High-Performance γ-Al2O3 Multichannel Tube-Type Tight Ultrafiltration Membrane Using a Modified Sol-Gel Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Boehmite Sol
2.2. Characterization
3. Results
3.1. Effect of PVA Addition on the Thickness of γ-Al2O3 Membranes
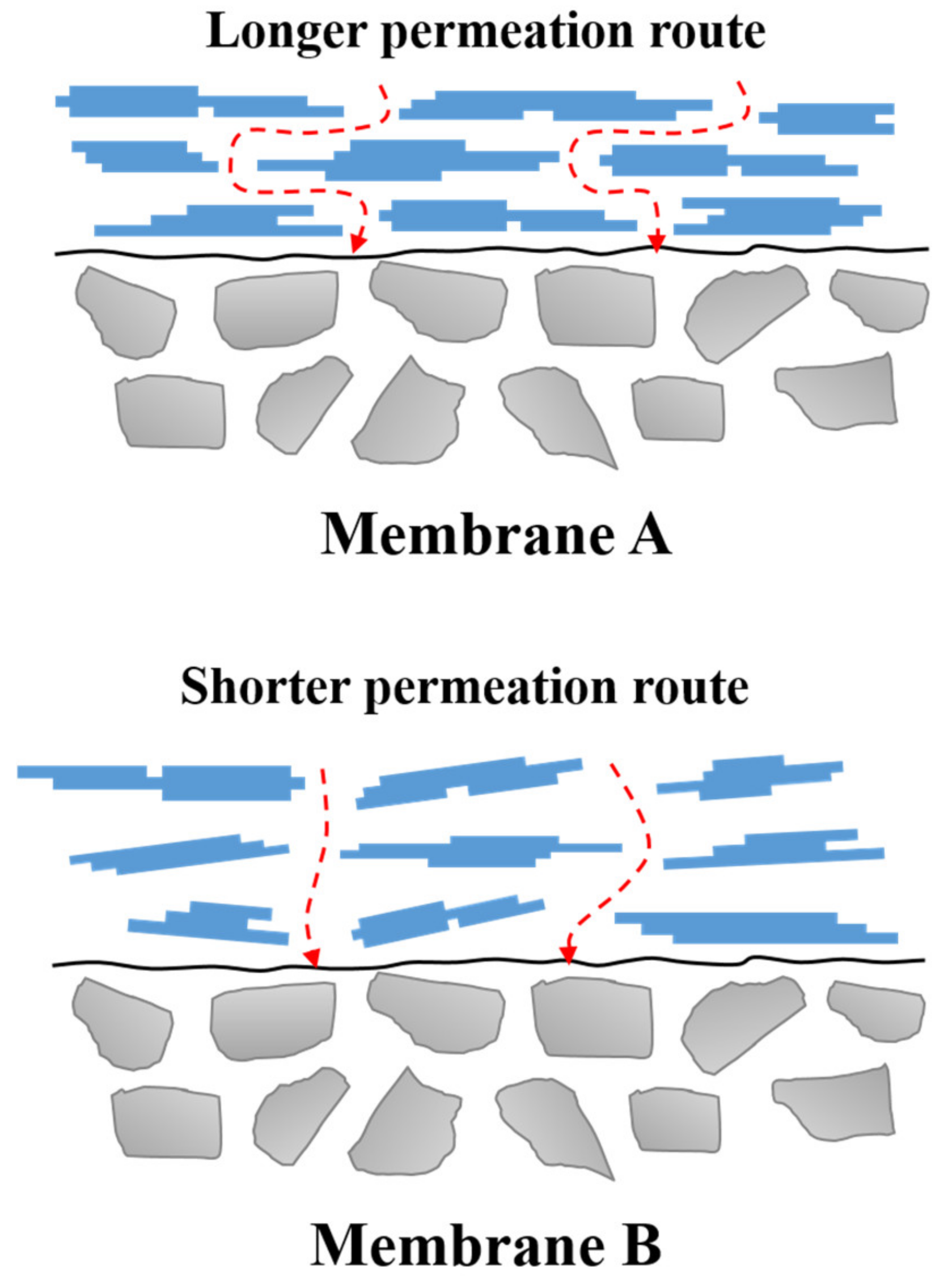
3.2. Influence of the Fabrication Routes on the Properties of γ-Al2O3 Membranes
3.3. Performance of the γ-Al2O3 Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Chen, P.; Zhou, M.; Zhong, Z.; Zhang, F.; Xing, W. Tight ultrafiltration ceramic membrane for separation of dyes and mixed salts (both NaCl/Na2SO4) in textile wastewater treatment. Ind. Eng. Chem. Res. 2017, 56, 7070–7079. [Google Scholar] [CrossRef]
- Khemakhem, M.; Khemakhem, S.; Ayedi, S.; Amar, R. Ben Study of ceramic ultrafiltration membrane support based on phosphate industry subproduct: Application for the cuttlefish conditioning effluents treatment. Ceram. Int. 2011, 37, 3617–3625. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J. Alpha-Alumina Inorganic Membrane Support and Method of Making the Same. U.S. Patent Application 12/001,263, 12 June 2008. [Google Scholar]
- Ebrahimi, M.; Kerker, S.; Schmitz, O.; Schmidt, A.A.; Czermak, P. Evaluation of the fouling potential of ceramic membrane configurations designed for the treatment of oilfield produced water. Sep. Sci. Technol. 2018, 53, 349–363. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.M.; Xu, Z.L.; Cao, Y.; Dong, Z.Q.; Shi, X.L. Preparation, characterization and solvent resistance of γ-Al2O3/α-Al2O3 inorganic hollow fiber nanofiltration membrane. J. Memb. Sci. 2016, 503, 69–80. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.X.; Luo, C.; Sun, L.D.; Zhang, Y.W.; Duan, W.T.; Liu, H.C.; Yan, C.H. Facile synthesis for ordered mesoporous γ-aluminas with high thermal stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, Y.; Vidal, L.; Valtchev, V.; Lebeau, B. Preparation of γ-Al2O3 film by high temperature transformation of nanosized γ-AlOOH precursors. New J. Chem. 2009, 33, 2255–2260. [Google Scholar] [CrossRef]
- Lee, H.C.; Kim, H.J.; Rhee, C.H.; Lee, K.H.; Lee, J.S.; Chung, S.H. Synthesis of nanostructured γ-alumina with a cationic surfactant and controlled amounts of water. Microporous Mesoporous Mater. 2005, 79, 61–68. [Google Scholar] [CrossRef]
- Bayat, A.; Mahdavi, H.R.; Kazemimoghaddam, M.; Mohammadi, T. Preparation and characterization of γ-alumina ceramic ultrafiltration membranes for pretreatment of oily wastewater. Desalin. Water Treat. 2016, 57, 24322–24332. [Google Scholar] [CrossRef]
- Qi, H.; Niu, S.; Jiang, X.; Xu, N. Enhanced performance of a macroporous ceramic support for nanofiltration by using α-Al2O3 with narrow size distribution. Ceram. Int. 2013, 39, 2463–2471. [Google Scholar] [CrossRef]
- Schaep, J.; Vandecasteele, C.; Peeters, B.; Luyten, J.; Dotremont, C.; Roels, D. Characteristics and retention properties of a mesoporous γ-Al2O3 membrane for nanofiltration. J. Memb. Sci. 1999, 163, 229–237. [Google Scholar] [CrossRef]
- Facciotti, M.; Boffa, V.; Magnacca, G.; Jørgensen, L.B.; Kristensen, P.K.; Farsi, A.; König, K.; Christensen, M.L.; Yue, Y. Deposition of thin ultrafiltration membranes on commercial SiC microfiltration tubes. Ceram. Int. 2014, 40, 3277–3285. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Ten Elshof, J.E.; Benes, N.E.; Keizer, K. Development and comparative study of different nanofiltration membranes for recovery of highly charged large ions. Desalination 2002, 144, 41–46. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Schmuhl, R.; Keizer, K.; Ten Elshof, J.E.; Blank, D.H.A. Pore size and surface chemistry effects on the transport of hydrophobic and hydrophilic solvents through mesoporous γ-alumina and silica MCM-48. J. Memb. Sci. 2003, 225, 177–186. [Google Scholar] [CrossRef]
- Van Gestel, T.; Kruidhof, H.; Blank, D.H.A.; Bouwmeester, H.J.M. ZrO2 and TiO2 membranes for nanofiltration and pervaporation. Part 1. Preparation and characterization of a corrosion-resistant ZrO2 nanofiltration membrane with a MWCO < 300. J. Memb. Sci. 2006, 284, 128–136. [Google Scholar] [CrossRef]
- Othman, M.R.; Ahmad, A.L.; Mukhtar, A.H. Characteristics of unsupported alumina membrane prepared using sol-gel technique. ASEAN J. Sci. Technol. Dev. 2017, 18. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Zamani, F.; Ashrafizadeh, S.N. Removal of oily hydrocarbon contaminants from wastewater by γ-alumina nanofiltration membranes. Desalin. Water Treat. 2010, 20, 80–85. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Y.; Chen, X.; Qiu, M.; Fan, Y. Modified colloidal sol-gel process for fabrication of titania nanofiltration membranes with organic additives. J. Memb. Sci. 2015, 476, 432–441. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Lin, Y.; Cai, Y.; Qiu, M.; Fan, Y. Preparation of high-flux γ-alumina nanofiltration membranes by using a modified sol-gel method. Microporous Mesoporous Mater. 2015, 214, 195–203. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.; Page, K.L.; Pearce, C.I.; Bowden, M.E.; Graham, T.R.; Shen, Z.; Li, P.; Wang, Z.; Kerisit, S.; et al. Size and morphology controlled synthesis of boehmite nanoplates and crystal growth mechanisms. Cryst. Growth Des. 2018, 18, 3596–3606. [Google Scholar] [CrossRef]
- Naseer, D.; Ha, J.-H.; Lee, J.; Park, C.; Song, I.-H. Effect of the peptization process and thermal treatment on the sol-gel preparation of mesoporous α-alumina membranes. Membranes 2022, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H. Inorganic membranes-synthesis, characteristics and applications. J. Memb. Sci. 1997, 137, 277. [Google Scholar] [CrossRef]
- Ecsedi, Z.; Lazǎu, I.; Pǎcurariu, C. Microstructural analysis of the effects of polyvinyl alcohol content on the porosity of sol-gel derived alumina ceramics. Microporous Mesoporous Mater. 2009, 118, 453–457. [Google Scholar] [CrossRef]
- Park, J.; Lee, D.; Park, K.; Kim, T.; Sung, J.; Song, K. Effect of PVA addition on the properties of alumina membranes. Korean Chem. Eng. Res. 2004, 42, 570–576. [Google Scholar]
- Perez-Catan, S.; Guraya, M. High Porous Gamma-Alumina Synthesized by a Modified Sol-Gel Technique. Int. J. Mater. Sci. 2015, 5, 33–39. [Google Scholar] [CrossRef]
- Naseer, D.; Ha, J.-H.; Lee, J.; Song, I.-H. Preparation of Al2O3 multichannel cylindrical-tube-type microfiltration membrane with surface modification. Appl. Sci. 2022, 12, 7993. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, J.; Wang, X.; Tao, H.; Ge, L. Formation of poly(vinylidene fluoride) (PVDF) membranes via thermally induced phase separation. Desalination 2006, 192, 160–167. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z.; Wong, F.S. A new method for the prediction of pore size distribution and MWCO of ultrafiltration membranes. J. Memb. Sci. 2006, 279, 558–569. [Google Scholar] [CrossRef]
- Gu, Y.; Meng, G. A model for ceramic membrane formation by dip-coating. J. Eur. Ceram. Soc. 1999, 19, 1961–1966. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ray, P. Studies on surface tension of poly (vinyl alcohol): Effect of concentration, temperature, and addition of chaotropic agents. J. Appl. Polym. Sci. 2004, 93, 122–130. [Google Scholar] [CrossRef]
- Levänen, E.; Mäntylä, T.; Mikkola, P.; Rosenholm, J.B. Influence of additives on capillary absorption of aqueous solutions into asymmetric porous ceramic substrate. J. Colloid Interface Sci. 2001, 234, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, P.; Levänen, E.; Rosenholm, J.B.; Mäntylä, T. Colloidal processing of aluminum oxide powder for membrane applications. Ceram. Int. 2003, 29, 393–401. [Google Scholar] [CrossRef]
- Qin, W.; Guan, K.; Lei, B.; Liu, Y.; Peng, C.; Wu, J. One-step coating and characterization of α-Al2O3 microfiltration membrane. J. Memb. Sci. 2015, 490, 160–168. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Rajabi, L.; Zinadini, S.; Derakhshan, A.A. Boehmite nanoparticles as a new nanofiller for preparation of antifouling mixed matrix membranes. J. Memb. Sci. 2012, 401–402, 132–143. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Chibowski, S.; Urban, T.; Sternik, D. Investigation of the alumina properties with adsorbed polyvinyl alcohol. J. Therm. Anal. Calorim. 2011, 103, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Marek, K. Chemical Properties of Material Surfaces; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Pisani, L. Simple expression for the tortuosity of porous media. Transp. Porous Media 2011, 88, 193–203. [Google Scholar] [CrossRef]
- Ardeshiri, F.; Salehi, S.; Peyravi, M.; Jahanshahi, M.; Amiri, A.; Rad, A.S. PVDF membrane assisted by modified hydrophobic ZnO nanoparticle for membrane distillation. Asia-Pac. J. Chem. Eng. 2018, 13, e2196. [Google Scholar] [CrossRef]
- Damak, K.; Ayadi, A.; Zeghmati, B.; Schmitz, P. A new Navier-Stokes and Darcy’s law combined model for fluid flow in crossflow filtration tubular membranes. Desalination 2004, 161, 67–77. [Google Scholar] [CrossRef]
- Shang, R.; Goulas, A.; Tang, C.Y.; de Frias Serra, X.; Rietveld, L.C.; Heijman, S.G.J. Atmospheric pressure atomic layer deposition for tight ceramic nanofiltration membranes: Synthesis and application in water purification. J. Memb. Sci. 2017, 528, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, P.; Butté, A.; Livingston, A.G. An improved phenomenological model for prediction of solvent permeation through ceramic NF and UF membranes. J. Memb. Sci. 2012, 415–416, 444–458. [Google Scholar] [CrossRef]
- Alami-Younssi, S.; Larbot, A.; Persin, M.; Sarrazin, J.; Cot, L. Gamma alumina nanofiltration membrane. Application to the rejection of metallic cations. J. Memb. Sci. 1994, 91, 87–95. [Google Scholar] [CrossRef]
- Larbot, A.; Alami-Younssi, S.; Persin, M.; Sarrazin, J.; Cot, L. Preparation of a γ-alumina nanofiltration membrane. J. Memb. Sci. 1994, 97, 167–173. [Google Scholar] [CrossRef]









| Sintering Temperature | Specific Surface Area | Pore Size | Thickness | Permeability | Reference |
|---|---|---|---|---|---|
| (°C) | (m2/g) | (nm) | (μm) | (LMH/bar) | |
| 500 | - | 4.4 | - | 4.5 | [11] |
| 600 | 271 | 5.5 | ~7 | 5 | [12] |
| 600 | 205 | 5.5 | 5.6 | 9 | [13] |
| 600 | - | 2.8 | ~1 | 1.1 | [15] |
| 500 | - | 1.9 | 1.5 | 5.4 | [18] |
| 600 | 260 | 2.7 | ~2 | 7.4 | [20] |
| 600 | 329.3 | 2.7 | ~1.8 | 18.2 | Our work |
| Sample | |||
|---|---|---|---|
| Membrane S | Membrane A | Membrane B | |
| Specific surface area (m2/g) | 226.17 | 293.84 | 329.36 |
| Mean pore size (nm) | 7.3329 | 7.0191 | 7.4434 |
| Total pore volume (cm3/g) | 0.4146 | 0.5413 | 0.6129 |
| Sample | Porosity (%) |
|---|---|
| Membrane S | 29.2 |
| Membrane A | 37.3 |
| Membrane B | 40.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseer, D.; Ha, J.-H.; Lee, J.; Lee, H.J.; Song, I.-H. High-Performance γ-Al2O3 Multichannel Tube-Type Tight Ultrafiltration Membrane Using a Modified Sol-Gel Method. Membranes 2023, 13, 405. https://doi.org/10.3390/membranes13040405
Naseer D, Ha J-H, Lee J, Lee HJ, Song I-H. High-Performance γ-Al2O3 Multichannel Tube-Type Tight Ultrafiltration Membrane Using a Modified Sol-Gel Method. Membranes. 2023; 13(4):405. https://doi.org/10.3390/membranes13040405
Chicago/Turabian StyleNaseer, Danyal, Jang-Hoon Ha, Jongman Lee, Hong Joo Lee, and In-Hyuck Song. 2023. "High-Performance γ-Al2O3 Multichannel Tube-Type Tight Ultrafiltration Membrane Using a Modified Sol-Gel Method" Membranes 13, no. 4: 405. https://doi.org/10.3390/membranes13040405






