Application of Turbiscan Stability Index for the Preparation of Alumina Photocatalytic Membranes for Dye Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Suspension Preparation and Characterization
2.3. Photocatalytic Membranes: Preparation and Characterization
2.4. Photocatalytic Membrane Tests
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Adams, J.B.; Rubidge, G. The state of persistent organic pollutants in South African estuaries: A review of environmental exposure and source. Ecotoxicol. Environ. Saf. 2021, 219, 112316. [Google Scholar] [CrossRef]
- Rafaqata, S.; Ali, N.; Torres, C.; Rittmann, B. Recent progress in treatment of dyes wastewater using microbial-electro-Fenton technology. RSC Adv. 2022, 12, 17104–17137. [Google Scholar] [CrossRef] [PubMed]
- Eltaboni, F.; Bader, N.; El-Kailany, R.; Elsharif, N.; Ahmida, A. Chemistry and applications of azo dyes: A comprehensive review. J. Chem. Rev. 2022, 4, 313–330. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Kurian, M. Advanced oxidation processes and nanomaterials-a review. Clean. Eng. Technol. 2021, 2, 100090. [Google Scholar] [CrossRef]
- García-Rodríguez, O.; Banuelos, J.A.; Rico-Zavala, A.; Godínez, L.A.; Rodríguez-Valadez, F.J. Electrocatalytic activity of three carbon materials for the In-situ production of hydrogen peroxide and Its application to the electro-Fenton heterogeneous process. Int. J. Chem. React. Eng. 2016, 14, 843–850. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; Gaayda, J.E.; Akbour, R.A.; Nidheesh, P.V.; Hamdan, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. Process Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Photocatalytic reduction of acetophenone in membrane reactors under UV and visible light using TiO2 and Pd/TiO2 catalysts. Chem. Eng. J. 2015, 274, 307–316. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B 2004, 491, 1–14. [Google Scholar] [CrossRef]
- Dave, S.; Das, J. Technological model on advanced stages of oxidation of wastewater effluent from food industry. In Advanced Oxidation Processes for Effluent Treatment Plants; Shah, M.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–49. [Google Scholar]
- Gao, B.; Yap, P.S.; Lim, T.M.; Lim, T.T. Adsorption-photocatalytic degradation of Acid Red 88 by supported TiO2: Effect of activated carbon support and aqueous anions. Chem. Eng. 2011, 171, 1098–1107. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, P.; Wang, H. Photocatalytic membrane reactors for produced water treatment and reuse: Fundamentals, affecting factors, rational design, and evaluation metrics. J. Hazard. Mater. 2022, 15, 127493. [Google Scholar] [CrossRef] [PubMed]
- Ashley, A.; Thrope, B.; Choudhury, M.R.; Pinto, A.H. Emerging investigator series: Photocatalytic membrane reactors: Fundamentals and advances in preparation and application in wastewater treatment. Environ. Sci. Water Res. Technol. 2022, 8, 22–46. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhang, J.; Wang, Z.; Xu, L.; Fan, Z. Influence of azo dye-TiO2 interactions on the filtration performance in a hybrid photocatalysis/ultrafiltration process. J. Colloid Interface Sci. 2013, 389, 273–283. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. The Evolution of Photocatalytic Membrane Reactors over the Last 20 Years: A State of the Art Perspective. Catalysts 2021, 11, 775. [Google Scholar] [CrossRef]
- Algieri, C.; Drioli, E. Zeolite membranes: Synthesis and applications. Sep. Purif. Technol. 2022, 278, 119295. [Google Scholar] [CrossRef]
- Singh, R.; Hankins, N.P. Introduction to Membrane Processes for Water Treatment. In Emerging Membrane Technology for Sustainable Water Treatment; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Athanasekou, C.P.; Romanos, G.E.; Katsaros, F.K.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Membr. Sci. 2012, 392–393, 192–203. [Google Scholar] [CrossRef]
- Pereira, V.R.; Isloor, A.M.; Zulhairun, A.K.; Subramaniam, M.N.; Lau, W.J.; Ismail, A.F. Preparation of polysulfone-based PANI–TiO2 nanocomposite hollow fiber membranes for industrial dye rejection applications. RSC Adv. 2016, 6, 99764–99773. [Google Scholar] [CrossRef]
- Erdei, L.; Arecrachakul, N.; Vigneswaran, S. A combined photocatalytic slurry reactor-immersed membrane module system for advanced wastewater treatment. Sep. Purif. Technol. 2008, 62, 382–388. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Katsaros, F.K.; Kanellopoulos, N.K.; Dionysiou, D.D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2012, 211–212, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Algieri, C.; Macedonio, F.; Drioli, E. Zeolite Membranes for Desalination in Sustainable Materials and Systems for Water Desalination; Inamuddin, A.K., Ed.; Springer: Cham, Switzerland, 2021; ISBN 978-3-030-72873-1. [Google Scholar]
- Deepracha, S.; Atfane, L.; Ayral, A.; Ogawa, M. Simple and efficient method for functionalizing photocatalytic ceramic membranes and assessment of its applicability for wastewater treatment in up-scalable membrane reactors. Sep. Purif. Technol. 2021, 262, 118307. [Google Scholar] [CrossRef]
- Phattepur, H.; Hiremath, P.G. Fabrication of Al2O3 supported TiO2 membranes for photocatalytic applications. Mater. Today Proc. 2002, 65, 3694–3699. [Google Scholar] [CrossRef]
- Ting, P.H.; Jing, J.F.; Zhenglong, Y.; Biao, Y.; Xin, L. Effects of polyvinylpyrrolidone and carbon nanotubes on magnetorheological properties of iron-based magnetorheological fluids. J. Appl. Polym. Sci. 2006, 102, 1653–1657. [Google Scholar] [CrossRef]
- De Paola, M.G.; Paletta, R.; Lopresto, C.G.; Lio, G.E.; De Luca, A.; Chakraborty, S.; Calabrò, V. Stability of film-forming dispersions: Affects the morphology and optical properties of polymeric films. Polymers 2021, 13, 1464. [Google Scholar] [CrossRef]
- De Paola, M.G.; Arcuri, N.; Calabro, V.; De Simone, M. Thermal and stability investigation of phase change material dispersions for thermal energy storage by T-history and optical methods. Energies 2017, 10, 354. [Google Scholar] [CrossRef]
- Asiri, A.M.; Petrosino, F.; Pugliese, V.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Algieri, C.; Chakraborty, S. Synthesis and characterization of blended cellulose acetate membranes. Polymers 2022, 14, 4. [Google Scholar] [CrossRef]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. Sci. Eng. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, A.; Batory, D.; Cichomski, M.; Miletic, A.; Czerniak-Reczulska, M.; Niedzielski, P.; Dudeka, M. Formation of anatase and srilankite mixture as a result of the thermally induced transformation of the a-C:H:TiOx coating. Surf. Coat. Technol. 2020, 400, 126230. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?–Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 44043. [Google Scholar] [CrossRef] [PubMed]
- Satoru, I.; Chu, S.Z.; Wada, K.; Li, D.; Haneda, H. New roots to formation of nanostructures on glass surface through anodic oxidation of sputtered aluminum. Sci. Technol. Adv. Mater. 2003, 4, 269–276. [Google Scholar] [CrossRef]
- Othman, H.; Rashid, S.A.; Ghazi, T.I.M.; Abdullah, N. Dispersion and stabilization of photocatalytic TiO2 nanoparticles in aqueous suspension for coatings applications. J. Nanomater. 2012, 718214. [Google Scholar] [CrossRef]
- Huertas, S.P.; Terpiłowski, K.; Wiśniewska, M.; Zarko, V. Influence of polyvinylpyrrolidone adsorption on stability of silica aqueous suspension–effects of polymer concentration and solid content. Physicochem. Probl. Miner. Process. 2017, 53, 121–135. [Google Scholar] [CrossRef]
- Candamano, S.; Sgambitterra, E.; Lamuta, C.; Pagnotta, L.; Chakraborty, S.; Crea, F. Graphene nanoplatelets in geopolymeric systems: A new dimension of nanocomposites. Mater. Lett. 2019, 236, 550–553. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- De Paola, M.G.; Lopresto, C.G. Waste oils and their transesterification products as novel bio-based phase change materials. J. Phase Change Mater. 2021, 1, 1. [Google Scholar] [CrossRef]
- Rangan, K.; Forbesa, H.; Kumar, N. Inorganic salt hydrates-hydrogel composites as phase change materials for energy storage in buildings. J. Phase Change Mater. 2022, 2, 25–40. [Google Scholar] [CrossRef]
- Meißner, T.; Potthoff, A.; Richter, V. Suspension characterization as important key for toxicological investigations. J. Phys. Conf. Ser. 2009, 170, 012012. [Google Scholar] [CrossRef]
- Franksm, G.V.; Meagher, L. The isoelectric points of sapphire crystals and alpha-alumina powder. Coll. Surf. A Physicochem. Eng. Asp. 2003, 214, 99–110. [Google Scholar] [CrossRef]
- Djafer, L.; Ayral, A.; Ouagued, A. Robust synthesis and performance of a titania based ultrafiltration membrane with photocatalytic properties. Sep. Purif. Technol. 2010, 75, 198–203. [Google Scholar] [CrossRef]
- Grilli, R.; Di Camillo, D.; Lozzi, L.; Horovitz, I.; Mamane, H.; Avisar, D.; Baker, M.A. Surface Characterization and photocatalytic performance of N-doped TiO2 thin films deposited onto 200 nm pore size alumina membranes by sol–gel methods. Mater. Chem. Phys. 2015, 159, 25–37. [Google Scholar] [CrossRef]
- Ma, N.; Quan, X.; Zhang, Y.; Chen, S.; Zhao, H. Integration of separation and photocatalysis using an inorganic membrane modified with Si-doped TiO2 for water purification. J. Membr. Sci. 2009, 335, 58–67. [Google Scholar] [CrossRef]
- Lau, Y.Y.; Wong, Y.S.; Teng, T.T.; Morad, N.; Rafatullah, M.; Ong, S.A. Degradation of cationic and anionic dyes in coagulation–flocculation process using bi-functionalized silica hybrid with aluminum-ferric as auxiliary agent. RSC Adv. 2015, 42, 34206. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Azuwa, M. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mater. Sci. Semicond. Process. 2017, 57, 157. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Likodimos, V.; Aloupogiannis, P.; Falaras, P. Hybrid Ultrafiltration/Photocatalytic Membranes for Efficient Water Treatment. Ind. Eng. Chem. Res. 2013, 52, 13938–13947. [Google Scholar] [CrossRef]
- Moustakas, N.G.; Katsaros, F.K.; Kontos, A.G.; Romanos, G.E.; Dionysiou, D.D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Curcio, S. Applications of ANNs on photocatalytic reactors: A brief overview. J. Phase Change Mater. 2022, 2, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Shi, F.; Huang, W.; Fan, C. Synthesis of high quality TiO2 membranes on alum.ina supports and their photocatalytic activity. Thin Solid Films 2012, 520, 2488. [Google Scholar] [CrossRef]
- Mathumba, P.; Maziya, K.; Kuvarega, A.T.; Dlamini, L.N.; Malinga, S.P. Photocatalytic degradation of a basic dye in water by nanostructured HPEI/TiO2 containing membranes. Water SA 2020, 46, 500–505. [Google Scholar] [CrossRef]
- Lei, P.; Wang, F.; Gao, X.; Ding, Y.; Zhang, S.; Zhao, J.; Liu, S.; Yang, M. Immobilization of TiO2 nanoparticles in polymeric substrates by chemical bonding for multi-cycle photodegradation of organic pollutants. J. Hazard. Mater. 2012, 227–228, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Ouyang, L. Photocatalytic activity and hydroxyl radical formation of carbon-doped TiO2 nanocrystalline: Effect of calcination temperature. Chem. Eng. J. 2009, 148, 248–253. [Google Scholar] [CrossRef]
- Canute, S.; Nieto-Maestre, J.; Kanel, S. Developing Novel Electro-catalysts for Green Hydrogen Generation. J. Phase Change Mater. 2022, 2, 52–58. [Google Scholar] [CrossRef]
- Das, P.; Saha, S. Characterization of CdS, CdXZn1-XS and ZnS Nanocrystallites Grown by Chemical Route. J. Phase Change Mater. 2022, 2, 4–11. [Google Scholar] [CrossRef]


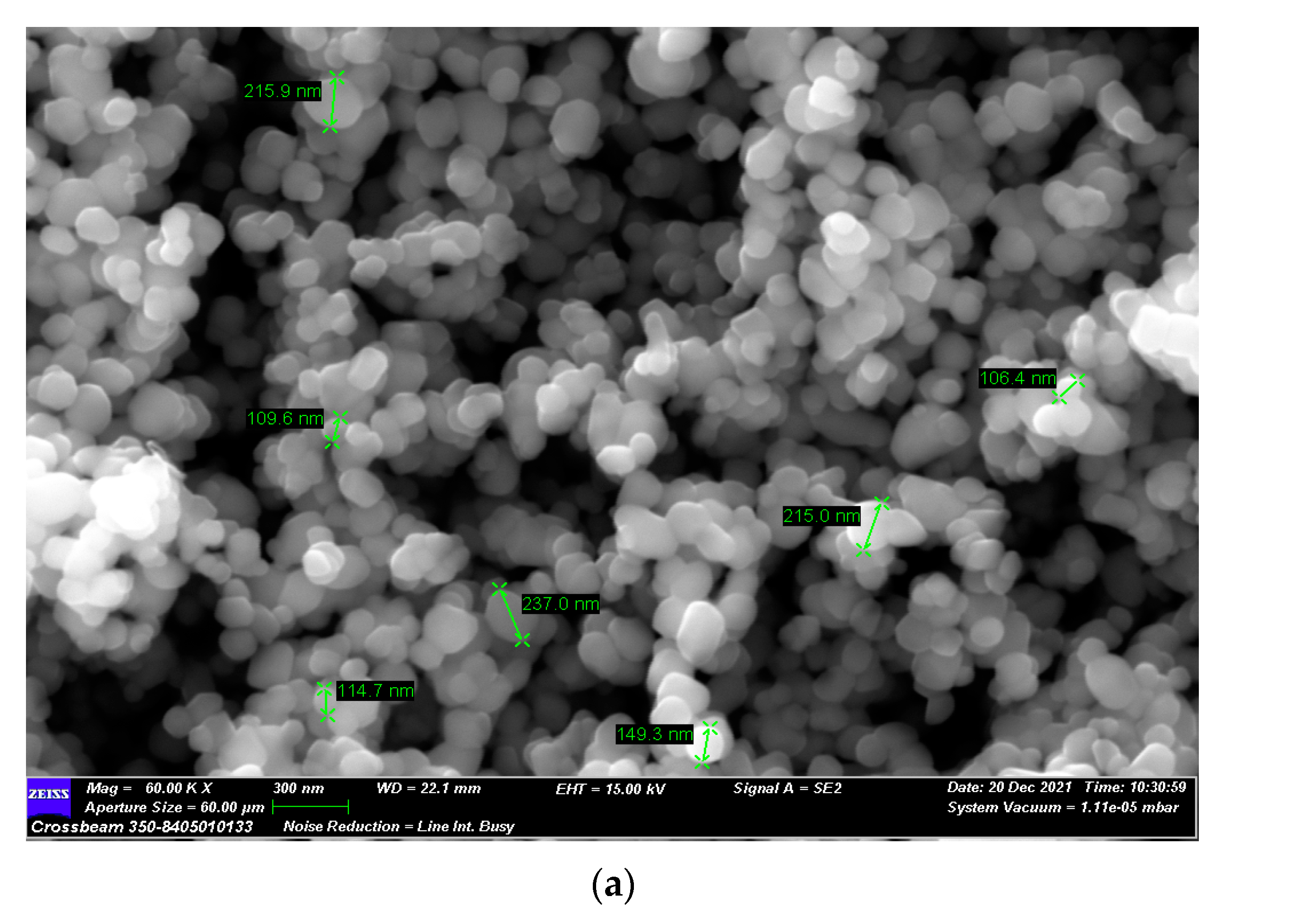
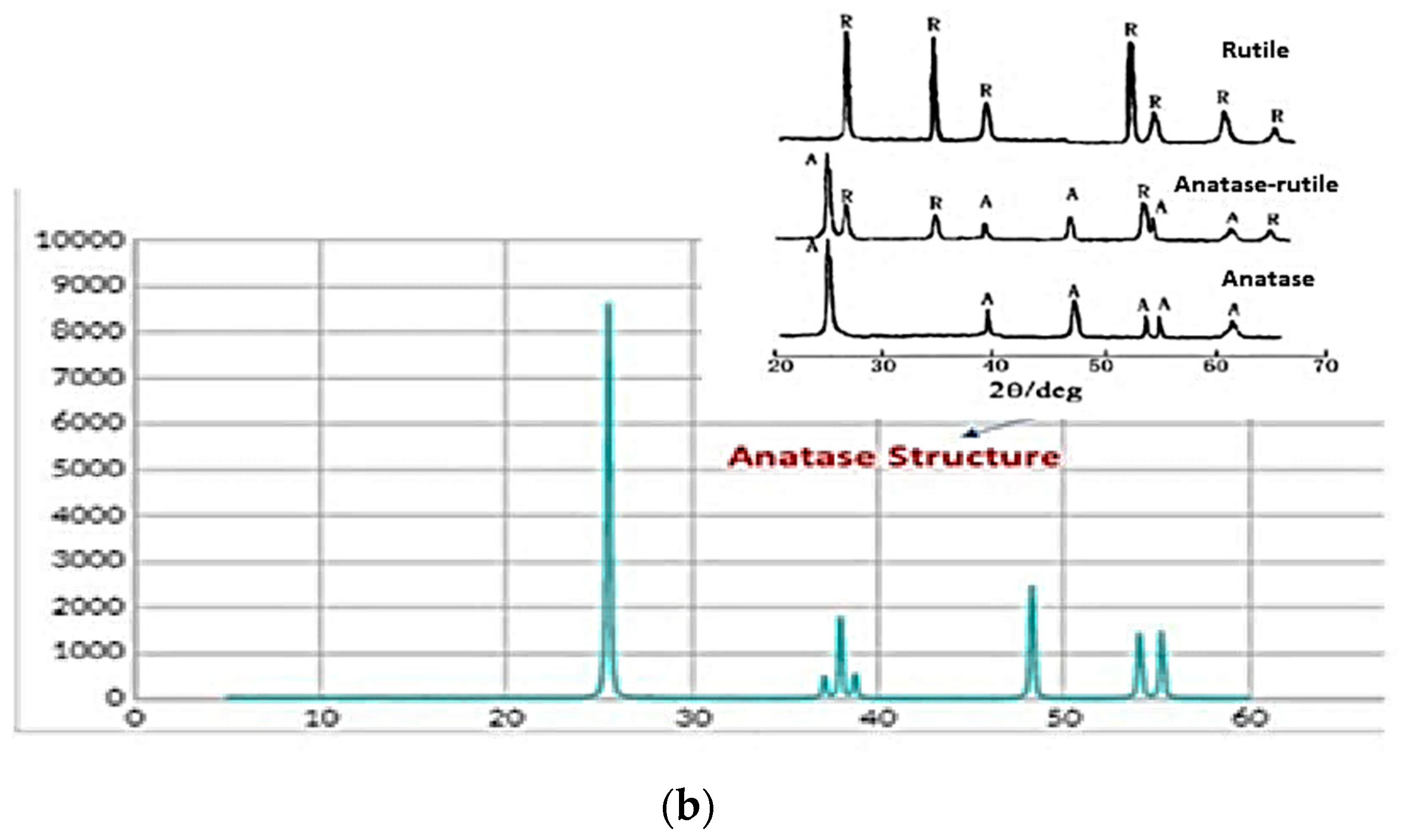
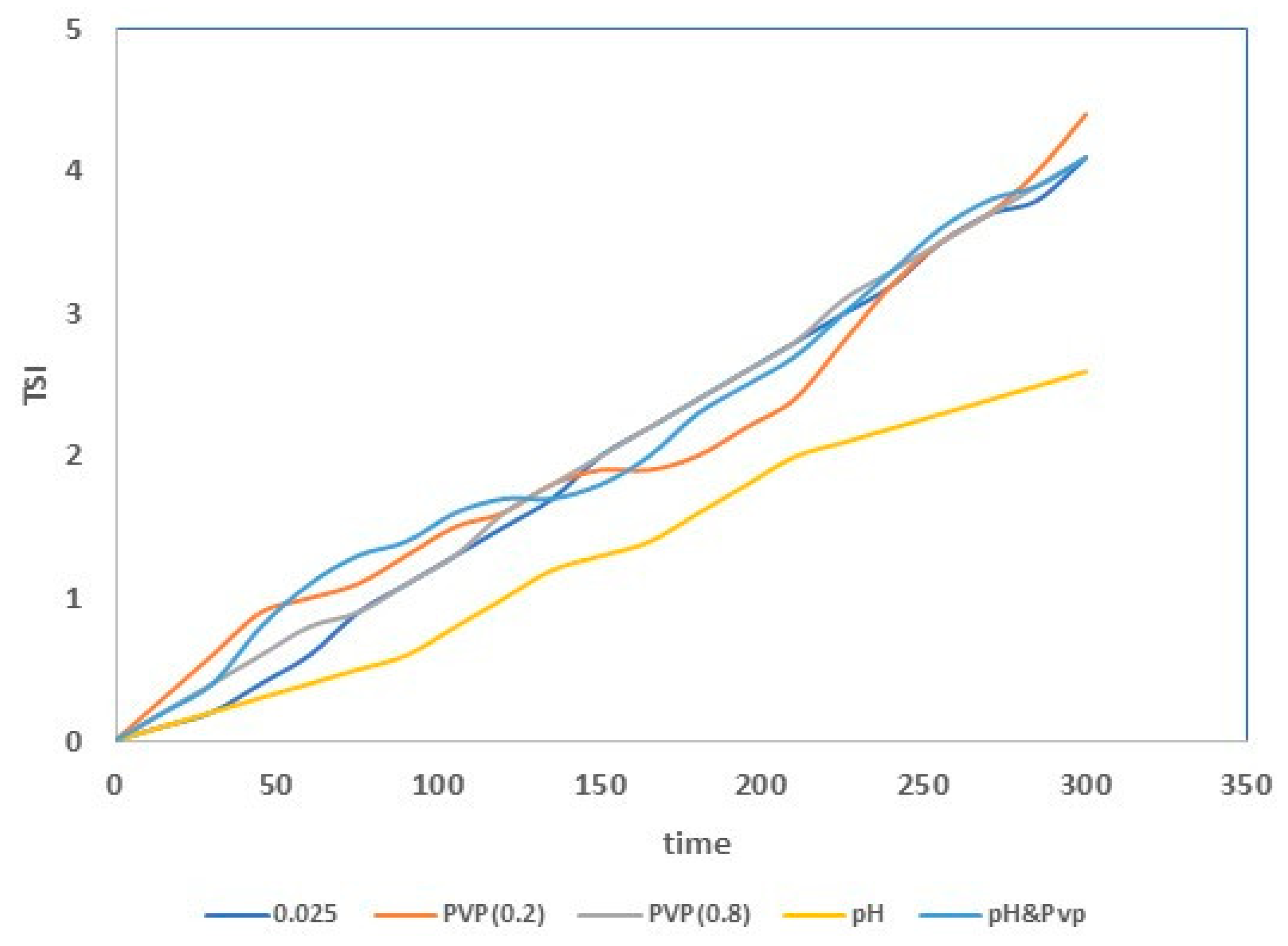
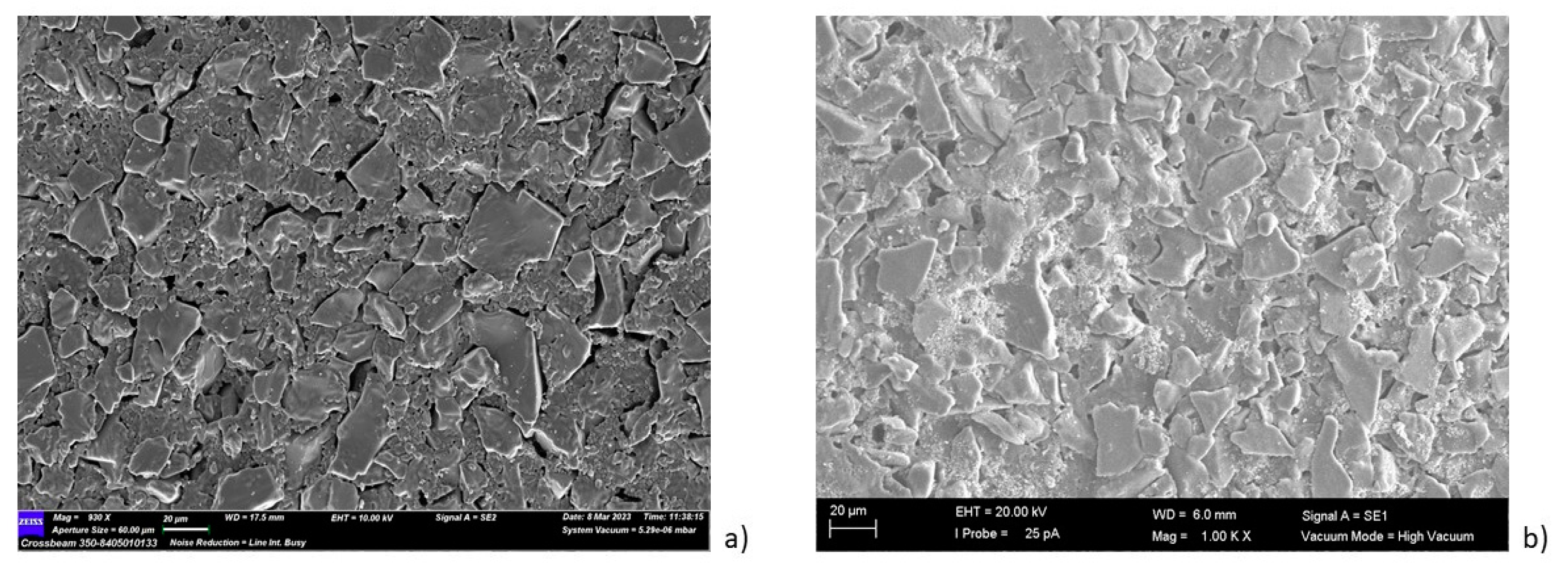
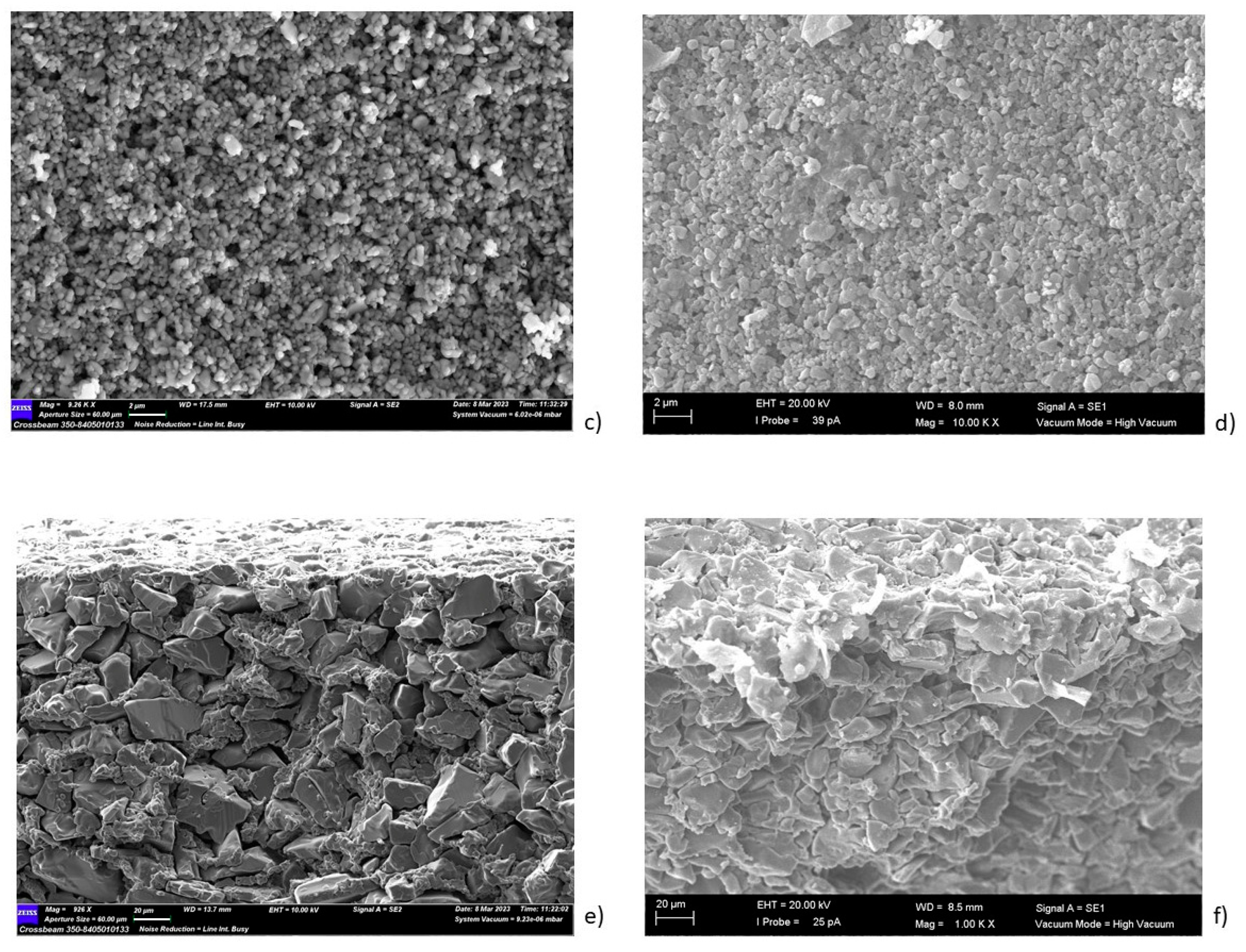
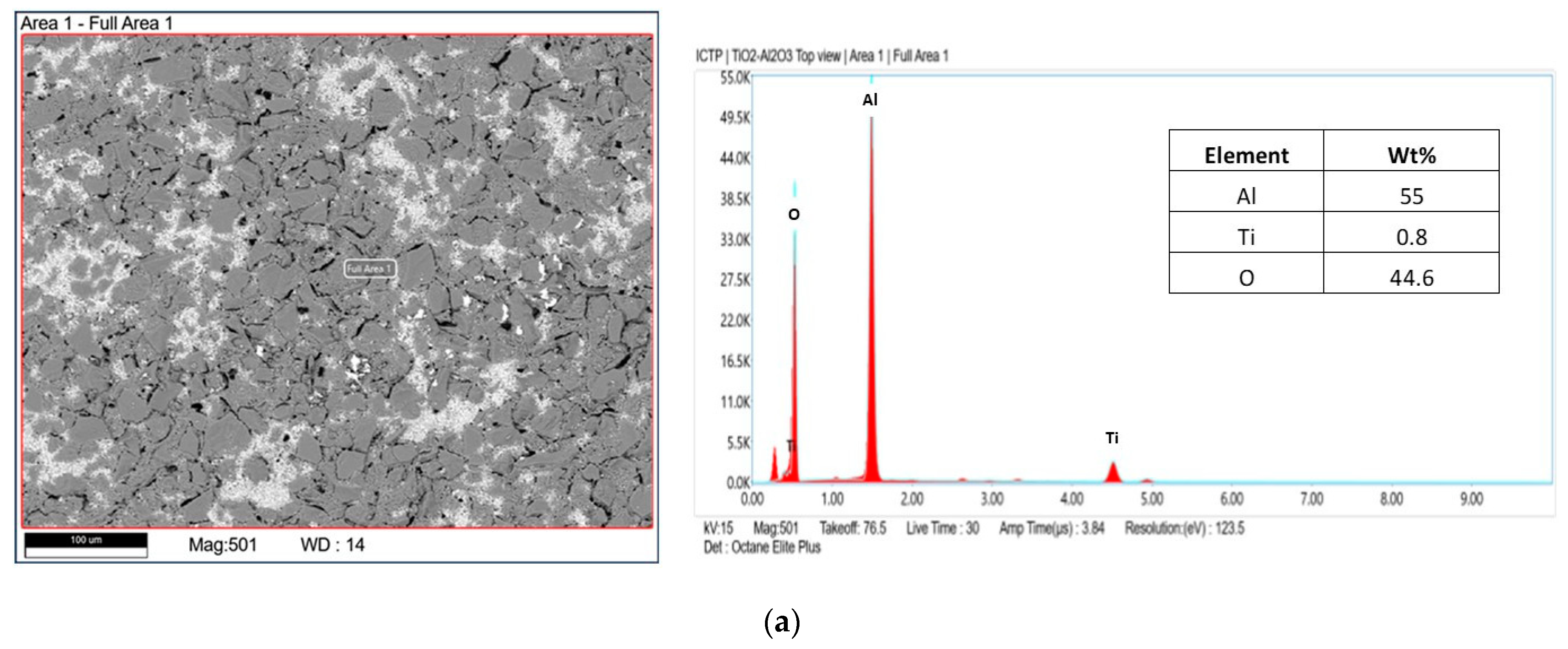

| Sample | Inorganic Powder | Liquid | TiO2 Concentration (wt%) | Sonication | PVP Solution (V = 5 mL) | HNO3 (wt. %) | pH |
|---|---|---|---|---|---|---|---|
| 1 | TiO2 | H2O | 0.025 | No | No | No | 6.5 |
| 2 | TiO2 | H2O | 0.25 | No | No | No | 6.5 |
| TiO2 | H2O | 0.10 | No | No | No | 6.5 | |
| 4 | TiO2 | H2O | 0.05 | No | No | No | 6.5 |
| 5 | TiO2 | H2O | 0.025 | Yes | No | No | 6.5 |
| 6 | TiO2 | H2O | 0.025 | No | PVP(0.2 wt. %) | No | 6.5 |
| 7 | TiO2 | H2O | 0.025 | No | PVP (0.8 wt %) | No | 6.5 |
| 8 | TiO2 | H2O | 0.025 | No | No | Yes | 4.5 |
| TiO2 Suspension (wt. %) | TSI (3 h) | TSI (5 h) |
|---|---|---|
| 0.025 | 2.4 | 4.1 |
| 0.05 | 2.6 | 4.5 |
| 0.10 | 4.3 | 6.7 |
| 0.25 | 4.4 | 7.0 |
| Sample | Zeta Potential [mV] |
|---|---|
| TiO2 suspension (0.025 wt. %; pH = 6.5) | −20.8 |
| TiO2-PVP suspension (0.025 wt. %; pH = 6.5; PVP = 0.2 wt. %) | −24.4 |
| TiO2-HNO3 suspension (0.025 wt. %; pH = 4.5) | −37.6 |
| Sample | Before Coating Hydraulic Permeance (Lm−2 h−1 bar1) | After Coating Hydraulic Permeance (Lm−2 h−1 bar1) | Reduction (%) | Ref. |
|---|---|---|---|---|
| TiO2 film on α-Al2O3 membrane (pore size = 0.2 μm) | 1800 | 150 | 92 | [46] |
| N-doped TiO2 film on α-Al2O3 membrane (pore size = 0.2 μm) | 3800 | 160 | 58 | [47] |
| Si-doped TiO2 film on α-Al2O3 membrane (pore size = 0.1 μm) | 1950 | 340 | 83 | [48] |
| TiO2 on α-Al2O3membrane (asymmetric support: external pore size = 3 µm, internal pore size = 0. 2 µm) | 1500 | 1330 | 11 | This work |
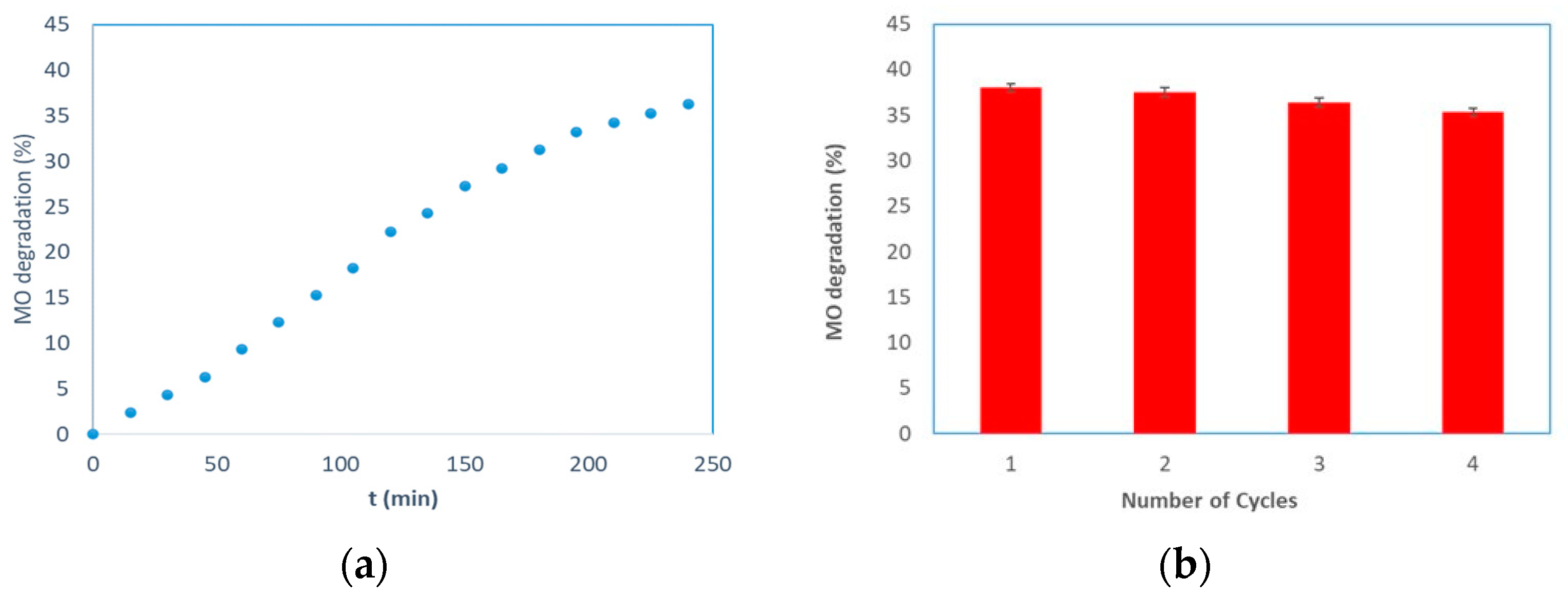
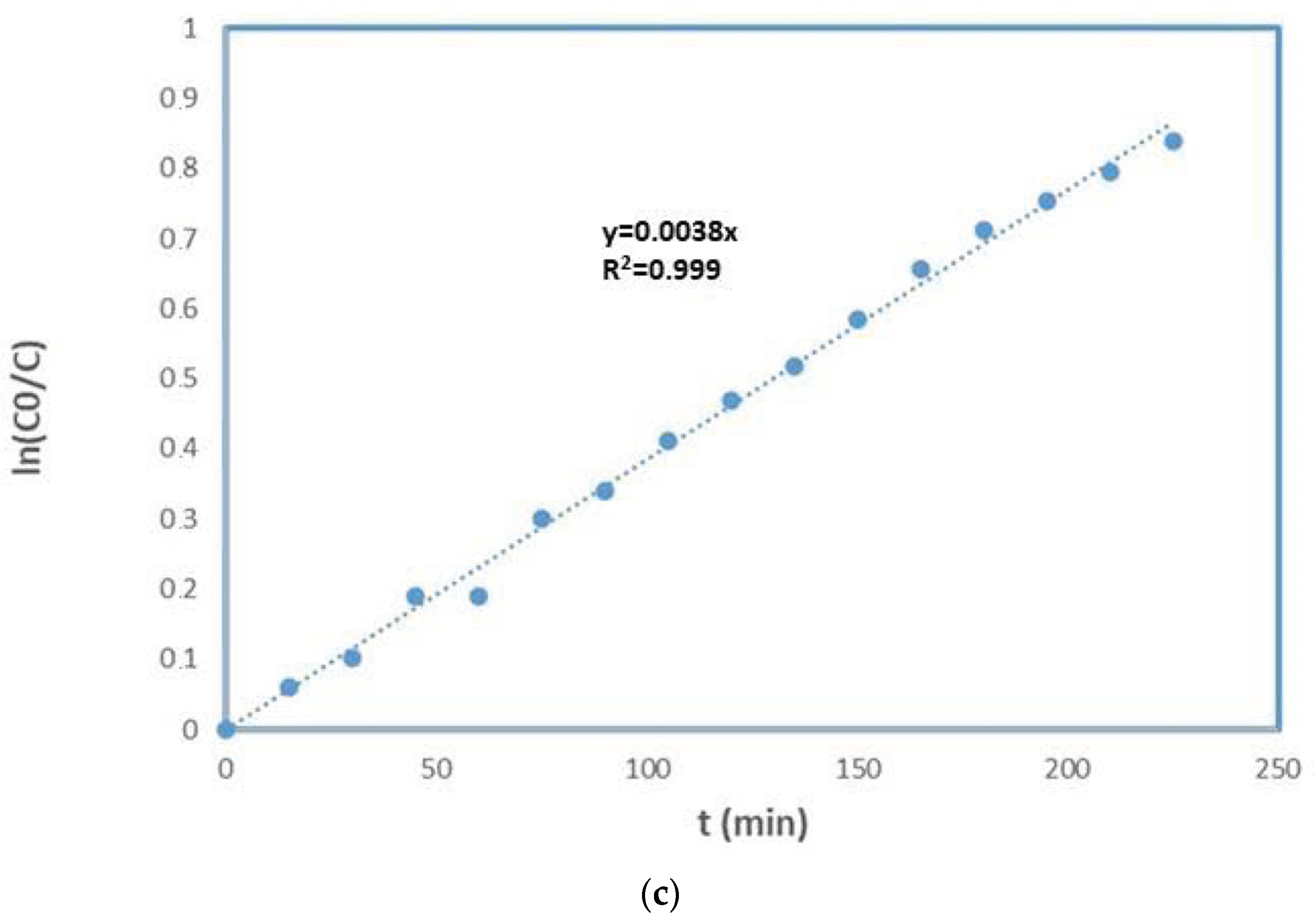
| Sample | MO (mg/L) | TiO2 (mg) | Irradiation Time (min) | MO Degradation (%) | Ref |
|---|---|---|---|---|---|
| PES-TiO2 | 10 | 1000 | 240 | 25 | [55] |
| α-Al2O3-TiO2 * | 6.5 | 54 | 60 | 20 | [56] |
| γ-Al2O3-TiO2 * | 2 | 20 | - | 50 | [57] |
| PS-TiO2 | 1 | 0.3 | 180 | 4 | [58] |
| α-Al2O3-TiO2 * | 7.8 | - | 360 | 60 | [59] |
| α-Al2O3-TiO2 ** | 1 | 10 | 240 | 36 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blasi, M.; Algieri, C.; Chakraborty, S.; Calabrò, V. Application of Turbiscan Stability Index for the Preparation of Alumina Photocatalytic Membranes for Dye Removal. Membranes 2023, 13, 400. https://doi.org/10.3390/membranes13040400
Blasi M, Algieri C, Chakraborty S, Calabrò V. Application of Turbiscan Stability Index for the Preparation of Alumina Photocatalytic Membranes for Dye Removal. Membranes. 2023; 13(4):400. https://doi.org/10.3390/membranes13040400
Chicago/Turabian StyleBlasi, Marida, Catia Algieri, Sudip Chakraborty, and Vincenza Calabrò. 2023. "Application of Turbiscan Stability Index for the Preparation of Alumina Photocatalytic Membranes for Dye Removal" Membranes 13, no. 4: 400. https://doi.org/10.3390/membranes13040400
APA StyleBlasi, M., Algieri, C., Chakraborty, S., & Calabrò, V. (2023). Application of Turbiscan Stability Index for the Preparation of Alumina Photocatalytic Membranes for Dye Removal. Membranes, 13(4), 400. https://doi.org/10.3390/membranes13040400












