Preparation and Characterization of a Janus Membrane with an “Integrated” Structure and Adjustable Hydrophilic Layer Thickness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method Used for Membrane Preparation
2.2.1. Hydrophobic Membrane Preparation
2.2.2. Preparation of Janus Membrane



2.3. Membrane Characterization
2.4. Wettability of Membrane
2.5. Separation of Oil-Water Emulsions
3. Results and Discussion
3.1. Morphological Structure
3.2. Chemical Composition
3.3. Asymmetric Wettability
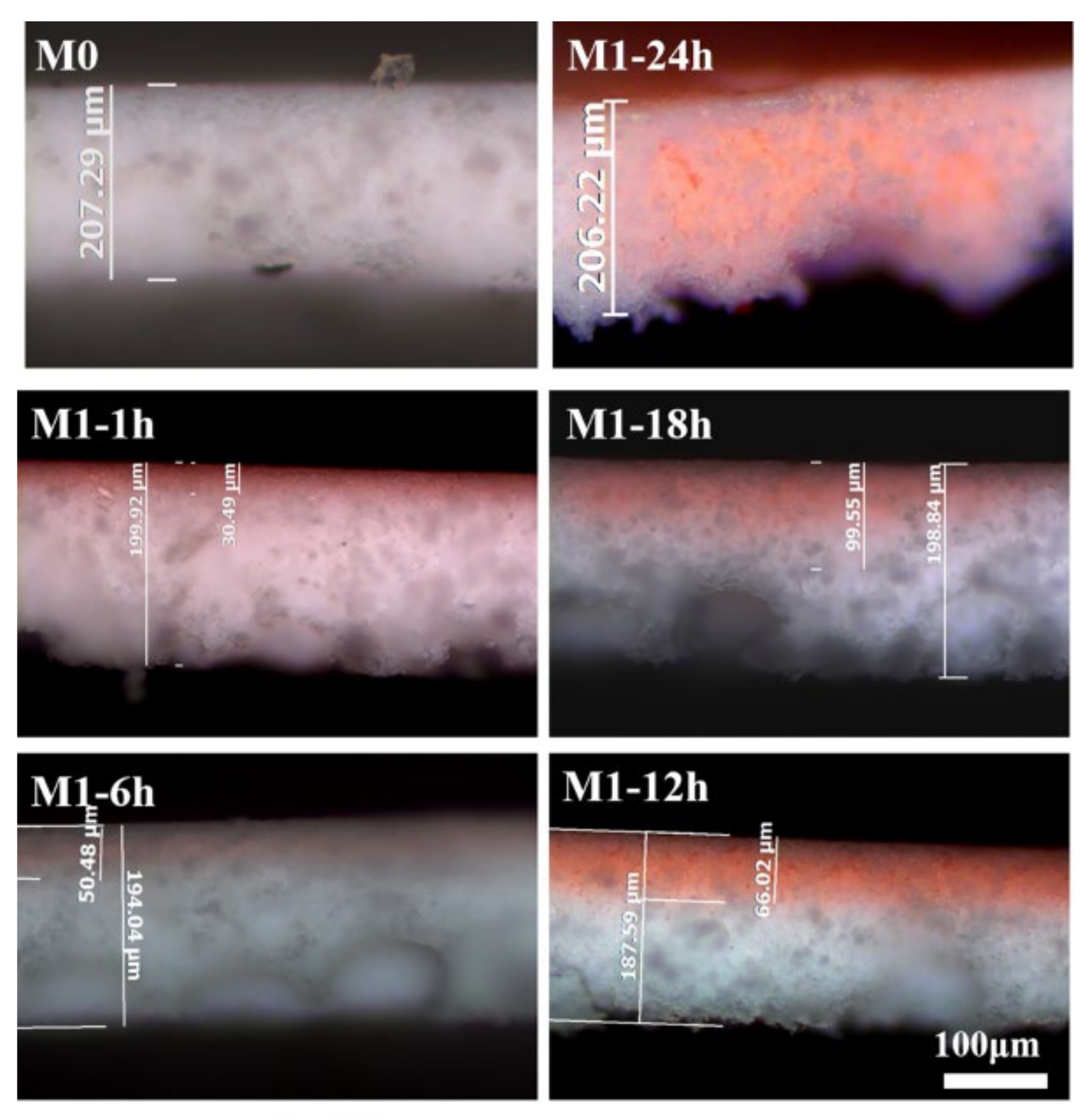
3.4. Hydrophilic Layer Thickness Control
3.5. Oil–Water Emulsions Separation
3.6. The Membrane-Stability Test
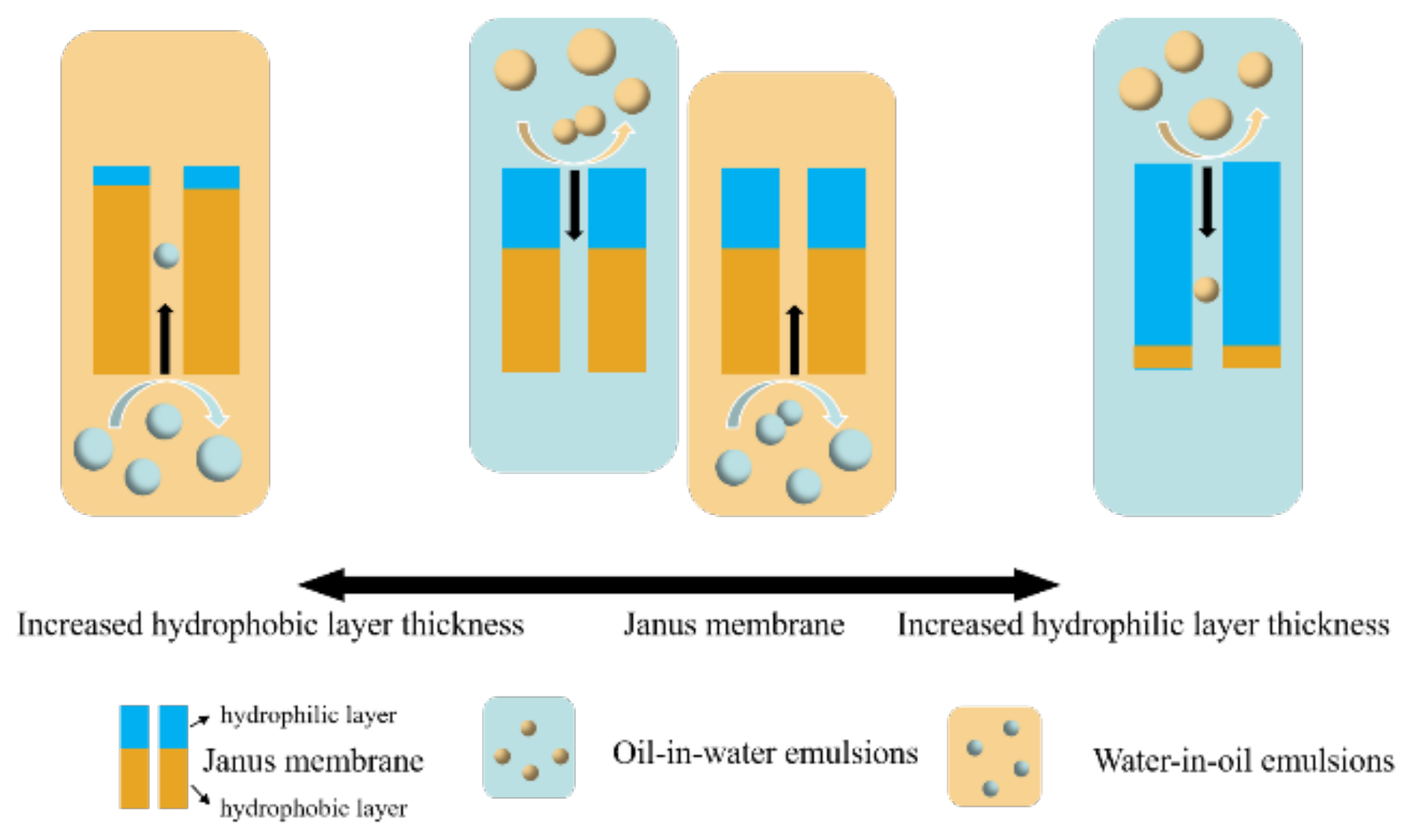
3.7. Separation Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, W.; Huang, J.; Li, S.; Ge, M.; Teng, L.; Chen, Z.; Lai, Y. Advanced materials with special wettability toward intelligent oily wastewater remediation. ACS Appl. Mater. Interfaces 2020, 13, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Tanudjaja, H.J.; Ouda, M.; Hasan, S.W.; Chew, J.W. Membrane fouling mitigation techniques for oily wastewater: A short review. J. Water Process Eng. 2021, 43, 102293. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Saad, M.; Kamil, M.; Abdurahman, N.; Yunus, R.M.; Awad, O.I. An overview of recent advances in state-of-the-art techniques in the demulsification of crude oil emulsions. Processes 2019, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Abdulredha, M.M.; Hussain, S.A.; Abdullah, L.C.; Tee Lee, H. Water-in-oil emulsion stability and demulsification via surface-active compounds: A review. J. Pet. Sci. Eng. 2021, 209, 109848. [Google Scholar] [CrossRef]
- Yuan, X.-T.; Xu, C.-X.; Geng, H.-Z.; Ji, Q.; Wang, L.; He, B.; Jiang, Y.; Kong, J.; Li, J. Multifunctional PVDF/CNT/GO mixed matrix membranes for ultrafiltration and fouling detection. J. Hazard. Mater. 2020, 384, 120978. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhao, X.; Sun, M.; Hao, H. Research Advances in the Synthesis, Application, Assembly, and Calculation of Janus Materials. Ind. Eng. Chem. Res. 2021, 60, 1071–1095. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, K.-J.; Japip, S.; Chung, T.-S. Can composite Janus membranes with an ultrathin dense hydrophilic layer resist wetting in membrane distillation? Environ. Sci. Technol. 2020, 54, 12713–12722. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Kharraz, J.A.; Choi, P.J.; Guo, J.; Deka, B.J.; An, A.K. Superhydrophobic membrane by hierarchically structured PDMS-POSS electrospray coating with cauliflower-shaped beads for enhanced MD performance. J. Membr. Sci. 2020, 597, 117638. [Google Scholar] [CrossRef]
- Li, X.; Cao, M.; Shan, H.; Handan Tezel, F.; Li, B. Facile and scalable fabrication of superhydrophobic and superoleophilic PDMS-co-PMHS coating on porous substrates for highly effective oil/water separation. Chem. Eng. J. 2019, 358, 1101–1113. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, J.; Wang, F.; Li, Z.; Yu, J.; Ding, B. Superhydrophilic and underwater superoleophobic nanofibrous membrane with hierarchical structured skin for effective oil-in-water emulsion separation. J. Mater. Chem. A 2017, 5, 497–502. [Google Scholar] [CrossRef]
- Tang, L.; Zeng, Z.; Wang, G.; Shen, L.; Zhu, L.; Zhang, Y.; Xue, Q. Study of oil dewetting ability of superhydrophilic and underwater superoleophobic surfaces from air to water for high-effective self-cleaning surface designing. ACS Appl. Mater. Interfaces 2019, 11, 18865–18875. [Google Scholar] [CrossRef]
- Lv, Y.; Ding, Y.; Wang, J.; He, B.; Yang, S.; Pan, K.; Liu, F. Carbonaceous microsphere/nanofiber composite superhydrophilic membrane with enhanced anti-adhesion property towards oil and anionic surfactant: Membrane fabrication and applications. Sep. Purif. Technol. 2020, 235, 116189. [Google Scholar] [CrossRef]
- Pornea, A.M.; Puguan, J.M.C.; Deonikar, V.G.; Kim, H. Robust Janus nanocomposite membrane with opposing surface wettability for selective oil-water separation. Sep. Purif. Technol. 2020, 236, 116297. [Google Scholar] [CrossRef]
- Lin, Y.; Salem, M.S.; Zhang, L.; Shen, Q.; El-shazly, A.H.; Nady, N.; Matsuyama, H. Development of Janus membrane with controllable asymmetric wettability for highly-efficient oil/water emulsions separation. J. Membr. Sci. 2020, 606, 118141. [Google Scholar] [CrossRef]
- Wu, M.-B.; Yang, H.-C.; Wang, J.-J.; Wu, G.-P.; Xu, Z.-K. Janus membranes with opposing surface wettability enabling oil-to-water and water-to-oil emulsification. ACS Appl. Mater. Interfaces 2017, 9, 5062–5066. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, Q.; Hou, Y.; Wang, B.; Zhang, T. Facile preparation of an asymmetric wettability Janus cellulose membrane for switchable emulsions’ separation and antibacterial property. ACS Sustain. Chem. Eng. 2019, 7, 15002–15011. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, Z. Superwetting Janus membranes: Focusing on unidirectional transport behaviors and multiple applications. J. Mater. Chem. A 2019, 7, 12921–12950. [Google Scholar] [CrossRef]
- Yue, G.; Wang, Y.; Li, D.; Hou, L.; Cui, Z.; Li, Q.; Wang, N.; Zhao, Y. Bioinspired surface with special wettability for liquid transportation and separation. Sustain. Mater. Technol. 2020, 25, e00175. [Google Scholar] [CrossRef]
- Chew NG, P.; Zhang, Y.; Goh, K.; Ho, J.S.; Xu, R.; Wang, R. Hierarchically structured Janus membrane surfaces for enhanced membrane distillation performance. ACS Appl. Mater. Interfaces 2019, 11, 25524–25534. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, C.; Jia, Y.; Yuan, J.; Song, G.; Zhou, X.; Sun, S.; Zhou, C.; Zhao, L.; Yang, S. Solar-powered Janus membrane for one-step conversion of sewage to clean water. Chem. Eng. J. 2020, 387, 124131. [Google Scholar] [CrossRef]
- Hu, R.; Wang, N.; Hou, L.; Cui, Z.; Liu, J.; Li, D.; Li, Q.; Zhang, H.; Zhao, Y. A bioinspired hybrid membrane with wettability and topology anisotropy for highly efficient fog collection. J. Mater. Chem. A 2019, 7, 124–132. [Google Scholar] [CrossRef]
- Han, M.; Dong, T.; Hou, D.; Yao, J.; Han, L. Carbon nanotube based Janus composite membrane of oil fouling resistance for direct contact membrane distillation. J. Membr. Sci. 2020, 607, 118078. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Hou, Y.; Li, P.; Sun, H.; Niu, Q.J. Fabrication of a superhydrophilic PVDF membrane with excellent chemical and mechanical stability for highly efficient emulsion separation. Sep. Purif. Technol. 2020, 251, 117408. [Google Scholar] [CrossRef]
- Yang, H.-C.; Zhong, W.; Hou, J.; Chen, V.; Xu, Z.-K. Janus hollow fiber membrane with a mussel-inspired coating on the lumen surface for direct contact membrane distillation. J. Membr. Sci. 2017, 523, 1–7. [Google Scholar] [CrossRef]
- Han, L.; Tan, Y.Z.; Xu, C.; Xiao, T.; Trinh, T.A.; Chew, J.W. Zwitterionic grafting of sulfobetaine methacrylate (SBMA) on hydrophobic PVDF membranes for enhanced anti-fouling and anti-wetting in the membrane distillation of oil emulsions. J. Membr. Sci. 2019, 588, 117196. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Du, X.; Tong, T.; Cath, T.Y.; Lee, J. Antiwetting and antifouling Janus membrane for desalination of saline oily wastewater by membrane distillation. ACS Appl. Mater. Interfaces 2019, 11, 18456–18465. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hou, D.; Lin, S. Composite membrane with underwater-oleophobic surface for anti-oil-fouling membrane distillation. Environ. Sci. Technol. 2016, 50, 3866–3874. [Google Scholar] [CrossRef]
- Ardeshiri, F.; Akbari, A.; Peyravi, M.; Jahanshahi, M. PDADMAC/PAA semi-IPN hydrogel-coated PVDF membrane for robust anti-wetting in membrane distillation. J. Ind. Eng. Chem. 2019, 74, 14–25. [Google Scholar] [CrossRef]
- Hou, D.; Wang, Z.; Wang, K.; Wang, J.; Lin, S. Composite membrane with electrospun multiscale-textured surface for robust oil-fouling resistance in membrane distillation. J. Membr. Sci. 2018, 546, 179–187. [Google Scholar] [CrossRef]
- Shah, A.A.; Heo, H.J.; Park, S.; Yi, E.; Cho, Y.; Nam, S.-E.; Park, Y.-I.; Kim, H.; Sohn, E.-H.; Park, H.; et al. Origin of Fluoropolymer Affinity toward Water and Its Impact on Membrane Performance. ACS Appl. Polym. Mater. 2020, 2, 5249–5258. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Zhong, L.; Song, C.; Shi, W.; Cui, F.; Wang, W. Breathable and asymmetrically superwettable Janus membrane with robust oil-fouling resistance for durable membrane distillation. J. Membr. Sci. 2018, 563, 602–609. [Google Scholar] [CrossRef]
- Tao, M.; Liu, F.; Xue, L. Persistently hydrophilic microporous membranes based on in situ cross-linking. J. Membr. Sci. 2015, 474, 224–232. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An intelligent superwetting PVDF membrane showing switchable transport performance for oil/water separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef]
- Qiu, L.; Sun, Y.; Guo, Z. Designing novel superwetting surfaces for high-efficiency oil–water separation: Design principles, opportunities, trends and challenges. J. Mater. Chem. A 2020, 8, 16831–16853. [Google Scholar] [CrossRef]
- Söz, C.K.; Trosien, S.; Biesalski, M. Janus interface materials: A critical review and comparative study. ACS Mater. Lett. 2020, 2, 336–357. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, J.H.; Choi, C.H. Cotton fabrics with single-faced superhydrophobicity. Langmuir 2012, 28, 17426–17434. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Surface segregation of fluorinated modifying macromolecule for hydrophobic/hydrophilic membrane preparation and application in air gap and direct contact membrane distillation. J. Membr. Sci. 2012, 417, 163–173. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Cheng, Z.; Liu, Y.; Shao, L.; Jiang, L. Simply realizing “water diode” Janus membranes for multifunctional smart applications. Mater. Horiz. 2017, 4, 701–708. [Google Scholar] [CrossRef]
- Hu, L.; Gao, S.; Zhu, Y.; Zhang, F.; Jiang, L.; Jin, J. An ultrathin bilayer membrane with asymmetric wettability for pressure responsive oil/water emulsion separation. J. Mater. Chem. A 2015, 3, 23477–23482. [Google Scholar] [CrossRef]
- Li, H.N.; Yang, J.; Xu, Z.K. Asymmetric surface engineering for Janus membranes. Adv. Mater. Interfaces 2020, 7, 1902064. [Google Scholar] [CrossRef]


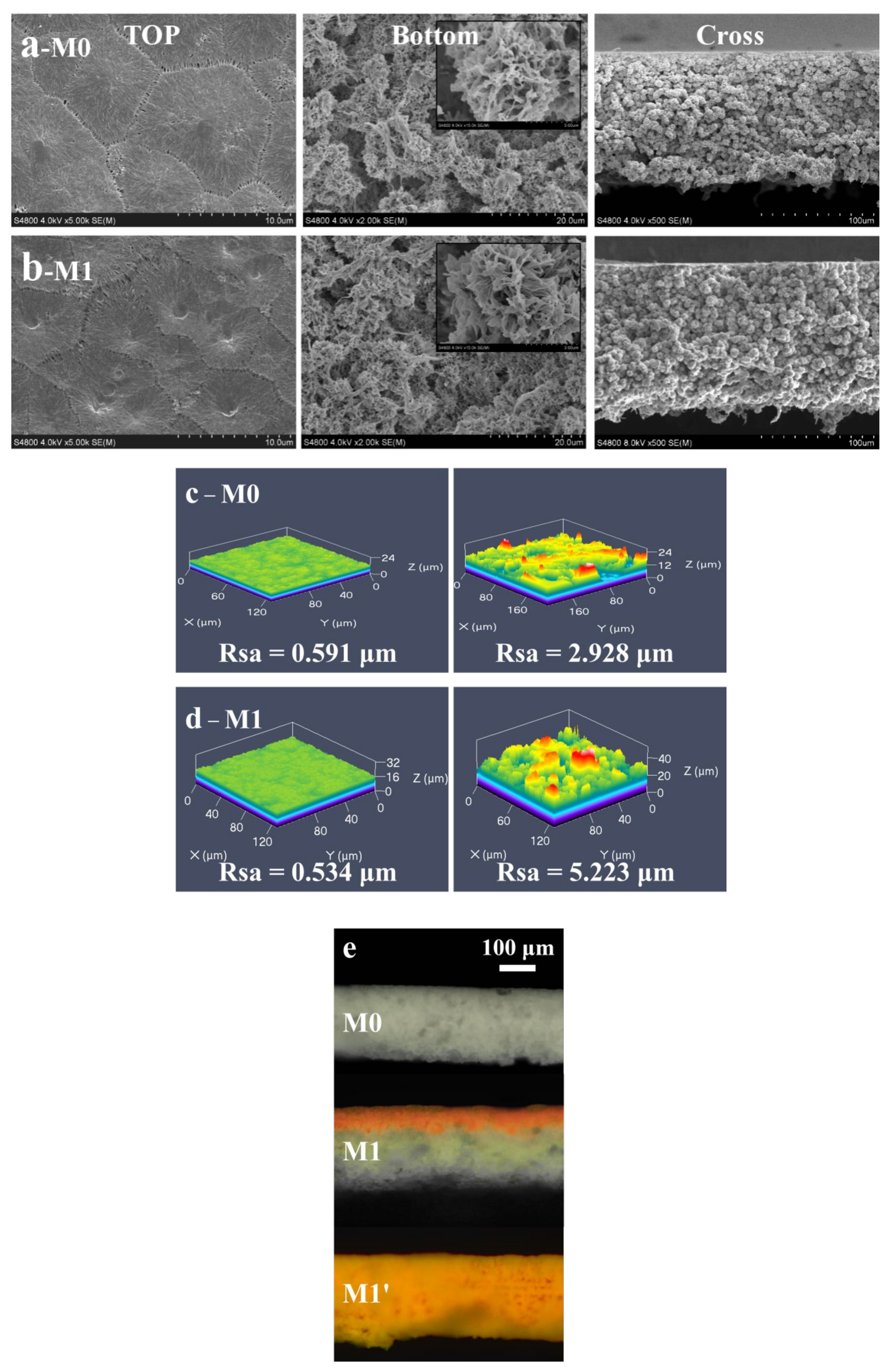
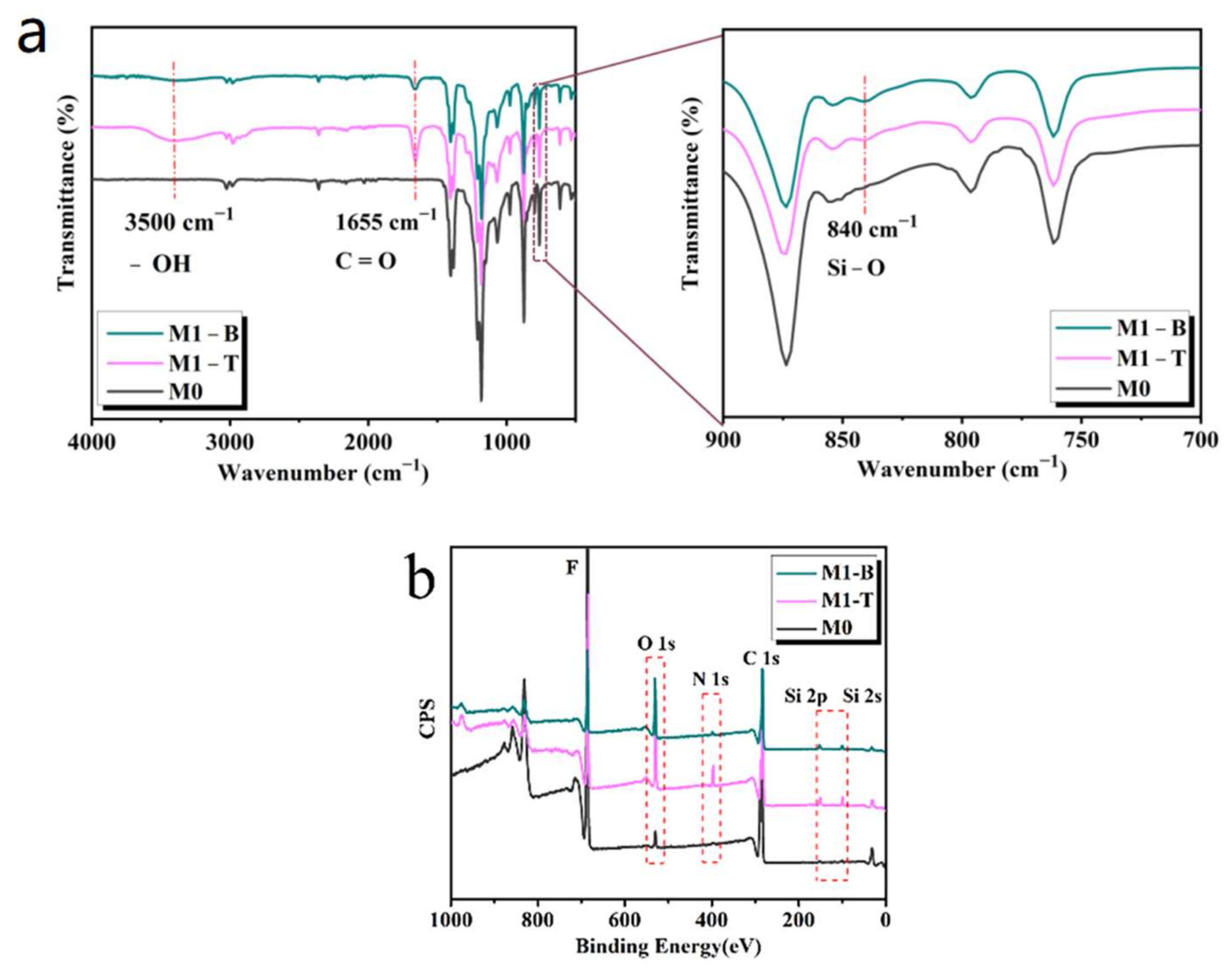



| Membrane | Thickness (μm) | Mean Pore Size (μm) | * Porosity (%) | Tensile Strength (MPa) |
|---|---|---|---|---|
| M0 | 200 ± 10 | 0.38 | 74.71 | 0.85 |
| M1 | 200 ± 10 | 0.33 | 73.26 | 1.16 |
| At (%) | C | F | N | O | Si |
|---|---|---|---|---|---|
| M0 | 50.1 | 49.9 | |||
| M1-T | 60.6 | 20.4 | 3.6 | 13.1 | 2.3 |
| M1-B | 56.4 | 32.5 | 1.5 | 8.2 | 1.4 |
| Literatures | Top Surface Angle (θ1, deg) | Bottom Surface Angle (θ2, deg) | Δθ (θ1–θ2, deg) |
|---|---|---|---|
| Functional cotton fabric with single-faced superhydrophobicity [38] | 140 | 40 | 100 |
| Surface segregation of fluorinated modifying macromolecule for hydrophobic/hydrophilic membrane [39] | 94 | 78 | 15 |
| PET/PTFE@TA-DETA multifunctional Janus membranes [40] | 115 | 16 | 99 |
| PDA/SWCNT bilayer membranes [41] | 104 | 48 | 56 |
| This study | 0 | 142 | 142 |
| Modification Time (h) | Total Membrane Thickness (μm) | Hydrophilic Layer Thickness (μm) | Hydrophilic Layer Percentage (%) | The Mean Percentage (%) |
|---|---|---|---|---|
| 1 | 199.92 | 30.49 | 15.25 | 15.57 ± 2.71 |
| 6 | 194.04 | 50.48 | 26.02 | 23.66 ± 2.75 |
| 12 | 187.59 | 66.02 | 35.19 | 37.94 ± 2.55 |
| 18 | 198.84 | 99.55 | 50.07 | 54.18 ± 6.09 |
| 24 | 206.22 | 206.22 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Deng, C.; Hou, X.; Li, T.; Lu, Y.; Liu, F. Preparation and Characterization of a Janus Membrane with an “Integrated” Structure and Adjustable Hydrophilic Layer Thickness. Membranes 2023, 13, 415. https://doi.org/10.3390/membranes13040415
Zhang R, Deng C, Hou X, Li T, Lu Y, Liu F. Preparation and Characterization of a Janus Membrane with an “Integrated” Structure and Adjustable Hydrophilic Layer Thickness. Membranes. 2023; 13(4):415. https://doi.org/10.3390/membranes13040415
Chicago/Turabian StyleZhang, Ruixian, Chengyu Deng, Xueyi Hou, Tiantian Li, Yanyue Lu, and Fu Liu. 2023. "Preparation and Characterization of a Janus Membrane with an “Integrated” Structure and Adjustable Hydrophilic Layer Thickness" Membranes 13, no. 4: 415. https://doi.org/10.3390/membranes13040415





