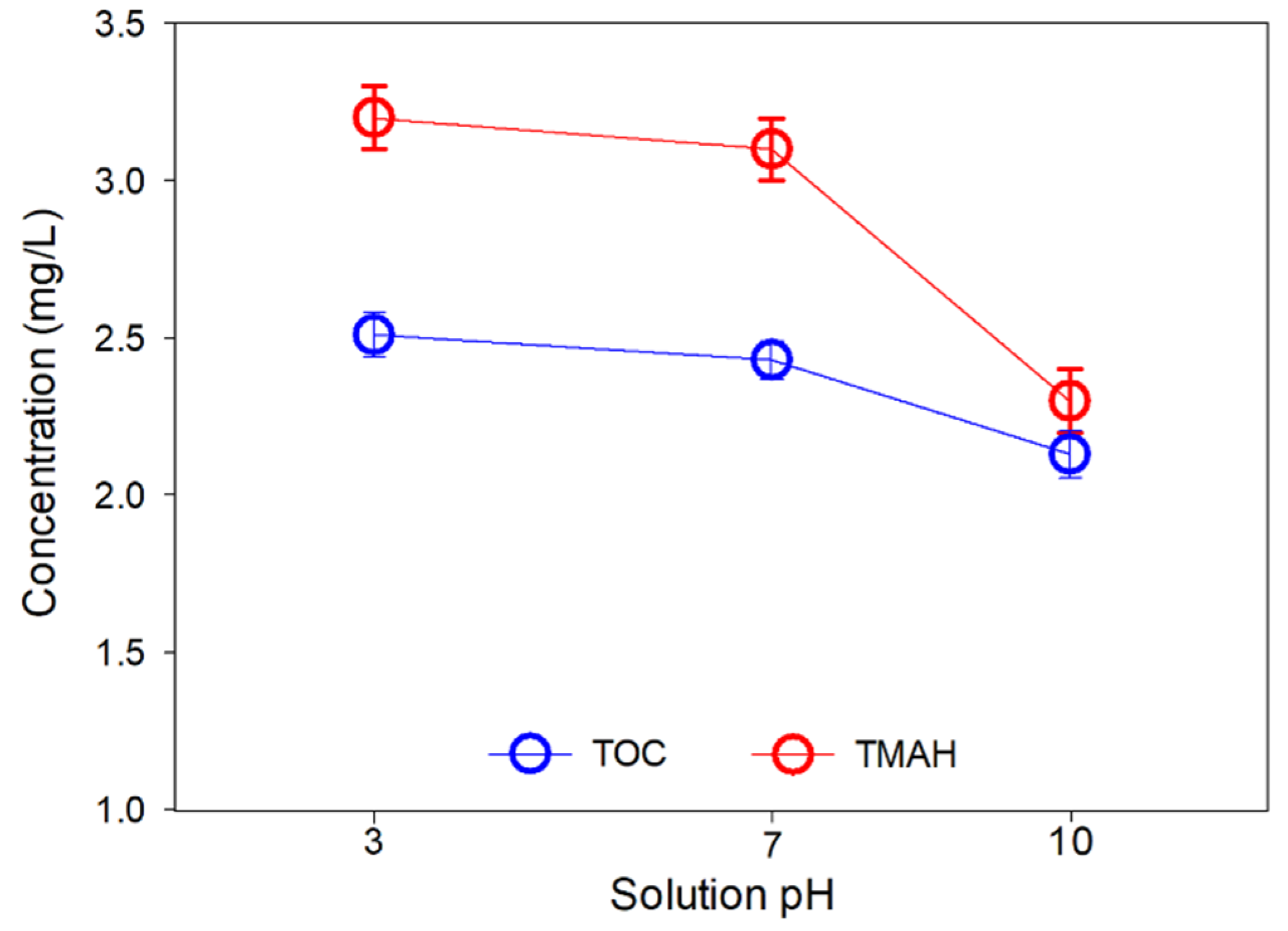
3.4. MCDI Test #3: Effect of pH on Contaminant Removal
4. Conclusions
- The NF experimental test shows that it is inadequate to treat TMAH in the wastewater due to its limited molecular weight cut-off;
- The RO process effectively removed TDS ~23.9 mg/L and TMAH ~3.0 mg/L from the semiconductor wastewater. However, this process only accounted for 56% of the TOC removal from the wastewater;
- On the other hand, MCDI showed its sufficient ability to treat the semiconductor wastewater by its adequate removal efficiency of TDS, TOC, and TMAH;
- In addition, it was found that the MCDI process can remove the monovalent ions including TMA ions more effectively than the multivalent ions due to their inherent ionic hydrate radii;
- Furthermore, the TMAH removal capability of MCDI can be improved by pH adjustment. In particular, this improvement was more pronounced when the pH of the semiconductor wastewater was higher.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Automotive Semiconductor Market (By Component: Processor, Discrete Power, Sensor, Memory, and Others; By Vehicle Type: Passenger Vehicle, Light Commercial Vehicle, and Heavy Commercial Vehicle; By Application: Chassis, Powertrain, Safety, Telematics & Infotainment, and Body Electronics)—Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2022–2030. Available online: https://www.precedenceresearch.com/automotive-semiconductor-market (accessed on 8 January 2023).
- Imtisal-e-Noor; Coenen, J.; Martin, A.; Dahl, O.; Aslin, M. Experimental investigation and techno-economic analysis of tetramethylammonium hydroxide removal from wastewater in nano-electronics manufacturing via membrane distillation. J. Membr. Sci. 2019, 579, 283–293. [Google Scholar] [CrossRef]
- Innocenzi, V.; Zueva, S.; Prisciandaro, M.; De Michelis, I.; Di Renzo, A.; di Celso, G.M.; Veglio, F. Treatment of TMAH solutions from the microelectronics industry: A combined process scheme. J. Water Process. Eng. 2019, 31, 100780. [Google Scholar] [CrossRef]
- Hirano, K.; Okamura, J.; Taira, T.; Sano, K.; Toyoda, A.; Ikeda, M. An efficient treatment technique for TMAH wastewater by catalytic oxidation. IEEE T Semiconduct. M 2001, 14, 202–206. [Google Scholar] [CrossRef]
- Nishi, Y.; Doering, R. Handbook of Semiconductor Manufacturing Technology; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Park, S.H.; Pak, J.; You, K.H.; Shin, H.C.; Kim, H.O. Tetramethylammonium Hydroxide Poisoning during a Pallet Cleaning Demonstration. J. Occup. Health 2013, 55, 120–124. [Google Scholar] [CrossRef]
- Hu, T.-H.; Whang, L.-M.; Liu, P.-W.G.; Hung, Y.-C.; Chen, H.-W.; Lin, L.-B.; Chen, C.-F.; Chen, S.-K.; Hsu, S.F.; Shen, W.; et al. Biological treatment of TMAH (tetra-methyl ammonium hydroxide) in a full-scale TFT-LCD wastewater treatment plant. Bioresour. Technol. 2012, 113, 303–310. [Google Scholar] [CrossRef]
- Lei, C.-N.; Whang, L.-M.; Chen, P.-C. Biological treatment of thin-film transistor liquid crystal display (TFT-LCD) wastewater using aerobic and anoxic/oxic sequencing batch reactors. Chemosphere 2010, 81, 57–64. [Google Scholar] [CrossRef]
- Kim, T.K.; Kim, T.; Lee, I.; Choi, K.; Zoh, K.D. Removal of tetramethylammonium hydroxide (TMAH) in semiconductor wastewater using the nano-ozone H2O2 process. J. Hazard. Mater. 2021, 409, 123759. [Google Scholar] [CrossRef]
- Wu, C.L.; Su, S.B.; Chen, J.L.; Lin, H.J.; Guo, H.R. Mortality from dermal exposure to tetramethylammonium hydroxide. J. Occup. Health 2008, 50, 99–102. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, C.C.; Ger, J.; Deng, J.F.; Hung, D.Z. Tetramethylammonium hydroxide poisoning. Clin. Toxicol. 2010, 48, 213–217. [Google Scholar] [CrossRef]
- Mori, I.C.; Arias-Barreiro, C.R.; Koutsaftis, A.; Ogo, A.; Kawano, T.; Yoshizuka, K.; Inayat-Hussain, S.H.; Aoyama, I. Toxicity of tetramethylammonium hydroxide to aquatic organisms and its synergistic action with potassium iodide. Chemosphere 2015, 120, 299–304. [Google Scholar] [CrossRef]
- Innocenzi, V.; Zueva, S.B.; Ferella, F.; Prisciandaro, M.; Vegli, F. A review of the existing and emerging technologies for wastewaters containing tetramethyl ammonium hydroxide (TMAH) and waste management systems in micro-chip microelectronic industries. Chemosphere 2022, 307, 135913. [Google Scholar] [CrossRef] [PubMed]
- European Union Life Program under Grant Agreement N. LIFE 15 ENV/IT 000332.Pilot Technology for Aerobic Biodegradation of Spent TMAH Photoresist Solution in Semiconductor Industries 2021. Available online: https://webgate.ec.europa.eu/life/publicWebsite/index.cfm?fuseaction=search.dspPage&n_proj_id=5773#RM (accessed on 5 January 2023).
- PubChem Compound Summary for CID 60966. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/60966 (accessed on 20 January 2023).
- Chen, S.-Y.; Lu, L.-A.; Lin, J.-G. Biodegradation of tetramethylammonium hydroxide (TMAH) in completely autotrophic nitrogen removal over nitrite (CANON) process. Bioresour. Technol. 2016, 210, 88–93. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Irmayani, L.; Setiyawan, A.A.; Whang, L.-M. Aerobic degradation of high tetramethylammonium hydroxide (TMAH) and its impacts on nitrification and microbial community. Chemosphere 2020, 258, 127146. [Google Scholar] [CrossRef] [PubMed]
- Den, W.; Fu-Hsiang, K.; Tiao-Yuan, H. Treatment of organic wastewater discharged from semiconductor manufacturing process by ultraviolet/hydrogen peroxide and biodegradation. IEEE Trans. Semicond. Manuf. 2002, 15, 540–551. [Google Scholar] [CrossRef]
- Chang, H.M.; Chen, S.S.; Hsiao, S.S.; Chang, W.S.; Chien, I.C.; Duong, C.C.; Nguyen, T.X.Q. Water reclamation and microbial community investigation: Treatment of tetramethylammonium hydroxide wastewater through an anaerobic osmotic membrane bioreactor hybrid system. J. Hazard. Mater. 2022, 427, 128200. [Google Scholar] [CrossRef]
- Dizge, N.; Keskinler, B.; Barlas, H. Sorption of Ni(II) ions from aqueous solution by Lewatit cation-exchange resin. J. Hazard. Mater. 2009, 167, 915–926. [Google Scholar] [CrossRef]
- Citraningrum, H.M.; Liu, J.C.; Chern, J.M. Removal of Tetramethylammonium Hydroxide from Solution Using Ion Exchange. IEEE Trans. Semicond. Manuf. 2013, 26, 214–220. [Google Scholar] [CrossRef]
- Tortora, F.; Innocenzi, V.; Prisciandaro, M.; De Michelis, I.; Vegliò, F.; Mazziotti di Celso, G. Removal of tetramethyl ammonium hydroxide from synthetic liquid wastes of electronic industry through micellar enhanced ultrafiltration. J. Dispers. Sci. Technol. 2018, 39, 207–213. [Google Scholar] [CrossRef]
- Nishihama, S.; Tsutsumi, Y.; Yoshizuka, K. Separation of tetramethyl ammonium hydroxide using an MFI-type zeolite-coated membrane. Sep. Purif. Technol. 2013, 120, 129–133. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-d.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Johnson, A.; Newman, J. Desalting by means of porous carbon electrodes. J. Electrochem. Soc. 1971, 118, 510. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, X.; Li, J.; Li, Y.; Wang, K.; Lu, T.; Hossain, M.S.A.; Amin, M.A.; Zhang, S.; Pan, L.; et al. Nanoarchitectonics from 2D to 3D: MXenes-derived nitrogen-doped 3D nanofibrous architecture for extraordinarily-fast capacitive deionization. Chem. Eng. J. 2022, 430, 133161. [Google Scholar] [CrossRef]
- Lee, K.S.; Cho, Y.; Choo, K.Y.; Yang, S.; Han, M.H.; Kim, D.K. Membrane-spacer assembly for flow-electrode capacitive deionization. Appl. Surf. Sci. 2018, 433, 437–442. [Google Scholar] [CrossRef]
- Tang, W.W.; Liang, J.; He, D.; Gong, J.L.; Tang, L.; Liu, Z.F.; Wang, D.B.; Zeng, G.M. Various cell architectures of capacitive deionization: Recent advances and future trends. Water Res. 2019, 150, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Kang, H.-S. Scale formation by electrode reactions in capacitive deionization and its effects on desalination performance. Appl. Chem. Eng. 2016, 27, 74–79. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.R.; Kang, J.; Kim, S.; Kim, C.; Yoon, J. Capacitive deionization with Ca-alginate coated-carbon electrode for hardness control. Desalination 2016, 392, 46–53. [Google Scholar] [CrossRef]
- Lee, D.H.; Ryu, T.; Shin, J.; Ryu, J.C.; Chung, K.S.; Kim, Y.H. Selective lithium recovery from aqueous solution using a modified membrane capacitive deionization system. Hydrometallurgy 2017, 173, 283–288. [Google Scholar] [CrossRef]
- Shi, W.H.; Liu, X.Y.; Ye, C.Z.; Cao, X.H.; Gao, C.J.; Shen, J.N. Efficient lithium extraction by membrane capacitive deionization incorporated with monovalent selective cation exchange membrane. Sep. Purif. Technol. 2019, 210, 885–890. [Google Scholar] [CrossRef]
- Park, H.M.; Lim, J.E.; Kim, S.E.; Lee, Y.T. Surface modification of nanofiltration membrane with silane coupling agents for separation of dye. Membr. J. 2018, 28, 414–423. [Google Scholar] [CrossRef]
- Kong, C.; Heo, J.; Yoon, Y.; Han, J.; Her, N. Treatment of AP Solutions Extracted from Solid Propellant by NF/RO Membrane Process. Membr. J. 2012, 22, 235–242. [Google Scholar]
- Huang, C.K.; Hall, A.H.; Wu, M.L.; Yang, C.C.; Hung, D.Z.; Mao, Y.C.; Deng, J.F. Presentations of tetramethylammonium hydroxide dermal exposure and the valuable potential of diphoterine solution in decontamination: A retrospective observational study. BMC Pharmacol. Toxicol. 2020, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Epsztein, R.; Cheng, W.; Shaulsky, E.; Dizge, N.; Elimelech, M. Elucidating the mechanisms underlying the difference between chloride and nitrate rejection in nanofiltration. J. Membr. Sci. 2018, 548, 694–701. [Google Scholar] [CrossRef]
- Xiao, H.F.; Shao, D.D.; Wu, Z.L.; Peng, W.B.; Akram, A.; Wang, Z.Y.; Zheng, L.J.; Xing, W.H.; Sun, S.P. Zero liquid discharge hybrid membrane process for separation and recovery of ions with equivalent and similar molecular weights. Desalination 2020, 482, 114387. [Google Scholar] [CrossRef]
- Lu, D.; Yao, Z.; Jiao, L.; Waheed, M.; Sun, Z.; Zhang, L. Separation mechanism, selectivity enhancement strategies and advanced materials for mono-/multivalent ion-selective nanofiltration membrane. Adv. Membr. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Garcia-Tarres, L.; Guardia, E. Hydration and dynamics of a tetramethylammonium ion in water: A computer simulation study. J. Phys. Chem. B 1998, 102, 7448–7454. [Google Scholar] [CrossRef]
- Aue, D.H.; Webb, H.M.; Bowers, M.T. A thermodynamic analysis of solvation effects on the basicities of alkylamines. An electrostatic analysis of substituent effects. J. Am. Chem. Soc. 1976, 98, 318–329. [Google Scholar] [CrossRef]
- Wang, Y.; Weinstock, I.A. Polyoxometalate-decorated nanoparticles. Chem. Soc. Rev. 2012, 41, 7479–7496. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of Solvation of Ions.5. Gibbs Free-Energy of Hydration at 298.15-K. J. Chem. Soc. Faraday T 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Weinstock, I.A. Homogeneous-phase electron-transfer reactions of polyoxometalates. Chem. Rev. 1998, 98, 113–170. [Google Scholar] [CrossRef]
- Luo, J.; Ye, S.; Li, T.; Sarnello, E.; Li, H.; Liu, T. Distinctive trend of metal binding affinity via hydration shell breakage in nanoconfined cavity. J. Phys. Chem. C 2019, 123, 14825–14833. [Google Scholar] [CrossRef]
- Erdey-Grúz, T. Transport Phenomena in Aqueous Solutions; Akademiai Kiado: Budapest, Hungary, 1974. [Google Scholar]
- Lyklema, J. Fundamentals of Interface and Colloid Science; Academic: London, UK; San Diego, CA, USA, 1991. [Google Scholar]
- Ntalikwa, J. Determination of surface charge density of α-alumina by Acid-base titration. Bull. Chem. Soc. Ethiop. 2007, 21, 117–128. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Paul, S.; Choi, K.S.; Lee, D.J.; Sudhagar, P.; Kang, Y.S. Factors affecting the performance of supercapacitors assembled with polypyrrole/multi-walled carbon nanotube composite electrodes. Electrochim. Acta 2012, 78, 649–655. [Google Scholar] [CrossRef]
- Chang, S.T.; Lu, C.Y.; Lin, K.Y.A. Comparisons of kinetics, thermodynamics and regeneration of tetramethylammonium hydroxide adsorption in aqueous solution with graphene oxide, zeolite and activated carbon. Appl. Surf. Sci. 2015, 326, 187–194. [Google Scholar] [CrossRef]
- Wang, C.K.; Lin, C.K. Study on the degradation of tetramethyl ammonium hydroxide using ultrasonic and ozone procedures. Desalin. Water Treat. 2019, 145, 309–317. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Tran, T.D.; Suffet, I.M. Electrosorption of inorganic salts from aqueous solution using carbon aerogels. Environ. Sci. Technol. 2002, 36, 3010–3019. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Heil, D.; Wang, G. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology. Water Res. 2008, 42, 2605–2617. [Google Scholar] [CrossRef]
- Li, Y.; Stewart, T.C.; Tang, H.L. A comparative study on electrosorptive rates of metal ions in capacitive deionization. J. Water Process. Eng. 2018, 26, 257–263. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Jiang, Y.; Wang, T.-J.; Wang, H. Effects of the hydration ratio on the electrosorption selectivity of ions during capacitive deionization. Desalination 2016, 399, 171–177. [Google Scholar] [CrossRef]
- Wu, T.; Brant, J.A. Magnetic field effects on pH and electrical conductivity: Implications for water and wastewater treatment. Environ. Eng. Sci. 2020, 37, 717–727. [Google Scholar] [CrossRef]
- Quickenden, T.; Betts, D.; Cole, B.; Noble, M. Effect of magnetic fields on the pH of water. J. Phys. Chem. 1971, 75, 2830–2831. [Google Scholar] [CrossRef]
- Yamashita, M.; Duffield, C.; Tiller, W.A. Direct current magnetic field and electromagnetic field effects on the pH and Oxidation− Reduction potential equilibration rates of water. 1. Purified water. Langmuir 2003, 19, 6851–6856. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Saghravani, S.F. Influence of magnetic field on the thermal conductivity of the water based mixed Fe3O4/CuO nanofluid. J. Magn. Magn. Mater. 2017, 441, 366–373. [Google Scholar] [CrossRef]
- Chang, S.T.; Lin, K.Y.A.; Lu, C.S. Efficient adsorptive removal of Tetramethylammonium hydroxide (TMAH) from water using graphene oxide. Sep. Purif. Technol. 2014, 133, 99–107. [Google Scholar] [CrossRef]




| Organism | Test Type | Route | Dose |
|---|---|---|---|
| Mouse | LDLo | Subcutaneous | 19 mg/kg |
| Rabbit | LDLo | Intravenous | 1 mg/kg |
| Guinea pig | LD50 | Skin | 25 mg/kg |
| Frog | LDLo | parenteral | 5 mg/kg |
| RO | NF | MCDI | ||||
|---|---|---|---|---|---|---|
| Electrodes | IEMs | Spacer | ||||
| Material | Polyamide | Poly-piperazine amide | Material | Activated carbon, Graphite | Polyethylene | Polyethylene terephthalate |
| Water flux | 48 L/m2-h (at 15 bar) | 21.6 L/m2-h (at 5 bar) | Capacity | 16 mg/g | >1.6 meq/g | - |
| NaCl rejection | 99.7% (at 15 bar) | 40–70% (at 5 bar) | Areal resistance | - | <0.5 Ωcm2 | - |
| pH range | 3–10 | 3–10 | pH range | 3–12 | 3–12 | 3–12 |
| Roughness (Rrms) | 80.8 nm | 1.86 nm | Average thickness | 600 μm (±10) | 15 μm (±1) | 99 μm (±1) |
| Effective area | 7.1 cm2 | 7.1 cm2 | Effective area | 99.2 cm2 | 99.2 cm2 | - |
| TMAH | TOC | TDS | Na+ | Ca2+ | Cl− | SO42− | |
|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 39.8 | 12.7 | 181 | 44.1 | 13.4 | 26.3 | 23.2 |
| Parameter | Condition | |
|---|---|---|
| Contact duration | Charge | 220 s |
| Discharge | 220 s | |
| Rest | 10 s | |
| Electric potential | ±1.3 V | |
| Flow rate | 20 mL/min | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, S.; Choi, Y.; Lee, S. Treatment of Semiconductor Wastewater Containing Tetramethylammonium Hydroxide (TMAH) Using Nanofiltration, Reverse Osmosis, and Membrane Capacitive Deionization. Membranes 2023, 13, 336. https://doi.org/10.3390/membranes13030336
Lee J, Lee S, Choi Y, Lee S. Treatment of Semiconductor Wastewater Containing Tetramethylammonium Hydroxide (TMAH) Using Nanofiltration, Reverse Osmosis, and Membrane Capacitive Deionization. Membranes. 2023; 13(3):336. https://doi.org/10.3390/membranes13030336
Chicago/Turabian StyleLee, Juyoung, Song Lee, Yongjun Choi, and Sangho Lee. 2023. "Treatment of Semiconductor Wastewater Containing Tetramethylammonium Hydroxide (TMAH) Using Nanofiltration, Reverse Osmosis, and Membrane Capacitive Deionization" Membranes 13, no. 3: 336. https://doi.org/10.3390/membranes13030336
APA StyleLee, J., Lee, S., Choi, Y., & Lee, S. (2023). Treatment of Semiconductor Wastewater Containing Tetramethylammonium Hydroxide (TMAH) Using Nanofiltration, Reverse Osmosis, and Membrane Capacitive Deionization. Membranes, 13(3), 336. https://doi.org/10.3390/membranes13030336









