Molecular Rearrangements in Protomembrane Models Probed by Laurdan Fluorescence
Abstract
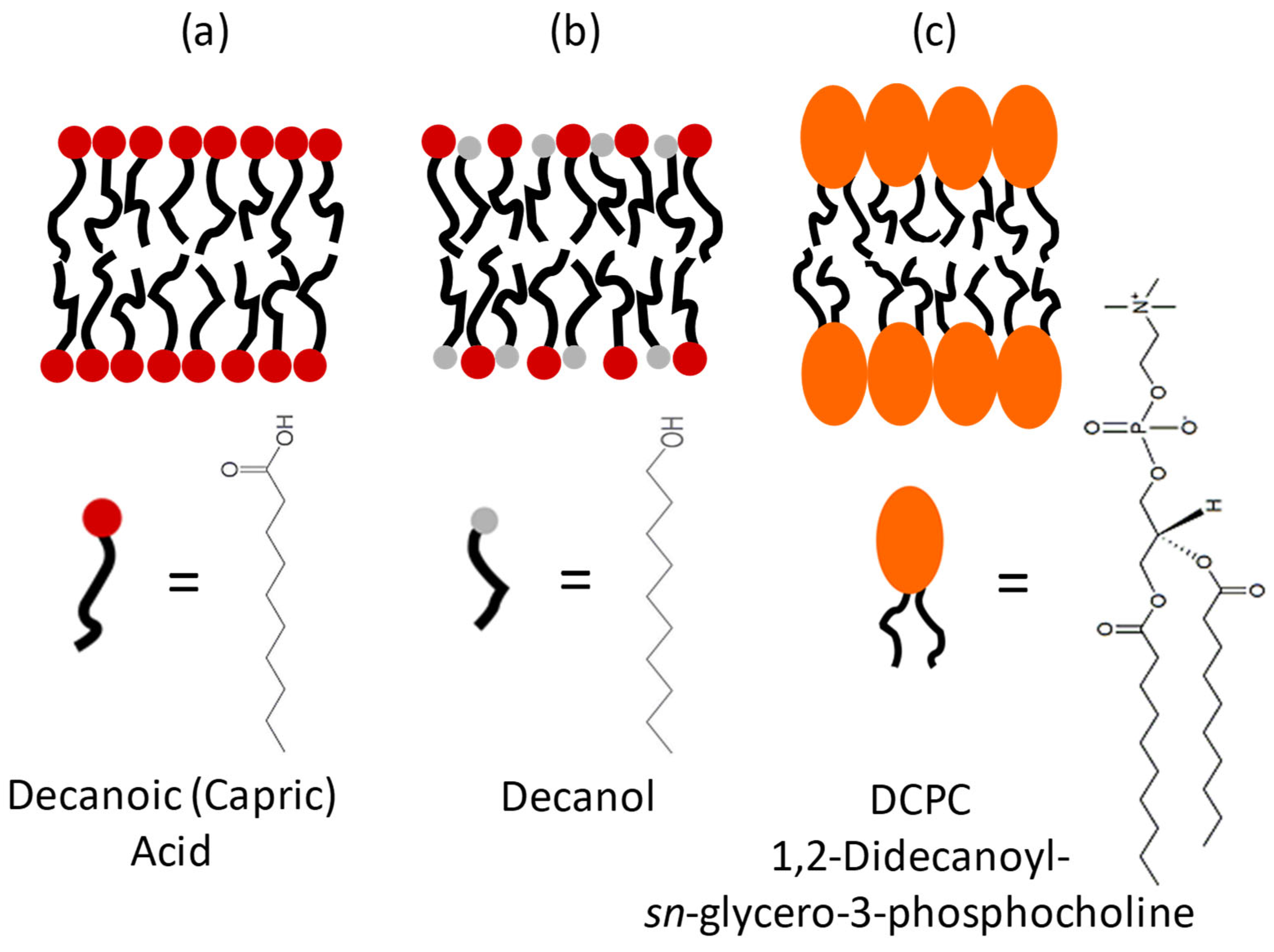
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Fluorescence with Laurdan
2.3. Small-Angle Neutron Scattering (SANS) Measurements
3. Results
3.1. Temperature Study
3.2. Concentration Dependent Measurements
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCollom, T.M.; Ritter, G.; Simoneit, B.R. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Monnard, P.A.; Deamer, D.W. Preparation of vesicles from nonphospholipid amphiphiles. Methods Enzym. 2003, 372, 133–151. [Google Scholar] [CrossRef]
- Miller, S.L.; Bada, J.L. Submarine hot springs and the origin of life. Nature 1988, 334, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. The Role of Lipid Membranes in Life’s Origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.L.; Deamer, D.W.; Mautner, M.N. Self-assembled vesicles of monocarboxylic acids and alcohols: Conditions for stability and for the encapsulation of biopolymers. BBA—Biomembr. 2002, 1559, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Misuraca, L.; Calio, A.; Grillo, I.; Grelard, A.; Oger, P.M.; Peters, J.; Demé, B. High-Temperature Behavior of Early Life Membrane Models. Langmuir 2020, 36, 13516–13526. [Google Scholar] [CrossRef] [PubMed]
- Misuraca, L.; Calio, A.; LoRicco, J.G.; Hoffman, I.; Winter, R.; Demé, B.; Peters, J.; Oger, P.M. Alkanes as Membrane Regulators of the Response of Early Membranes to Extreme Temperatures. Life 2022, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Misuraca, L.; Matsuo, T.; Cisse, A.; LoRicco, J.; Calio, A.; Zanotti, J.M.; Deme, B.; Oger, P.; Peters, J. High temperature molecular motions within a model protomembrane architecture. Phys. Chem. Chem. Phys. 2022, 24, 15083–15090. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, K.; Walde, P. Fatty acid vesicles. Curr. Opin. Coll. Inter. Sci. 2007, 75, 80. [Google Scholar] [CrossRef]
- Parasassi, T.; Gratton, E. Membrane lipid domains and dynamics as detected by Laurdan fluorescence. J. Fluoresc. 1995, 5, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.A.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Kapoor, S.; Berghaus, M.; Suladze, S.; Prumbaum, D.; Grobelny, S.; Degen, P.; Raunser, S.; Winter, R. Prebiotic cell membranes that survive extreme environmental pressure conditions. Angew. Chem. Int. Ed. Engl. 2014, 53, 8397–8401. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, C.; Grillo, I.; Honecker, D.; Bonnaud, M.; Jacques, M.; Amrouni, C.; Perillo-Marcone, A.; Manzin, G.; Cubitt, R. The small-angle neutron scattering instrument D33 at the Institut Laue–Langevin. J. Appl. Cryst. 2016, 49, 1–14. [Google Scholar] [CrossRef]
- Cristiglio, V.; Giroud, B.; Didier, L.; Demé, B. D16 is back to business: More neutrons, more space, more fun. Neutron. News 2015, 26, 22–24. [Google Scholar] [CrossRef]
- Zorila, B.; Bacalum, M.; Popescu, A.I.; Radu, M. Log-normal deconvolution of laurdan fluorescence spectra—A tool to assess lipid membrane fluidity. Rom. Rep. Phys. 2016, 68, 702–712. [Google Scholar]
- Veatch, S.L.; Keller, S.L. Seeing spots: Complex phase behavior in simple membranes. Biochim. Biophys. Acta 2005, 1746, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Porod, G.; Glatter, O.; Kratky, O.K.O.G. Small Angle X-ray Scattering; Glatter, O., Kratky, O., Eds.; Academic Press: London, UK, 1982; p. 17. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misuraca, L.; Winter, R.; Demé, B.; Oger, P.M.; Peters, J. Molecular Rearrangements in Protomembrane Models Probed by Laurdan Fluorescence. Membranes 2023, 13, 386. https://doi.org/10.3390/membranes13040386
Misuraca L, Winter R, Demé B, Oger PM, Peters J. Molecular Rearrangements in Protomembrane Models Probed by Laurdan Fluorescence. Membranes. 2023; 13(4):386. https://doi.org/10.3390/membranes13040386
Chicago/Turabian StyleMisuraca, Loreto, Roland Winter, Bruno Demé, Philippe M. Oger, and Judith Peters. 2023. "Molecular Rearrangements in Protomembrane Models Probed by Laurdan Fluorescence" Membranes 13, no. 4: 386. https://doi.org/10.3390/membranes13040386
APA StyleMisuraca, L., Winter, R., Demé, B., Oger, P. M., & Peters, J. (2023). Molecular Rearrangements in Protomembrane Models Probed by Laurdan Fluorescence. Membranes, 13(4), 386. https://doi.org/10.3390/membranes13040386






