Application of Photo-Fenton-Membrane Technology in Wastewater Treatment: A Review
Abstract
1. Introduction
2. Photo-Fenton Catalysts and Membranes
2.1. Photo-Fenton Catalysts
2.1.1. Fe-Based Materials
ZVI
Iron Oxides
Fe–Metal-Oxide Composites
Fe-Based MOFs
2.1.2. Non-Fe-Based Materials
2.2. Membranes
3. Photo-Fenton-Membrane Reactors
3.1. Immobilized Reactors
3.1.1. Membranes Made by Mixing with Catalysts
3.1.2. Membranes Made by Fixing Catalysts on the Surface
3.1.3. Membranes Made Independently of Catalysts
3.2. Suspension Reactors
4. Application of Photo-Fenton-Membrane Technology
4.1. Separation of Pollutants
| Membranes | WCA | OCA | Substances to Be Separated | Flux/(L·m−2·h−1·bar−1) | Separation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| GO/M88A | 0–77.6° | 126.6–161.7° | MB | 28.7 | 99.58 | [74] |
| RhB | 26.3 | 99.95 | ||||
| MO | 30.3 | 98.9 | ||||
| PVDF/NM88B | 62.3–84.2° | 108.9–151.3° | hexane | 679 | all above 99.4 | [45] |
| toluene | 1766 | |||||
| petroleum ether | 1970 | |||||
| soybean oil | 340 | |||||
| dichloroethane | 1359 | |||||
| PVDF/PPy/ZVI | 26.4° | 139.2° | soybean oil | 2672.7 | 97 | [24] |
| CS/PAN@FeOOH/g-C3N4 | 40° | / | MB | 130.5 | 68.49 | [52] |
| ERY | 182.7 | 72.58 | ||||
| GO-2 | 34.6 | / | MLB | 30 | 93.1 | [75] |
| prGO/Ag/M88A | 0–56.1° | / | EB | 85.8 | 95.8 | [36] |
| PS/HTCC-3/FcSO3 | ≈84° | / | BPS | 1.4 | 98.1 | [76] |
| PMIA/β-FeOOH | 0° | >150° | various oil–water mixtures | >13,925 | >97 | [77] |
| SPAN@GO/M88A | 17.8 ± 3.5° | 159.5 ± 2.1° | oily wastewater | 1569 ± 104 | 99.82 | [67] |
| PVP-coated porous potassium-doped g-C3N4 | 8.0° | 158.6° | toluene | 94.1 | 99.3 | [43] |
| n-hexane | 104.1 | 99.6 | ||||
| dichloromethane | 100.8 | 99.5 | ||||
| PVDF/TA-Fe (III)-HPO4 | 0° | 155.50° | oil-in-water | 1840.94 | / | [78] |
| PVDF/TA/β-FeOOH | 0° | 156.4° | petroleum ether | 1698.5 | 99.7 | [27] |
| NiFe2O4/TA/PVDF | 0° | 159.1° | toluene | 1477 | >99.35 | [29] |
| NM88B@QFM | 0° | 161.3° | petroleum ether | 67,900 | >99.4 | [79] |
| PVDF-g-PAA@FeOOH | 0° | / | bovine serum albumin | / | >90 | [13] |
| PAA@NM88B/GO | 21.8° | / | MB | ~62.5 | >97.5 | [80] |
| PSF + β-FeOOH | ~29° | / | CR | 100 | 92.6 | [62] |
| RB | 105.5 | 60.6 | ||||
| MB | 53.6 | 86 | ||||
| β-FeOOH/HNTs@PVDF | 0° | >150° | surfactant-free n-hexane oil-in-water emulsion | 7338 ± 222 | >99 | [81] |
| PVDF-MT | 0° | 151.38° | carbon tetrachloride | 6133.86 | / | [82] |
4.2. Degradation of Pollutants
| Membranes | Pollutants | Dosage of H2O2/(mmol·L−1) | Initial Concentration of Pollutants/(mg/L) | Light Conditions | Time/min | Degradation Rate (%) | Ref. |
|---|---|---|---|---|---|---|---|
| GO/MIL-88A(Fe) | BPA | 10 | 10 | visible light (λ ≥ 420 nm) | 40 | 97.27 | [75] |
| MB | 10 | 10 | visible light (λ ≥ 420 nm) | 40 | 98.81 | ||
| Fe2O3@CMWCNTs | phenol | 3 | 2.5 | xenon lamp | 120 | 93 | [25] |
| α-FeOOH-coated ceramic membrane | bovine serum albumin | 10 | 35 | UV lamp (401 μW·cm−2) | 60 | 86 | [89] |
| humic acid | 10 | 25 | UV lamp (401 μW·cm−2) | 60 | 76.4 | ||
| CS/PAN@FeOOH/g-C3N4 | MB | 50 | 50 | xenon lamp (λ > 400 nm) | 80 | 100 | [52] |
| ERY | 50 | 20 | xenon lamp (λ > 400 nm) | 140 | 100 | ||
| prGO/Ag/M88A | MB | 35 | 10 | visible light (300 W, λ ≥ 420 nm) | 60 | 98.8 | [36] |
| FeOCl-coated ceramic membrane | nitrobenzene | 8 | 1.23 | UV (λ = 254 nm) | 7 | 100 | [90] |
| (3D) CuO/TiO2 | RhB | 88 | 50 | mercury–xenon arc lamp | 10 | 100 | [38] |
| PS/HTCC-3/FcSO3 | BPS | 1 | 20 | UV lamp (6 W, 254 nm) | 60 | 99.5 | [76] |
| PMIA/β-FeOOH | MB | 30 wt%, 20 μL | 10 | LED light (400~800 nm) | 360 | 99.99 | [77] |
| RhB | 30 wt%, 20 μL | 10 | LED light (400~800 nm) | 480 | 99.75 | ||
| TiO2-GO-Fe3O4 | amoxicillin | 20 | 20 | visible light | 120 | 88.5 | [92] |
| SPAN@GO/M88A | MB | 30 wt%, 50 μL | 50 | xenon arc lamp (150 W) | 20 | >90 | [67] |
| TiO2-ZrO2 UF | caffeine | 1.5 | 0.1 | xenon lamp (300~800 nm) | 15 | 66 | [39] |
| imidacloprid | 1.5 | 0.1 | xenon lamp (300~800 nm) | 15 | 52 | ||
| thiacloprid | 1.5 | 0.1 | xenon lamp (300~800 nm) | 15 | 45 | ||
| carbamazepine | 1.5 | 0.1 | xenon lamp (300~800 nm) | 15 | 83 | ||
| Fe(III)-ZnS/g-C3N4 | p-nitrophenol | 5 | 10 | xenon lamp (500 W) | 240 | 91.6 | [61] |
| Fe-complex/CA | MB | 39 | 10 | xenon lamp (400~780 nm) | 60 | 100 | [53] |
| Co3O4@Fe3O4/cellulose | perfluorooctanoic acid | 30 | 20 | visible light | 180 | 94.5 | [93] |
| PVDF-MT | MB | 10 | 10 | xenon lamp | 70 | 98 | [57] |
| α-Fe2O3-CM | tetracycline hydrochloride | 19.4 | 20 | visible LED light | 200 | 82 | [26] |
4.3. Additional Function
4.4. Problems
4.4.1. The Service Life of Membrane
4.4.2. Small Treatment Scale
5. Conclusions
- The life of the membrane is unstable. The membrane is susceptible to damage to the membrane material and structure when exposed to light. Moreover, after the membrane is recycled several times, the loaded catalyst particles tend to fall off from the membrane surface, affecting the subsequent treatment effect and making it difficult to recover.
- Catalyst selection should be cautious. Careful selection of the appropriate photo-Fenton catalyst is required because when the catalysts are combined with the membranes through co-blending or loading, they can affect the hydrophilicity/hydrophobicity of the membranes and have an impact on the separation effect.
- The optimization of the process needs to be improved. The optimization of the photo-Fenton-membrane treatment process can be carried out through strategies such as objective function development and selection of decision variables.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Wu, M.; Chen, J.; Lin, H.; He, Y. Different fouling propensities of loosely and tightly bound extracellular polymeric substances (EPSs) and the related fouling mechanisms in a membrane bioreactor. Chemosphere 2020, 255, 126953. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Z.; Wang, P.; Tang, Y. An integration of photo-Fenton and membrane process for water treatment by a PVDF@CuFe2O4 catalytic membrane. J. Membr. Sci. 2019, 572, 419–427. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-based Fenton-like catalysts for water treatment: Catalytic mechanisms and applications. J. Mol. Liq. 2021, 332, 115755. [Google Scholar] [CrossRef]
- Sanabria, P.; Wilde, M.L.; Ruiz-Padillo, A.; Sirtori, C. Trends in Fenton and photo-Fenton processes for degradation of antineoplastic agents in water matrices: Current knowledge and future challenges evaluation using a bibliometric and systematic analysis. Environ. Sci. Pollut. Res. Int. 2022, 29, 42168–42184. [Google Scholar] [CrossRef]
- Gao, L.; Cao, Y.; Wang, L.; Li, S. A review on sustainable reuse applications of Fenton sludge during wastewater treatment. Front. Environ. Sci. Eng. 2021, 16, 77. [Google Scholar] [CrossRef]
- Sun, S.-P.; Zeng, X.; Li, C.; Lemley, A.T. Enhanced heterogeneous and homogeneous Fenton-like degradation of carbamazepine by nano-Fe3O4/H2O2 with nitrilotriacetic acid. Chem. Eng. J. 2014, 244, 44–49. [Google Scholar] [CrossRef]
- De Luca, A.; Dantas, R.F.; Esplugas, S. Study of Fe(III)-NTA chelates stability for applicability in photo-Fenton at neutral pH. Appl. Catal. B Environ. 2015, 179, 372–379. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Nowack, B. Environmental chemistry of aminopolycarboxylate chelating agents. Environ. Sci. Technol. 2002, 36, 4009–4016. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Meng, X.; Tian, Y.; Wang, X.; Zhu, J.; Zheng, H.; Wang, L. Fabrication of a superhydrophilic PVDF-g-PAA@FeOOH ultrafiltration membrane with visible light photo-fenton self-cleaning performance. J. Membr. Sci. 2020, 616, 118587. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional Carbon Nitride Materials in Photo-Fenton-Like Catalysis for Environmental Remediation. Adv. Funct. Mater. 2022, 32, 2201743. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: Mechanism study and research gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar] [CrossRef]
- Giannakis, S. Analogies and differences among bacterial and viral disinfection by the photo-Fenton process at neutral pH: A mini review. Environ. Sci. Pollut. Res. Int. 2018, 25, 27676–27692. [Google Scholar] [CrossRef]
- Gutierrez-Mata, A.G.; Velazquez-Martínez, S.; Álvarez-Gallegos, A.; Ahmadi, M.; Hernández-Pérez, J.A.; Ghanbari, F.; Silva-Martínez, S. Recent Overview of Solar Photocatalysis and Solar Photo-Fenton Processes for Wastewater Treatment. Int. J. Photoenergy 2017, 2017, 8528063. [Google Scholar] [CrossRef]
- Shao, D.D.; Yang, W.J.; Xiao, H.F.; Wang, Z.Y.; Zhou, C.; Cao, X.L.; Sun, S.P. Self-Cleaning Nanofiltration Membranes by Coordinated Regulation of Carbon Quantum Dots and Polydopamine. ACS Appl. Mater. Interfaces 2020, 12, 580–590. [Google Scholar] [CrossRef]
- Zhang, S.; Hedtke, T.; Zhu, Q.; Sun, M.; Weon, S.; Zhao, Y.; Stavitski, E.; Elimelech, M.; Kim, J.H. Membrane-Confined Iron Oxychloride Nanocatalysts for Highly Efficient Heterogeneous Fenton Water Treatment. Environ. Sci. Technol. 2021, 55, 9266–9275. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Silwana, N.; Calderon, B.; Ntwampe, S.K.O.; Fullana, A. Heterogeneous Fenton Degradation of Patulin in Apple Juice Using Carbon-Encapsulated Nano Zero-Valent Iron (CE-nZVI). Foods 2020, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Sciscenko, I.; Arques, A.; Escudero-Onate, C.; Roccamante, M.; Ruiz-Delgado, A.; Miralles-Cuevas, S.; Malato, S.; Oller, I. A Rational Analysis on Key Parameters Ruling Zerovalent Iron-Based Treatment Trains: Towards the Separation of Reductive from Oxidative Phases. Nanomaterials 2021, 11, 2948. [Google Scholar] [CrossRef] [PubMed]
- Minella, M.; Sappa, E.; Hanna, K.; Barsotti, F.; Maurino, V.; Minero, C.; Vione, D. Considerable Fenton and photo-Fenton reactivity of passivated zero-valent iron. RSC Adv. 2016, 6, 86752–86761. [Google Scholar] [CrossRef]
- Lee, S.; Bayarkhuu, B.; Han, Y.; Kim, H.-W.; Jeong, S.; Boo, C.; Byun, J. Multifunctional photo-Fenton-active membrane for solar-driven water purification. J. Membr. Sci. 2022, 660, 120832. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, X.; Wu, H.; Li, D.; Sun, Y. Self-cleaning ceramic membranes coated with low crystalline Fe2O3@CMWCNTs for highly efficient photo-Fenton removal of aromatic compounds. J. Environ. Chem. Eng. 2022, 10, 107455. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, Z.; Wei, J.; Xu, Q.; Zhang, X.; Wei, Z. Efficient degradation of antibiotics by photo-Fenton reactive ceramic membrane with high flux by a facile spraying method under visible LED light. J. Clean. Prod. 2022, 366, 132849. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Dai, J.; Lang, J.; Li, C.; Yan, Y. Photo-Fenton self-cleaning membranes with robust flux recovery for an efficient oil/water emulsion separation. J. Mater. Chem. A 2019, 7, 8491–8502. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, B.; Liu, S.-Q.; Meng, Z.; Chen, Z.-G.; Zou, C.-Y.; Liu, C.-B.; Chen, F.; Zhou, X. Photo-Fenton degradation of ammonia via a manganese–iron double-active component catalyst of graphene–manganese ferrite under visible light. Chem. Eng. J. 2016, 283, 266–275. [Google Scholar] [CrossRef]
- Gao, J.; Ma, S.; Xu, M.; Yuan, M.; Li, J.; Xue, J.; Wang, M. Photo-Fenton superwettable NiFe2O4/TA/PVDF composite membrane for organic pollutant degradation with successively oil-in-water separation. Chemosphere 2022, 286, 131705. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced Fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Gao, S.; Cen, W.; Li, Q.; Li, J.; Lu, Y.; Wang, H.; Wu, Z. A mild one-step method for enhancing optical absorption of amine-functionalized metal-organic frameworks. Appl. Catal. B Environ. 2018, 227, 190–197. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Liang, X.; Lei, C.; Cui, Y.; Wu, W.; Yang, Y.; Zhang, Z.; Lei, Z. Construction of heterostructured MIL-125/Ag/g-C3N4 nanocomposite as an efficient bifunctional visible light photocatalyst for the organic oxidation and reduction reactions. Appl. Catal. B Environ. 2017, 205, 42–54. [Google Scholar] [CrossRef]
- He, X.; Fang, H.; Gosztola, D.J.; Jiang, Z.; Jena, P.; Wang, W.N. Mechanistic Insight into Photocatalytic Pathways of MIL-100(Fe)/TiO2 Composites. ACS Appl. Mater. Interfaces 2019, 11, 12516–12524. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Chen, S.; Ye, F.; Quan, X.; Afzal, S.; Yu, H.; Zhao, X. Efficient photo-Fenton activity in mesoporous MIL-100(Fe) decorated with ZnO nanosphere for pollutants degradation. Appl. Catal. B Environ. 2019, 245, 428–438. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Chen, X.; Wang, L.; Cai, W. Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway. Chem. Eng. J. 2019, 369, 745–757. [Google Scholar] [CrossRef]
- Yue, R.; Raisi, B.; Rahmatinejad, J.; Ye, Z.; Barbeau, B.; Rahaman, M.S. A photo-Fenton nanocomposite ultrafiltration membrane for enhanced dye removal with self-cleaning properties. J. Colloid Interface Sci. 2021, 604, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, X.; Feng, W.; Li, W. Enhanced catalytic performance of a bio-templated TiO2 UV-Fenton system on the degradation of tetracycline. Appl. Surf. Sci. 2019, 465, 223–231. [Google Scholar] [CrossRef]
- Date, M.K.; Yang, L.H.; Yang, T.Y.; Wang, K.Y.; Su, T.Y.; Wu, D.C.; Cheuh, Y.L. Three-Dimensional CuO/TiO2 Hybrid Nanorod Arrays Prepared by Electrodeposition in AAO Membranes as an Excellent Fenton-Like Photocatalyst for Dye Degradation. Nanoscale Res. Lett. 2020, 15, 45. [Google Scholar] [CrossRef]
- Deemter, D.; Coelho, F.E.B.; Oller, I.; Malato, S.; Amat, A.M. Assessment of a Novel Photocatalytic TiO2-Zirconia Ultrafiltration Membrane and Combination with Solar Photo-Fenton Tertiary Treatment of Urban Wastewater. Catalysts 2022, 12, 552. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, S.; Zhou, L.; Hu, Y.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y.; Cui, C. Efficient removal of antibiotic-resistant bacteria and intracellular antibiotic resistance genes by heterogeneous activation of peroxymonosulfate on hierarchical macro-mesoporous Co3O4-SiO2 with enhanced photogenerated charges. J. Hazard. Mater. 2022, 430, 127414. [Google Scholar] [CrossRef]
- Mokoba, T.; Li, Z.; Zhang, T.C.; Yuan, S. Superwetting sea urchin-like BiOBr@Co3O4 nanowire clusters-coated copper mesh with efficient emulsion separation and photo-Fenton-like degradation of soluble dye. Appl. Surf. Sci. 2022, 594, 153497. [Google Scholar] [CrossRef]
- Lan, H.; Wang, F.; Lan, M.; An, X.; Liu, H.; Qu, J. Hydrogen-Bond-Mediated Self-Assembly of Carbon-Nitride-Based Photo-Fenton-like Membranes for Wastewater Treatment. Environ. Sci. Technol. 2019, 53, 6981–6988. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Saifur Rahaman, M. Hydrophilic and underwater superoleophobic porous graphitic carbon nitride (g-C3N4) membranes with photo-Fenton self-cleaning ability for efficient oil/water separation. J. Colloid Interface Sci. 2022, 608, 1960–1972. [Google Scholar] [CrossRef]
- Aouadja, F.; Bouzerara, F.; Guvenc, C.M.; Demir, M.M. Fabrication and properties of novel porous ceramic membrane supports from the (Sig) diatomite and alumina mixtures. Boletín Soc. Española Cerámica Vidr. 2022, 61, 531–540. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Photo-Fenton self-cleaning PVDF/NH2-MIL-88B(Fe) membranes towards highly-efficient oil/water emulsion separation. J. Membr. Sci. 2020, 595, 117499. [Google Scholar] [CrossRef]
- Bao, X.; Liu, Q.; Yang, J.; Wang, F.; Yu, F.; Yu, J.; Yang, Y. Cascading in-situ generation of H2O2 and Fenton-like reaction in photocatalytic composite ultrafiltration membrane for high self-cleaning performance in wastewater treatment. J. Membr. Sci. 2022, 660, 120866. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.J.; Wu, G.P.; Darling, S.B.; Xu, Z.K. Photocatalytic Nanofiltration Membranes with Self-Cleaning Property for Wastewater Treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- González-Bahamón, L.F.; Mazille, F.; Benítez, L.N.; Pulgarín, C. Photo-Fenton degradation of resorcinol mediated by catalysts based on iron species supported on polymers. J. Photochem. Photobiol. A Chem. 2011, 217, 201–206. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Bortot Coelho, F.E.; Deemter, D.; Candelario, V.M.; Boffa, V.; Malato, S.; Magnacca, G. Development of a photocatalytic zirconia-titania ultrafiltration membrane with anti-fouling and self-cleaning properties. J. Environ. Chem. Eng. 2021, 9, 106671. [Google Scholar] [CrossRef]
- Nazemidashtarjandi, S.; Mousavi, S.A.; Bastani, D. Preparation and characterization of polycarbonate/thermoplastic polyurethane blend membranes for wastewater filtration. J. Water Process Eng. 2017, 16, 170–182. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, H.; Tong, X.; Wang, Z.; Crittenden, J.C.; Huang, M. Integration of a Photo-Fenton Reaction and a Membrane Filtration using CS/PAN@FeOOH/g-C3N4 Electrospun Nanofibers: Synthesis, Characterization, Self-cleaning Performance and Mechanism. Appl. Catal. B Environ. 2021, 281, 119519. [Google Scholar] [CrossRef]
- Wang, D.; Yang, J.; Yang, H.; Zhao, P.; Shi, Z. Fe-complex modified cellulose acetate composite membrane with excellent photo-Fenton catalytic activity. Carbohydr. Polym. 2022, 296, 119960. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yin, J.; Tang, H.; Wang, H.; Liu, S.; Huang, T.; Fang, S.; Zhu, K.; Xie, Z. Fabrication of ZIF-67@PVDF ultrafiltration membrane with improved antifouling and separation performance for dye wastewater treatment via sulfate radical enhancement. Sep. Purif. Technol. 2021, 279, 119755. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Huang, Y.; Liu, J.; Wang, D.; Feng, S.; Huang, X.; Wang, H. Electrospinning preparation of PAN/TiO2/PANI hybrid fiber membrane with highly selective adsorption and photocatalytic regeneration properties. Chem. Eng. J. 2020, 399, 125749. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for mixed-matrix membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Zhao, G.; Long, X.; Hu, J.; Jiao, F. Photo-Fenton Antifouling Membrane Based on Hydrophilized MIL-88A for Sustainable Treatment of Colored Emulsions. Ind. Eng. Chem. Res. 2022, 61, 18503–18513. [Google Scholar] [CrossRef]
- Domenzain-Gonzalez, J.; Castro-Arellano, J.J.; Galicia-Luna, L.A.; Rodriguez-Cruz, M.; Hernandez-Lopez, R.T.; Lartundo-Rojas, L. Photocatalytic membrane reactor based on Mexican Natural Zeolite: RB5 dye removal by photo-Fenton process. J. Environ. Chem. Eng. 2021, 9, 105281. [Google Scholar] [CrossRef]
- Molina, R.; Segura, Y.; Martínez, F.; Melero, J.A. Immobilization of active and stable goethite coated-films by a dip-coating process and its application for photo-Fenton systems. Chem. Eng. J. 2012, 203, 212–222. [Google Scholar] [CrossRef]
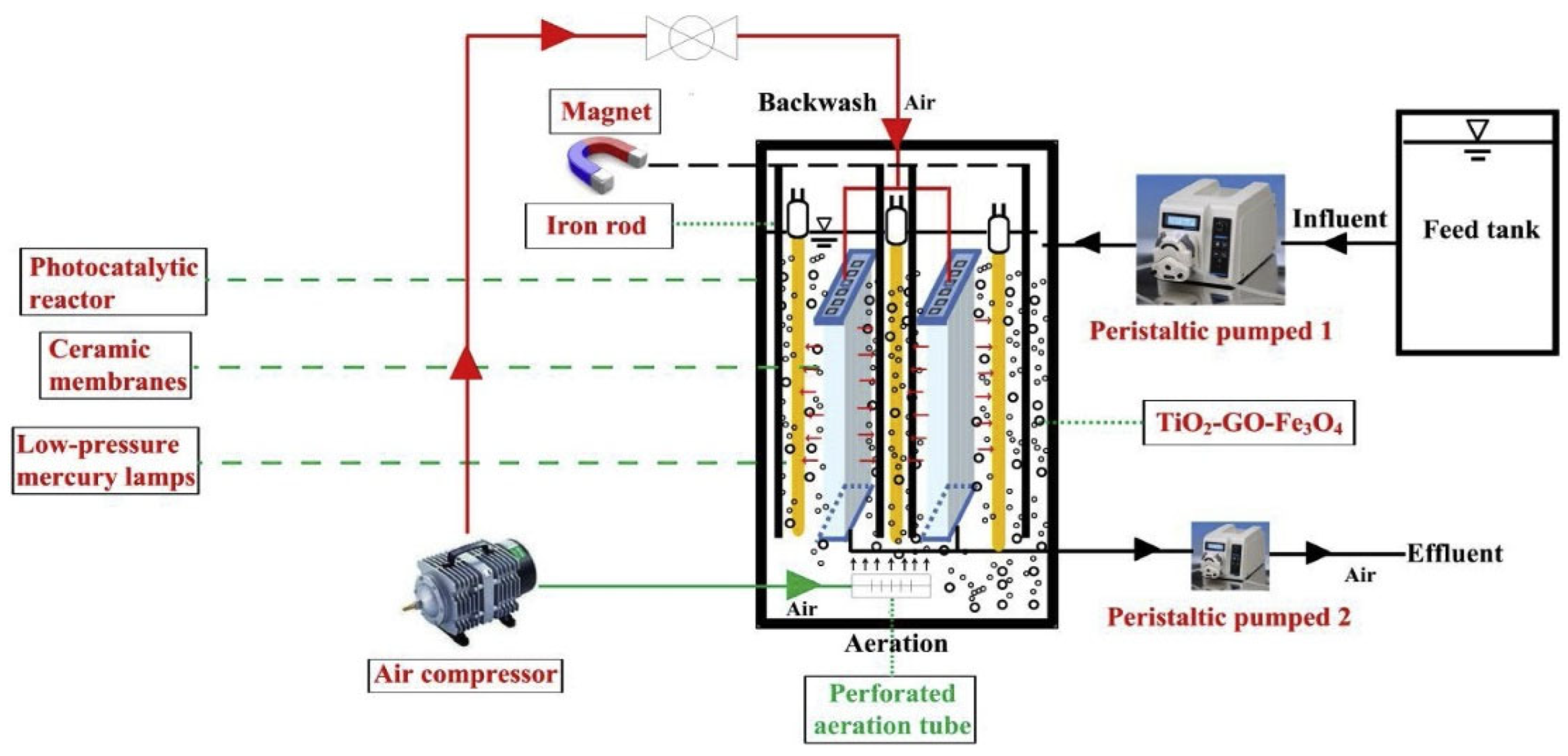
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 2019, 373, 437–446. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Xu, P.; Hu, L.; Wang, X.; Qu, J.; Zhang, G. Submerged membrane photocatalytic reactor for advanced treatment of p-nitrophenol wastewater through visible-light-driven photo-Fenton reactions. Sep. Purif. Technol. 2021, 256, 117783. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Hou, Y.; Li, P.; Sun, H.; Niu, Q.J. Photo-Fenton assisted self-cleaning hybrid ultrafiltration membranes with high-efficient flux recovery for wastewater remediation. Sep. Purif. Technol. 2020, 249, 117159. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, B.; Zhu, L.; Zeng, Z.; Wang, G.; Xiong, Z. Efficient Fenton-Like Catalysis Boosting the Antifouling Performance of the Heterostructured Membranes Fabricated via Vapor-Induced Phase Separation and In Situ Mineralization. ACS Appl. Mater. Interfaces 2021, 13, 43648–43660. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Superamphiphilic and underwater superoleophobic membrane for oil/water emulsion separation and organic dye degradation. J. Membr. Sci. 2020, 598, 117804. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, G.; Gao, S.; Jin, H.; Zhu, Y.; Zhang, F.; Jin, J. Cupric Phosphate Nanosheets-Wrapped Inorganic Membranes with Superhydrophilic and Outstanding Anticrude Oil-Fouling Property for Oil/Water Separation. ACS Nano 2018, 12, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, H.; Wei, B.; Zhang, T.C.; Chang, H.; Li, Y.; Tian, X.; Fan, Y.; Liang, Y.; Yuan, S. Multifunctional Switchable Nanocoated Membranes for Efficient Integrated Purification of Oil/Water Emulsions. ACS Appl. Mater. Interfaces 2021, 13, 54315–54323. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Luo, P.; Ma, L.; Li, S.; Nie, Y.; Zhong, F.; Wang, Y.; Chen, L. Photocatalytic GO/M88A “interceptor plate” assembled nanofibrous membrane with photo-Fenton self-cleaning performance for oil/water emulsion separation. Chem. Eng. J. 2022, 427, 130948. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Amphiphile grafted membranes for the separation of oil-in-water dispersions. J. Colloid Interface Sci. 2009, 329, 127–132. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. The impact of low-surface-energy functional groups on oil fouling resistance in membrane distillation. J. Membr. Sci. 2017, 527, 68–77. [Google Scholar] [CrossRef]
- Ma, Z.; Shu, G.; Lu, X. Preparation of an antifouling and easy cleaning membrane based on amphiphobic fluorine island structure and chemical cleaning responsiveness. J. Membr. Sci. 2020, 611, 118403. [Google Scholar] [CrossRef]
- Jing, J.; Liu, Z.-J.; Zhang, X.-G.; Ren, L.-N.; Wang, H.-Y. Research progress of superwetting oil-water separation membrane. Surf. Technol. 2023, 52, 172–182. (In Chinese) [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Jia, R.; Shao, J.; He, Y. Enhanced catalytic degradation of amoxicillin with TiO2-Fe3O4 composites via a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). RSC Adv. 2019, 9, 12538–12546. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes. Appl. Catal. B Environ. 2020, 264, 118548. [Google Scholar] [CrossRef]
- Yue, R.; Chen, T.; Ye, Z.; Barbeau, B.; Rahaman, M.S. A photo-Fenton graphene oxide membrane with improved perm-selectivity and self-cleaning ability for efficient dye removal under visible light irradiation. J. Water Process Eng. 2021, 44, 102443. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Chen, J.; Wen, S.; Li, D.; Wang, B.; Zhang, Q. Multifunctional ferrocene-based photo-Fenton membrane: An efficient integration of rejection and catalytic process. Sep. Purif. Technol. 2022, 298, 121557. [Google Scholar] [CrossRef]
- Piao, H.; Zhao, J.; Liu, M.; Zhang, S.; Huang, Q.; Liu, Y.; Xiao, C. Ultra-low power light driven lycopodium-like nanofiber membrane reinforced by PET braid tube with robust pollutants removal and regeneration capacity based on photo-Fenton catalysis. Chem. Eng. J. 2022, 450, 138204. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Wang, L.; Long, X.; Zhang, J.; Zuo, Y.; Jiao, F. Tannic acid-based complex coating modified membranes with photo-Fenton self-cleaning property for sustainable oil-in-water emulsion separation. Sep. Purif. Technol. 2021, 272, 118893. [Google Scholar] [CrossRef]
- Xie, A.; Wu, Y.; Liu, Y.; Xue, C.; Ding, G.; Cheng, G.; Cui, J.; Pan, J. Robust antifouling NH2-MIL-88B coated quartz fibrous membrane for efficient gravity-driven oil-water emulsion separation. J. Membr. Sci. 2022, 644, 120093. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, S.; He, Y.; Fan, Y.; Zhang, L.; Ma, J.; Hou, R.; Chen, L.; Chen, J. A photo-Fenton self-cleaning membrane based on NH2-MIL-88B (Fe) and graphene oxide to improve dye removal performance. J. Membr. Sci. 2021, 626, 119192. [Google Scholar] [CrossRef]
- Zhou, L.; Xiao, G.; He, Y.; Wu, J.; Shi, H.; Yin, X.; He, T.; Li, Z.; Chen, J. Multi-functional composite membrane with strong photocatalysis to effectively separate emulsified-oil/dyes from complex oily sewage. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128733. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Couto, C.F.; Maia, A.; Moravia, W.G.; Amaral, M.C.S. Process development for textile wastewater treatment towards zero liquid discharge: Integrating membrane separation process and advanced oxidation techniques. Process Saf. Environ. Prot. 2022, 157, 537–546. [Google Scholar] [CrossRef]
- Fan, D.; Ding, L.; Huang, H.; Chen, M.; Ren, H. Fluidized-bed Fenton coupled with ceramic membrane separation for advanced treatment of flax wastewater. J. Hazard. Mater. 2017, 340, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Arriaga, E.B.; Zepeda-Aviles, J.A.; García-Sánchez, L. Post-treatment of real oil refinery effluent with high concentrations of phenols using photo-ferrioxalate and Fenton’s reactions with membrane process step. Chem. Eng. J. 2016, 285, 508–516. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Michael, C.; Vakondios, N.; Drosou, K.; Xekoukoulotakis, N.P.; Diamadopoulos, E.; Fatta-Kassinos, D. Winery wastewater purification by reverse osmosis and oxidation of the concentrate by solar photo-Fenton. Sep. Purif. Technol. 2013, 118, 659–669. [Google Scholar] [CrossRef]
- Aydiner, C.; Mert, B.K.; Dogan, E.C.; Yatmaz, H.C.; Dagli, S.; Aksu, S.; Tilki, Y.M.; Goren, A.Y.; Balci, E. Novel hybrid treatments of textile wastewater by membrane oxidation reactor: Performance investigations, optimizations and efficiency comparisons. Sci. Total Environ. 2019, 683, 411–426. [Google Scholar] [CrossRef]
- Gholami, M.; Abbasi Souraki, B.; Pendashteh, A.; Bagherian Marzouni, M. Efficiency evaluation of the membrane/AOPs for paper mill wastewater treatment. Env. Technol. 2017, 38, 1127–1138. [Google Scholar] [CrossRef]
- Calik, C.; Cifci, D.I. Comparison of kinetics and costs of Fenton and photo-Fenton processes used for the treatment of a textile industry wastewater. J. Environ. Manag. 2022, 304, 114234. [Google Scholar] [CrossRef]
- Sun, S.; Yao, H.; Fu, W.; Hua, L.; Zhang, G.; Zhang, W. Reactive Photo-Fenton ceramic membranes: Synthesis, characterization and antifouling performance. Water Res. 2018, 144, 690–698. [Google Scholar] [CrossRef]
- Liu, F.; Yao, H.; Sun, S.; Tao, W.; Wei, T.; Sun, P. Photo-Fenton activation mechanism and antifouling performance of an FeOCl-coated ceramic membrane. Chem. Eng. J. 2020, 402, 125477. [Google Scholar] [CrossRef]
- Dogan, E.C.; Kilicoglu, O.; Narci, A.O.; Mert, B.K.; Durna, E.; Akbacak, U.A.; Aydiner, C. Fenton and photo-Fenton processes integrated with submerged ultrafiltration for the treatment of pulp and paper industry wastewater. J. Environ. Chem. Eng. 2021, 9, 105878. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Matsuura, T.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Othman, N.H.; Abdullah, N.; Paiman, S.H.; et al. Photocatalytic nanofiber-coated alumina hollow fiber membranes for highly efficient oilfield produced water treatment. Chem. Eng. J. 2019, 360, 1437–1446. [Google Scholar] [CrossRef]
- Gao, J.; Chen, W.; Shi, H.; Li, Z.; Jing, L.; Hou, C.; Wang, J.; Wang, Y. Co3O4@Fe3O4/cellulose blend membranes for efficient degradation of perfluorooctanoic acid in the visible light-driven photo-Fenton system. Surf. Interfaces 2022, 34, 102302. [Google Scholar] [CrossRef]
- Vilardi, G.; Di Palma, L.; Verdone, N. On the critical use of zero valent iron nanoparticles and Fenton processes for the treatment of tannery wastewater. J. Water Process Eng. 2018, 22, 109–122. [Google Scholar] [CrossRef]
- Giannakis, S.; Polo López, M.I.; Spuhler, D.; Sánchez Pérez, J.A.; Fernández Ibáñez, P.; Pulgarin, C. Solar disinfection is an augmentable, in situ -generated photo-Fenton reaction—Part 1: A review of the mechanisms and the fundamental aspects of the process. Appl. Catal. B Environ. 2016, 199, 199–223. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Comparative evaluation of Fe3+ and TiO2 photoassisted processes in solar photocatalytic disinfection of water. Appl. Catal. B Environ. 2006, 63, 222–231. [Google Scholar] [CrossRef]
- Barreca, S.; Velez Colmenares, J.J.; Pace, A.; Orecchio, S.; Pulgarin, C. Escherichia coli inactivation by neutral solar heterogeneous photo-Fenton (HPF) over hybrid iron/montmorillonite/alginate beads. J. Environ. Chem. Eng. 2015, 3, 317–324. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Barona, J.F.; Sienkiewicz, A.; Vélez, J.; Benítez, L.N.; Pulgarín, C. Bacterial inactivation with iron citrate complex: A new source of dissolved iron in solar photo-Fenton process at near-neutral and alkaline pH. Appl. Catal. B Environ. 2016, 180, 379–390. [Google Scholar] [CrossRef]
- Ortega-Gomez, E.; Esteban Garcia, B.; Ballesteros Martin, M.M.; Fernandez Ibanez, P.; Sanchez Perez, J.A. Inactivation of natural enteric bacteria in real municipal wastewater by solar photo-Fenton at neutral pH. Water Res. 2014, 63, 316–324. [Google Scholar] [CrossRef]
- Polo-López, M.I.; Oller, I.; Fernández-Ibáñez, P. Benefits of photo-Fenton at low concentrations for solar disinfection of distilled water. A case study: Phytophthora capsici. Catal. Today 2013, 209, 181–187. [Google Scholar] [CrossRef]
- Ortega-Gomez, E.; Fernandez-Ibanez, P.; Ballesteros Martin, M.M.; Polo-Lopez, M.I.; Esteban Garcia, B.; Sanchez Perez, J.A. Water disinfection using photo-Fenton: Effect of temperature on Enterococcus faecalis survival. Water Res. 2012, 46, 6154–6162. [Google Scholar] [CrossRef] [PubMed]
- Aurioles-López, V.; Polo-López, M.I.; Fernández-Ibáñez, P.; López-Malo, A.; Bandala, E.R. Effect of iron salt counter ion in dose–response curves for inactivation of Fusarium solani in water through solar driven Fenton-like processes. Phys. Chem. Earth Parts A/B/C 2016, 91, 46–52. [Google Scholar] [CrossRef]
- Giannakis, S.; Ruales-Lonfat, C.; Rtimi, S.; Thabet, S.; Cotton, P.; Pulgarin, C. Castles fall from inside: Evidence for dominant internal photo-catalytic mechanisms during treatment of Saccharomyces cerevisiae by photo-Fenton at near-neutral pH. Appl. Catal. B Environ. 2016, 185, 150–162. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Benítez, N.; Sienkiewicz, A.; Pulgarín, C. Deleterious effect of homogeneous and heterogeneous near-neutral photo-Fenton system on Escherichia coli. Comparison with photo-catalytic action of TiO2 during cell envelope disruption. Appl. Catal. B Environ. 2014, 160–161, 286–297. [Google Scholar] [CrossRef]
- Giannakis, S.; Voumard, M.; Rtimi, S.; Pulgarin, C. Bacterial disinfection by the photo-Fenton process: Extracellular oxidation or intracellular photo-catalysis? Appl. Catal. B Environ. 2018, 227, 285–295. [Google Scholar] [CrossRef]
- Xue, J.; Yuan, M.; Gao, J.; Zhang, Z.; Wang, M.; Ma, S. Photo-Fenton catalyst Fe(III)@PCN-222 grafted on PVDF membrane for multitasking applications: Oil/water separation, aromatic pollutants degradation and bacterial inactivation. Process Saf. Environ. Prot. 2023, 169, 746–756. [Google Scholar] [CrossRef]
- Ballesteros Martin, M.M.; Garrido, L.; Casas Lopez, J.L.; Sanchez, O.; Mas, J.; Maldonado, M.I.; Sanchez Perez, J.A. An analysis of the bacterial community in a membrane bioreactor fed with photo-Fenton pre-treated toxic water. J. Ind. Microbiol. Biotechnol. 2011, 38, 1171–1178. [Google Scholar] [CrossRef]
- Sakurai, K.S.I.; Neves, L.C.; Souza, J.B.D.; Vidal, C.M.D.S.; Souza, K.V.D. Pós-tratamento de efluente de indústria de papel e celulose empregando membranas de microfiltração e ultrafiltração combinadas com o processo foto-fenton. Sci. For. 2016, 44, 937–945. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef]
- Lee, M.J.; Ong, C.S.; Lau, W.J.; Ng, B.C.; Ismail, A.F.; Lai, S.O. Degradation of PVDF-based composite membrane and its impacts on membrane intrinsic and separation properties. J. Polym. Eng. 2016, 36, 261–268. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Ji, L.; Ma, Z.; Ren, Y.; Zhou, S.; Long, X.; Cao, C. Application of Photo-Fenton-Membrane Technology in Wastewater Treatment: A Review. Membranes 2023, 13, 369. https://doi.org/10.3390/membranes13040369
Liang L, Ji L, Ma Z, Ren Y, Zhou S, Long X, Cao C. Application of Photo-Fenton-Membrane Technology in Wastewater Treatment: A Review. Membranes. 2023; 13(4):369. https://doi.org/10.3390/membranes13040369
Chicago/Turabian StyleLiang, Lihua, Lin Ji, Zhaoyan Ma, Yuanyuan Ren, Shuyu Zhou, Xinchang Long, and Chenyang Cao. 2023. "Application of Photo-Fenton-Membrane Technology in Wastewater Treatment: A Review" Membranes 13, no. 4: 369. https://doi.org/10.3390/membranes13040369
APA StyleLiang, L., Ji, L., Ma, Z., Ren, Y., Zhou, S., Long, X., & Cao, C. (2023). Application of Photo-Fenton-Membrane Technology in Wastewater Treatment: A Review. Membranes, 13(4), 369. https://doi.org/10.3390/membranes13040369






