Single-Pass Tangential Flow Filtration (SPTFF) of Nanoparticles: Achieving Sustainable Operation with Dilute Colloidal Suspensions for Gene Therapy Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
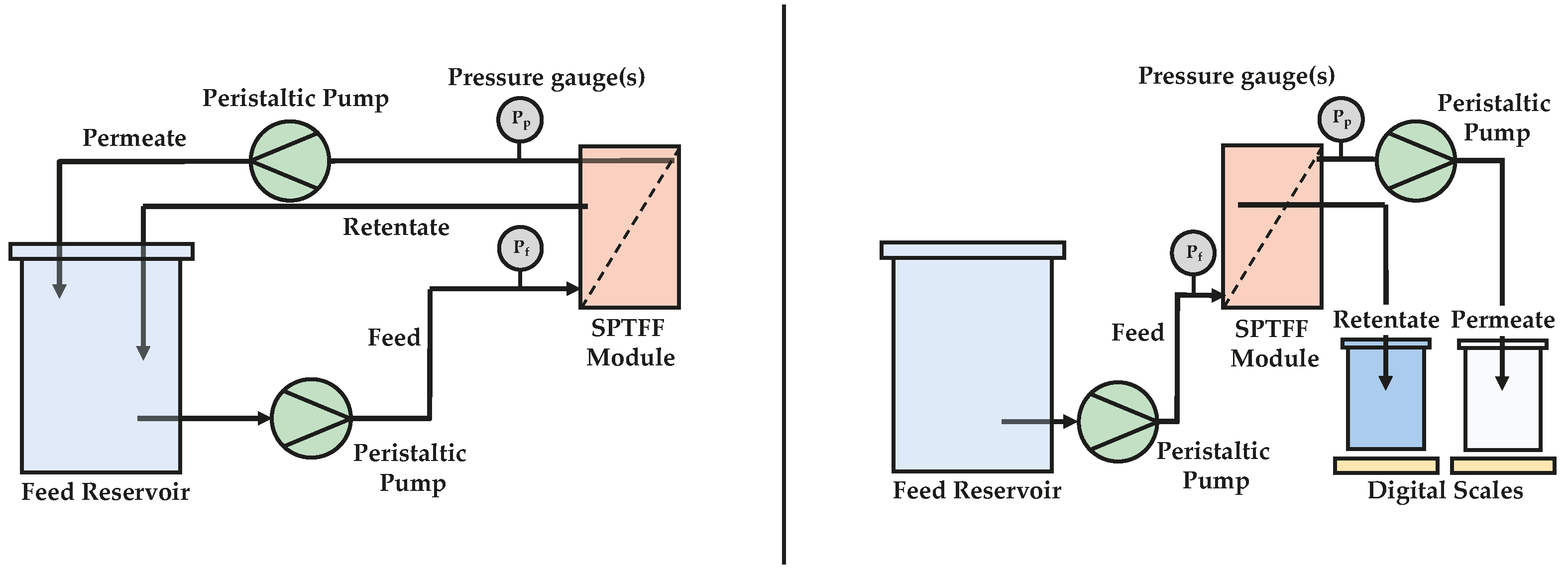
2.2. Experimental Setup
3. Results and Discussion
3.1. Nanoparticle Characterization
3.2. Particle Loss during SPTFF
3.3. Critical Flux Behavior
3.4. Concentration Polarization Model
3.5. Conversion
3.6. Long-Duration SPTFF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curreri, A.; Sankholkar, D.; Mitragotri, S.; Zhao, Z. RNA therapeutics in the clinic. Bioeng. Transl. Med. 2023, 8, 120527. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 30 January 2023).
- Weger, S. High-Level rAAV Vector Production by rAdV-Mediated Amplification of Small Amounts of Input Vector. Viruses 2022, 15, 64. [Google Scholar] [CrossRef]
- Connolly, J.B. Lentiviruses in gene therapy clinical research. Gene Ther. 2002, 9, 1730–1734. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, F.; Thompson, A.A.; Kwiatkowski, J.L.; Porter, J.B.; Thrasher, A.J.; Hongeng, S.; Sauer, M.G.; Thuret, I.; Lal, A.; Algeri, M.; et al. Betibeglogene Autotemcel Gene Therapy for Non–β0/β0 Genotype β-Thalassemia. New Engl. J. Med. 2021, 386, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Elivaldogene Autotemcel: First Approval. Mol. Diagn. Ther. 2021, 25, 803–809. [Google Scholar] [CrossRef]
- Bandeira, V.; Peixoto, C.; Rodrigues, A.F.; Cruz, P.E.; Alves, P.M.; Coroadinha, A.S.; Carrondo, M.J.T. Downstream Processing of Lentiviral Vectors: Releasing Bottlenecks. Hum. Gene Ther. Methods 2012, 23, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, K.; Scheibe, O.; Kocourek, A.; Muelich, J.; Jurkiewicz, E.; Pfeifer, A. Highly Efficient Concentration of Lenti- and Retroviral Vector Preparations by Membrane Adsorbers and Ultrafiltration. BMC Biotechnol. 2011, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, E.; Kontostathi, G.; Drakopoulou, E.; Georgomanoli, M.; Stamateris, E.; Vougas, K.; Vlahou, A.; Maloy, A.; Ware, M.; Anagnou, N.P. Characterization and Comparative Performance of Lentiviral Vector Preparations Concentrated by Either One-Step Ultrafiltration or Ultracentrifugation. Virus Res. 2013, 175, 1–11. [Google Scholar] [CrossRef]
- Rosales Gerpe, M.C.; van Lieshout, L.P.; Domm, J.M.; van Vloten, J.P.; Datu, J.; Ingrao, J.C.; Yu, D.L.; de Jong, J.; Moraes, T.J.; Krell, P.J.; et al. Optimized Pre-Clinical Grade Production of Two Novel Lentiviral Vector Pseudotypes for Lung Gene Delivery. Hum. Gene Ther. 2020, 31, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Valkama, A.J.; Oruetxebarria, I.; Lipponen, E.M.; Leinonen, H.M.; Käyhty, P.; Hynynen, H.; Turkki, V.; Malinen, J.; Miinalainen, T.; Heikura, T.; et al. Development of Large-Scale Downstream Processing for Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 2020, 17, 717–730. [Google Scholar] [CrossRef]
- Soldi, M.; Sergi, L.S.; Unali, G.; Kerzel, T.; Cuccovillo, I.; Capasso, P.; Annoni, A.; Biffi, M.; Rancoita, P.M.V.; Cantore, A.; et al. Laboratory-Scale Lentiviral Vector Production and Purification for Enhanced Ex Vivo and in Vivo Genetic Engineering. Mol. Ther. Methods Clin. Dev. 2020, 19, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; Rayat, A.C.M.E. Lentiviral Vector Bioprocessing. Viruses 2021, 13, 268. [Google Scholar] [CrossRef]
- Vandanjon, L.; Rossignol, N.; Jaouen, P.; Robert, J.M.; Quéméneur, F. Effects of shear on two microalgae species. Contribution of pumps and valves in tangential flow filtration systems. Biotechnol. Bioeng. 1999, 63, 1–9. [Google Scholar] [CrossRef]
- Wickramasinghe, S.R.; Kalbfuß, B.; Zimmermann, A.; Thom, V.; Reichl, U. Tangential Flow Microfiltration and Ultrafiltration for Human Influenza a Virus Concentration and Purification. Biotechnol. Bioeng. 2005, 92, 199–208. [Google Scholar] [CrossRef]
- Madsen, E.; Kaiser, J.; Krühne, U.; Pinelo, M. Single Pass Tangential Flow Filtration: Critical Operational Variables, Fouling, and Main Current Applications. Sep. Purif. Technol. 2022, 291, 120949. [Google Scholar] [CrossRef]
- Zydney, A.L. Continuous Downstream Processing for High Value Biological Products: A Review. Biotechnol. Bioeng. 2015, 113, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Elich, T.; Goodrich, E.; Lutz, H.; Mehta, U. Investigating the Combination of Single-Pass Tangential Flow Filtration and Anion Exchange Chromatography for Intensified MAb Polishing. Biotechnol. Prog. 2019, 35, e2862. [Google Scholar] [CrossRef] [PubMed]
- Casey, C.; Gallos, T.; Alekseev, Y.; Ayturk, E.; Pearl, S. Protein Concentration with Single-Pass Tangential Flow Filtration (SPTFF). J. Membr. Sci. 2011, 384, 82–88. [Google Scholar] [CrossRef]
- Arunkumar, A.; Singh, N.; Peck, M.; Borys, M.C.; Li, Z.J. Investigation of Single-Pass Tangential Flow Filtration (SPTFF) as an Inline Concentration Step for Cell Culture Harvest. J. Membr. Sci. 2017, 524, 20–32. [Google Scholar] [CrossRef]
- Dizon-Maspat, J.; Bourret, J.; D’Agostini, A.; Li, F. Single Pass Tangential Flow Filtration to Debottleneck Downstream Processing for Therapeutic Antibody Production. Biotechnol. Bioeng. 2011, 109, 962–970. [Google Scholar] [CrossRef]
- Yehl, C.J.; Zydney, A.L. Single-Use, Single-Pass Tangential Flow Filtration Using Low-Cost Hollow Fiber Modules. J. Membr. Sci. 2020, 595, 117517. [Google Scholar] [CrossRef]
- Field, R.W.; Pearce, G.K. Critical, Sustainable and Threshold Fluxes for Membrane Filtration with Water Industry Applications. Adv. Colloid Interface Sci. 2011, 164, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mänttäri, M.; Nyström, M. Critical Flux in NF of High Molar Mass Polysaccharides and Effluents from the Paper Industry. J. Membr. Sci. 2000, 170, 257–273. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Vigneswaran, S.; Fane, A.G.; Aim, R.B. Experimental Determination of Critical Flux in Cross-Flow Microfiltration. Sep. Purif. Technol. 2000, 19, 169–181. [Google Scholar] [CrossRef]
- Li, Z.; Zydney, A.L. Effect of Zinc Chloride and PEG Concentrations on the Critical Flux during Tangential Flow Microfiltration of BSA Precipitates. Biotechnol. Prog. 2017, 33, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, A.; Singh, N. Ultrafiltration Behavior of Recombinant Adeno Associated Viral Vectors Used in Gene Therapy. J. Membr. Sci. 2021, 620, 118812. [Google Scholar] [CrossRef]
- Wolf, T.; Rosengarten, J.; Härtel, I.; Stitz, J.; Barbe, S. A Hydrodynamic Approach to the Study of HIV Virus-like Particle (VLP) Tangential Flow Filtration. Membranes 2022, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, B.; Müller, P.; Uhl, W. Rejection of Submicron Sized Particles from Swimming Pool Water by a Monolithic SiC Microfiltration Membrane: Relevance of Steric and Electrostatic Interactions. J. Membr. Sci. 2016, 499, 92–104. [Google Scholar] [CrossRef]
- Michen, B.; Diatta, A.; Fritsch, J.; Aneziris, C.; Graule, T. Removal of Colloidal Particles in Ceramic Depth Filters Based on Diatomaceous Earth. Sep. Purif. Technol. 2011. [Google Scholar] [CrossRef]
- Bielefeldt, A.R.; Kowalski, K.; Schilling, C.; Schreier, S.; Kohler, A.; Summers, R.S. Removal of Virus to Protozoan Sized Particles in Point-of-Use Ceramic Water Filters. Water Research 2010, 44, 1482–1488. [Google Scholar] [CrossRef]
- Pazouki, M.; Noelle Wilton, A.; Latulippe, D.R. An Experimental Study on Sterile Filtration of Fluorescently Labeled Nanoparticles—The Importance of Surfactant Concentration. Sep. Purif. Technol. 2019, 218, 217–226. [Google Scholar] [CrossRef]
- Taylor, N.; Ma, W.; Kristopeit, A.; Wang, S.; Zydney, A.L. Evaluation of a Sterile Filtration Process for Viral Vaccines Using a Model Nanoparticle Suspension. Biotechnol. Bioeng. 2020, 118, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.-K.; Wickramasinghe, S.R.; Qian, X.; Zydney, A.L. Retention and Fouling during Nanoparticle Filtration: Implications for Membrane Purification of Biotherapeutics. Membranes 2022, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nature Reviews Drug Discovery 2020, 20, 1–24. [Google Scholar] [CrossRef]
- Robertson, J.D.; Rizzello, L.; Avila-Olias, M.; Gaitzsch, J.; Contini, C.; Magoń, M.S.; Renshaw, S.A.; Battaglia, G. Purification of Nanoparticles by Size and Shape. Scientific Reports 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Konan, Y.N.; Cerny, R.; Favet, J.; Berton, M.; Gurny, R.; Allémann, E. Preparation and Characterization of Sterile Sub-200 Nm Meso-Tetra(4-Hydroxylphenyl) Porphyrin-Loaded Nanoparticles for Photodynamic Therapy. Eur. J. Pharm. Biopharm. 2003, 55, 115–124. [Google Scholar] [CrossRef]
- Dalwadi, G.; Benson, H.A.E.; Chen, Y. Comparison of Diafiltration and Tangential Flow Filtration for Purification of Nanoparticle Suspensions. Pharm.l Res. 2005, 22, 2152–2162. [Google Scholar] [CrossRef]
- Anders, C.B.; Baker, J.D.; Stahler, A.C.; Williams, A.J.; Sisco, J.N.; Trefry, J.C.; Wooley, D.P.; Sizemore, I.E.P. Tangential Flow Ultrafiltration: A “Green” Method for the Size Selection and Concentration of Colloidal Silver Nanoparticles. J. Visualized Exp. 2012, 68. [Google Scholar] [CrossRef] [Green Version]
- Pansare, V.J.; Tien, D.; Thoniyot, P.; Prud’homme, R.K. Ultrafiltration of Nanoparticle Colloids. J. Membr. Sci. 2017, 538, 41–49. [Google Scholar] [CrossRef]
- Marques, S.S.; Ramos, I.I.; Fernandes, S.R.; Barreiros, L.; Lima, S.A.C.; Reis, S.; Domingues, M.R.M.; Segundo, M.A. Insights on Ultrafiltration-Based Separation for the Purification and Quantification of Methotrexate in Nanocarriers. Molecules 2020, 25, 1879. [Google Scholar] [CrossRef] [Green Version]
- Kumru, O.S.; Wang, Y.; Gombotz, C.W.R.; Kelley-Clarke, B.; Cieplak, W.; Kim, T.; Joshi, S.B.; Volkin, D.B. Physical Characterization and Stabilization of a Lentiviral Vector against Adsorption and Freeze-Thaw. J. Pharm. Sci. 2018, 107, 2764–2774. [Google Scholar] [CrossRef]
- Trettin, D.R.; Doshi, M.R. Limiting flux in ultrafiltration of macromolecular solutions. Chem. Eng. Commun. 1980, 4, 507–522. [Google Scholar] [CrossRef]
- Da Costa, A.R.; Fane, A.G.; Wiley, D.E. Spacer Characterization and Pressure Drop Modelling in Spacer-Filled Channels for Ultrafiltration. J. Membr. Sci. 1994, 87, 79–98. [Google Scholar] [CrossRef]
- Jabra, M.G.; Lipinski, A.M.; Zydney, A.L. Single Pass Tangential Flow Filtration (SPTFF) of Monoclonal Antibodies: Experimental Studies and Theoretical Analysis. J. Membr. Sci. 2021, 637, 119606. [Google Scholar] [CrossRef]
- Minervini, M.; Zydney, A.L. Effect of Module Geometry on the Sustainable Flux during Microfiltration of Precipitated IgG. J. Membr. Sci. 2022, 660, 120834. [Google Scholar] [CrossRef]






| Nanoparticles | Lentivirus | |
|---|---|---|
| Diameter (DLS) | 122 ± 2 nm | 123 ± 8 nm |
| Diameter (NTA) | 104 ± 35 nm | 113 ± 2 nm |
| Zeta potential (tris buffer) | −25.3 ± 1.1 mV | −17.7 ± 10.2 mV |
| Zeta potential (PBS) | −54.0 ± 1.3 mV | N/A |
| Permeate Flux | Concentration Factor | Predicted Operating Time |
|---|---|---|
| 55 LMH | 2.2 | ~6 weeks |
| 82 LMH | 5.1 | ~11 days |
| 99 LMH | 34.0 | ~7 hours |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaubal, A.S.; Zydney, A.L. Single-Pass Tangential Flow Filtration (SPTFF) of Nanoparticles: Achieving Sustainable Operation with Dilute Colloidal Suspensions for Gene Therapy Applications. Membranes 2023, 13, 433. https://doi.org/10.3390/membranes13040433
Chaubal AS, Zydney AL. Single-Pass Tangential Flow Filtration (SPTFF) of Nanoparticles: Achieving Sustainable Operation with Dilute Colloidal Suspensions for Gene Therapy Applications. Membranes. 2023; 13(4):433. https://doi.org/10.3390/membranes13040433
Chicago/Turabian StyleChaubal, Akshay S., and Andrew L. Zydney. 2023. "Single-Pass Tangential Flow Filtration (SPTFF) of Nanoparticles: Achieving Sustainable Operation with Dilute Colloidal Suspensions for Gene Therapy Applications" Membranes 13, no. 4: 433. https://doi.org/10.3390/membranes13040433
APA StyleChaubal, A. S., & Zydney, A. L. (2023). Single-Pass Tangential Flow Filtration (SPTFF) of Nanoparticles: Achieving Sustainable Operation with Dilute Colloidal Suspensions for Gene Therapy Applications. Membranes, 13(4), 433. https://doi.org/10.3390/membranes13040433






