Selective Separation of Singly Charged Chloride and Dihydrogen Phosphate Anions by Electrobaromembrane Method with Nanoporous Membranes
Abstract
1. Introduction
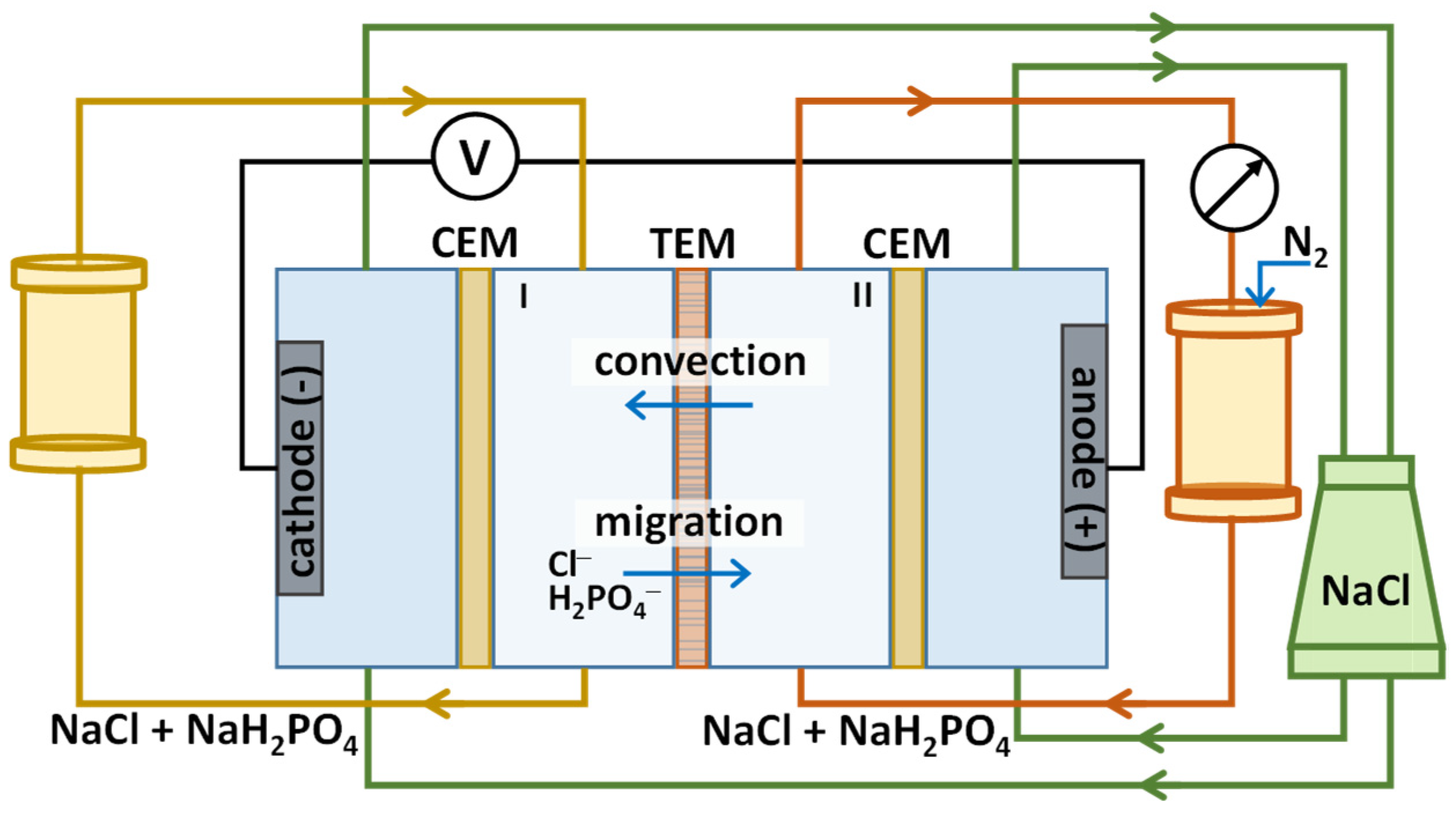
2. Materials and Methods
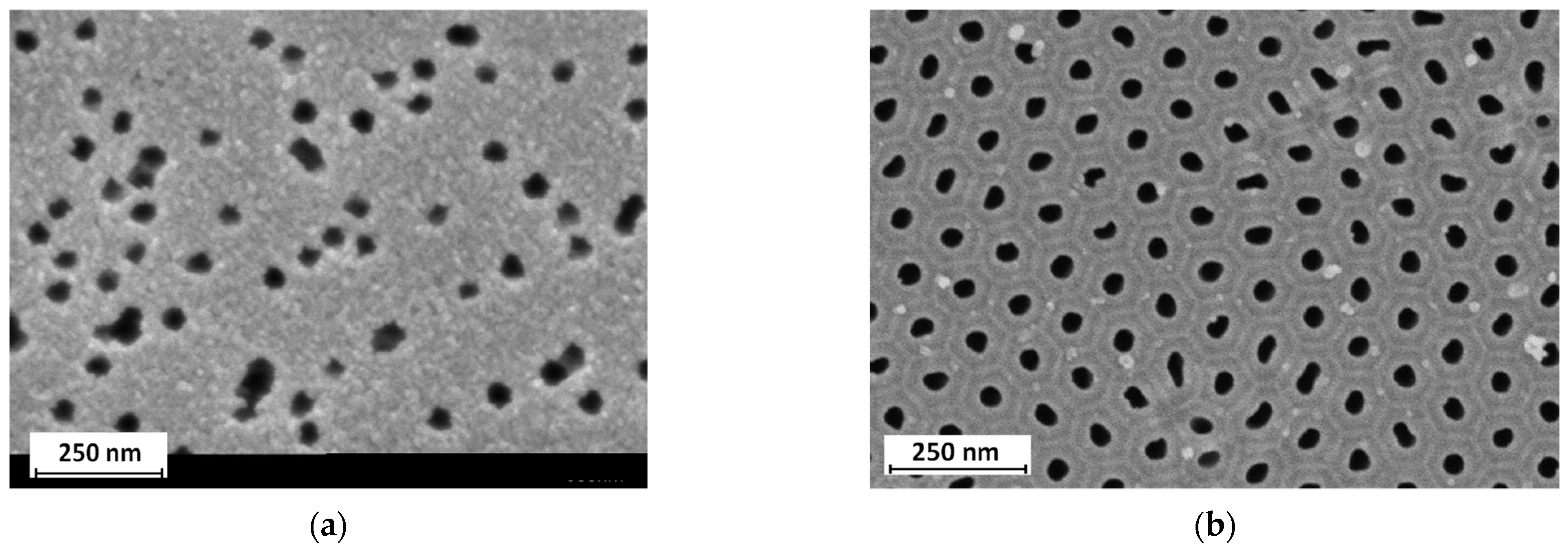
- Preparation of porous anodic alumina membranes
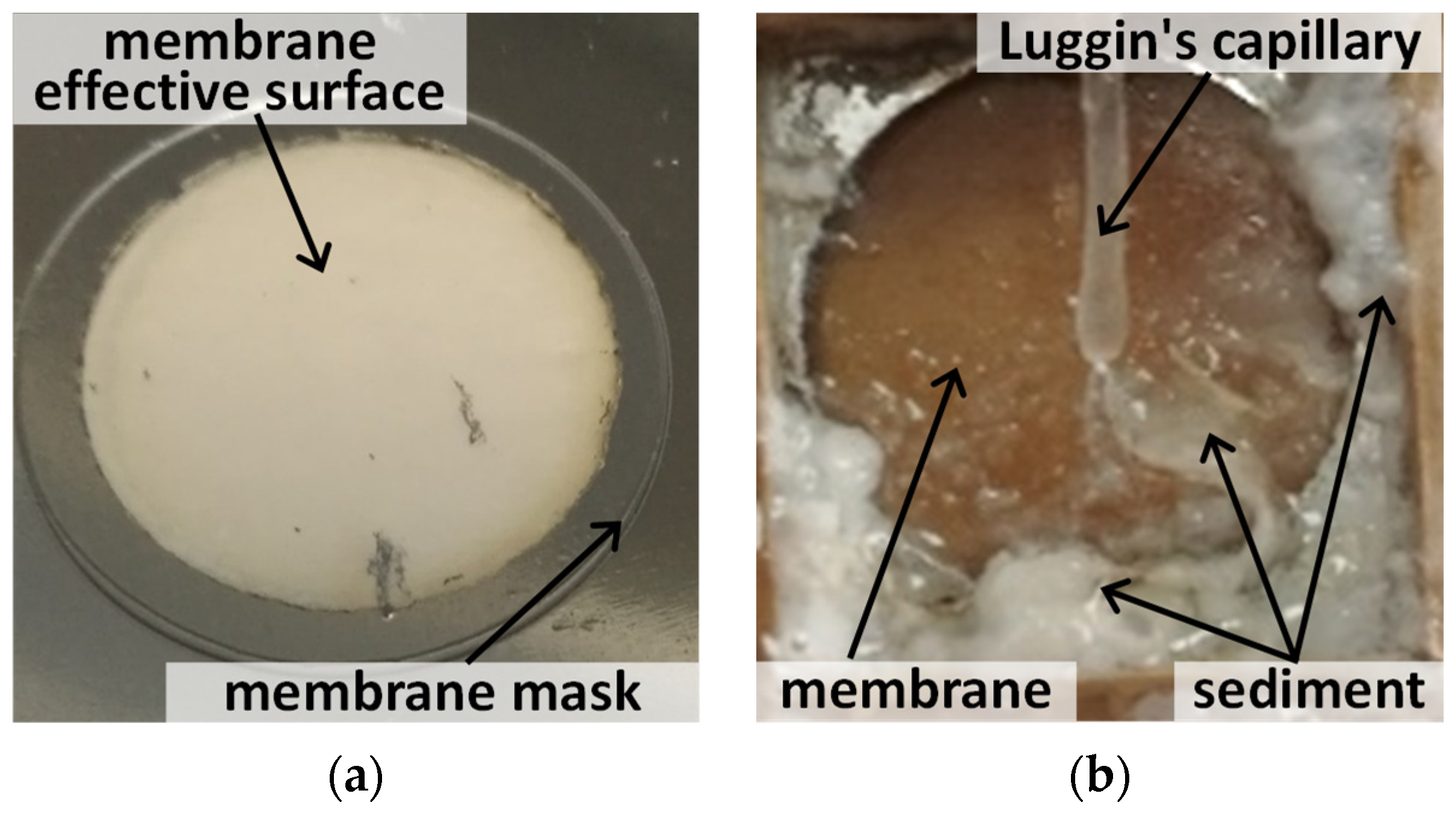
- Determination of membrane ion separation coefficient
3. Results
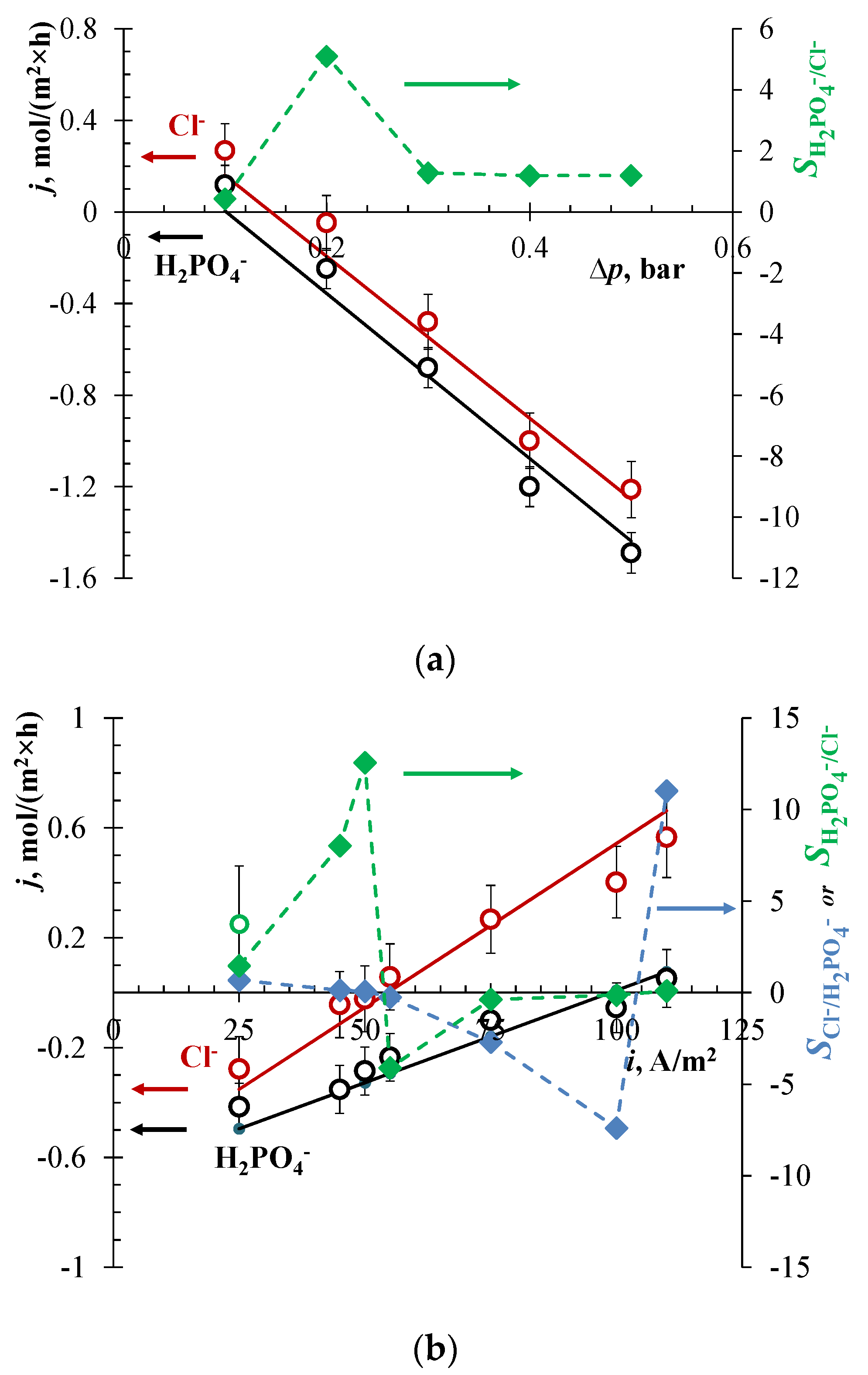
3.1. Track-Etched Membrane
3.2. Anodic Alumina Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Coman, V.; Robotin, B.; Ilea, P. Nickel recovery/removal from industrial wastes: A review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Tsygurina, K.; Nikonenko, V. Recovery of Nutrients from Residual Streams Using Ion-Exchange Membranes: Current State, Bottlenecks, Fundamentals and Innovations. Membranes 2022, 12, 497. [Google Scholar] [CrossRef]
- Butylskii, D.Y.; Dammak, L.; Larchet, C.; Pismenskaya, N.D.; Nikonenko, V.V. Selective recovery and re-utilization of lithium: Prospects for the use of membrane methods. Russ. Chem. Rev. 2023, 92, RCR5074. [Google Scholar] [CrossRef]
- Li, X.; Shen, S.; Xu, Y.; Guo, T.; Dai, H.; Lu, X. Application of membrane separation processes in phosphorus recovery: A review. Sci. Total Environ. 2021, 767, 144346. [Google Scholar] [CrossRef]
- Kovalev, N.V.; Karpenko, T.V.; Sheldeshov, N.V.; Zabolotsky, V.I. Electrochemical Characteristics of Modified Heterogeneous Bipolar Membrane and Electromembrane Process of Nitric Acid and Sodium Hydroxide Recuperation from Sodium Nitrate and Boric Acid Solution. Russ. J. Electrochem. 2021, 57, 122–133. [Google Scholar] [CrossRef]
- Nir, O.; Sengpiel, R.; Wessling, M. Closing the cycle: Phosphorus removal and recovery from diluted effluents using acid resistive membranes. Chem. Eng. J. 2018, 346, 640–648. [Google Scholar] [CrossRef]
- Oren, Y.; Korngold, E.; Daltrophe, N.; Messalem, R.; Volkman, Y.; Aronov, L.; Weismann, M.; Bouriakov, N.; Glueckstern, P.; Gilron, J. Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination 2010, 261, 321–330. [Google Scholar] [CrossRef]
- Sanin, F.D.; Clarkson, W.W.; Vesilind, P.A. Sludge Engineering: The Treatment and Disposal of Wastewater Sludges; DEStech Publications, Inc: Lancaster, PA, USA, 2011; ISBN 978-1-932078-87-9. [Google Scholar]
- HELCOM Thematic Assessment of Eutrophication 2011–2016. Supplementary Report to the ‘State of the Baltic Sea’ Report. Available online: https://helcom.fi/baltic-sea-trends/holistic-assessments/state-of-the-baltic-sea-2018/reports-and-materials/ (accessed on 14 February 2023).
- Fang, L.; Li, J.; Guo, M.Z.; Cheeseman, C.R.; Tsang, D.C.W.; Donatello, S.; Poon, C.S. Phosphorus recovery and leaching of trace elements from incinerated sewage sludge ash (ISSA). Chemosphere 2018, 193, 278–287. [Google Scholar] [CrossRef]
- Sengupta, S.; Pandit, A. Selective removal of phosphorus from wastewater combined with its recovery as a solid-phase fertilizer. Water Res. 2011, 45, 3318–3330. [Google Scholar] [CrossRef]
- Awual, M.R.; Jyo, A. Assessing of phosphorus removal by polymeric anion exchangers. Desalination 2011, 281, 111–117. [Google Scholar] [CrossRef]
- Acelas, N.Y.; Martin, B.D.; López, D.; Jefferson, B. Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media. Chemosphere 2015, 119, 1353–1360. [Google Scholar] [CrossRef]
- Ostara Nutrient Management Solutions. Available online: https://ostara.com/phosphates-production/ (accessed on 25 October 2022).
- Gysin, A.; Lycke, D.; Wirtel, S. The Pearl® and WASSTRIP® processes (Canada). In Phosphorus: Polluter and Resource of the Future—Removal and Recovery from Wastewater; International Water Association: London, UK, 2018; pp. 359–365. [Google Scholar]
- Orner, K.D.; Camacho-Céspedes, F.; Cunningham, J.A.; Mihelcic, J.R. Assessment of nutrient fluxes and recovery for a small-scale agricultural waste management system. J. Environ. Manag. 2020, 267, 110626. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Q.; Wang, X.; Yang, M.; Fang, Y.; Lu, Y. An efficient strategy of phosphorus recovery: Electrochemical pretreatment enhanced the anaerobic fermentation of waste activated sludge. Chemosphere 2021, 268, 129391. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Zhang, Q.; Gu, J.-D.; Zhang, Y.; Liu, Y.; Wang, L.; Xiao, Y.; Shen, F.; Deng, S.; et al. Highly efficient removal of phosphorus from agricultural runoff by a new akadama clay barrier-vegetated drainage ditch system (VDD) and its mechanism. J. Environ. Manag. 2021, 290, 112575. [Google Scholar] [CrossRef]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef]
- van Voorthuizen, E.M.; Zwijnenburg, A.; Wessling, M. Nutrient removal by NF and RO membranes in a decentralized sanitation system. Water Res. 2005, 39, 3657–3667. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Licon, E.; Valderrama, C.; Gibert, O.; Cortina, J.L. Evaluating the integration of nanofiltration membranes in advanced water reclamation schemes using synthetic solutions: From phosphorous removal to phosphorous circularity. Sep. Purif. Technol. 2022, 290, 120914. [Google Scholar] [CrossRef]
- Masse, L.; Masse, D.; Pellerin, Y. The effect of pH on the separation of manure nutrients with reverse osmosis membranes. J. Memb. Sci. 2008, 325, 914–919. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Qalyoubi, L.; Al-Othman, A.; Qasim, M.; Shirazi, M. Insights on the development of enhanced antifouling reverse osmosis membranes: Industrial applications and challenges. Desalination 2023, 553, 116460. [Google Scholar] [CrossRef]
- Niewersch, C.; Meier, K.; Wintgens, T.; Melin, T. Selectivity of polyamide nanofiltration membranes for cations and phosphoric acid. Desalination 2010, 250, 1021–1024. [Google Scholar] [CrossRef]
- Ghyselbrecht, K.; Jongbloet, A.; Pinoy, L.; Meesschaert, B. Optimization of the configuration of the anion selectrodialysis stack for fractionation of phosphate from UASB effluent in batch mode on lab scale and pilot scale. J. Environ. Chem. Eng. 2020, 8, 104492. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Solonchenko, K.V.; Butylskii, D.Y.; Nikonenko, V.V.; Pismenskaya, N.D. Effect of the Parameters of Pulsed Electric Fields on the Average Current Density in the Electrodialysis Desalination of a Phosphate-Containing Solution. Membr. Membr. Technol. 2022, 4, 385–397. [Google Scholar] [CrossRef]
- Simons, R. Water splitting in ion exchange membranes. Electrochim. Acta 1985, 30, 275–282. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Sharafan, M.V.; Nikonenko, V.V.; Pismenskaya, N.D. Two mechanisms of H+/OH− ion generation in anion-exchange membrane systems with polybasic acid salt solutions. J. Memb. Sci. 2022, 651, 120449. [Google Scholar] [CrossRef]
- Tang, C.; Bondarenko, M.P.; Yaroshchuk, A.; Bruening, M.L. Highly selective ion separations based on counter-flow electromigration in nanoporous membranes. J. Memb. Sci. 2021, 638, 119684. [Google Scholar] [CrossRef]
- Butylskii, D.Y.; Pismenskaya, N.D.; Apel, P.Y.; Sabbatovskiy, K.G.; Nikonenko, V.V. Highly selective separation of singly charged cations by countercurrent electromigration with a track-etched membrane. J. Memb. Sci. 2021, 635, 119449. [Google Scholar] [CrossRef]
- Kislyi, A.G.; Butylskii, D.Y.; Mareev, S.A.; Nikonenko, V.V. Model of Competitive Ion Transfer in an Electro-Baromembrane System with Track-Etched Membrane. Membr. Membr. Technol. 2021, 3, 131–137. [Google Scholar] [CrossRef]
- Brewer, A.K.; Madorsky, S.L.; Westhaver, J.W. The Concentration of 39K and 41K by Balanced. Ion Migration in a Counterflowing Electrolyte. Science 1946, 104, 156–157. [Google Scholar] [CrossRef]
- Brewer, A.K.; Madorsky, S.L.; Taylor, J.K.; Dibeler, V.H.; Bradt, P.; Parham, O.L.; Britten, R.J.; Reid, J.G. Concentration of isotopes of potassium by the counter-current electromigration method. J. Res. Natl. Bur. Stand. 1947, 38, 137–168. [Google Scholar] [CrossRef]
- Forssell, P.; Kontturi, K. Experimental Verification of Separation of Ions Using Countercurrent Electrolysis in a Thin, Porous Membrane. Sep. Sci. Technol. 1983, 18, 205–214. [Google Scholar] [CrossRef]
- Kontturi, K.; Ojala, T.; Forssell, P. Transport of acetate and chloroacetate weak electrolytes through a thin porous membrane in counter-current electrolysis. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1984, 80, 3379. [Google Scholar] [CrossRef]
- Kontturi, K.; Forssell, P.; Ekman, A. Separation of Ions Using Countercurrent Electrolysis in a Thin, Porous Membrane. Sep. Sci. Technol. 1982, 17, 1195–1204. [Google Scholar] [CrossRef]
- Ekman, A.; Forssell, P.; Kontturi, K.; Sundholm, G. Transport of ions in a porous membrane in the case of a ternary electrolyte system with simultaneous convection and electric current. J. Memb. Sci. 1982, 11, 65–77. [Google Scholar] [CrossRef]
- Kontturi, K. Countercurrent Electrolysis in a Cell Where Porous Membranes Have Been Connected in Series with Ion-Exchange Membranes. Part 1. Method. Sep. Sci. Technol. 1988, 23, 227–234. [Google Scholar] [CrossRef]
- Kontturi, K.; Westerberg, L.-M. Countercurrent Electrolysis in a Cell Where Porous Membranes Have Been Connected in Series with Ion-Exchange Membranes. Part 2. Experimental Verification. Sep. Sci. Technol. 1988, 23, 235–241. [Google Scholar] [CrossRef]
- Tang, C.; Yaroshchuk, A.; Bruening, M.L. Ion Separations Based on Spontaneously Arising Streaming Potentials in Rotating Isoporous Membranes. Membranes 2022, 12, 631. [Google Scholar] [CrossRef]
- Marcus, Y. Ions in Water and Biophysical Implications; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-4646-6. [Google Scholar]
- Apel, P.Y. Track-Etching. In Encyclopedia of Membrane Science and Technology; Hoek, E.M.V., Tarabara, V.V., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 332–355. [Google Scholar]
- Petukhov, D.I.; Buldakov, D.A.; Tishkin, A.A.; Lukashin, A.V.; Eliseev, A.A. Liquid permeation and chemical stability of anodic alumina membranes. Beilstein J. Nanotechnol. 2017, 8, 561–570. [Google Scholar] [CrossRef]
- Ryzhkov, I.I.; Kharchenko, I.A.; Mikhlina, E.V.; Minakov, A.V.; Guzei, D.V.; Nemtsev, I.V.; Volochaev, M.N.; Korobko, A.V.; Simunin, M.M. Growth of carbon nanotubes inside porous anodic alumina membranes: Simulation and experiment. Int. J. Heat Mass Transf. 2021, 176, 121414. [Google Scholar] [CrossRef]
- Lillo, M.; Losic, D. Pore opening detection for controlled dissolution of barrier oxide layer and fabrication of nanoporous alumina with through-hole morphology. J. Memb. Sci. 2009, 327, 11–17. [Google Scholar] [CrossRef]
- Sata, T.; Sata, T.; Yang, W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. J. Memb. Sci. 2002, 206, 31–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.; Tang, H. Membrane technologies for Li+/Mg2+ separation from salt-lake brines and seawater: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Wang, W.; Liu, R.; Tan, M.; Sun, H.; Niu, Q.J.; Xu, T.; Nikonenko, V.; Zhang, Y. Evaluation of the ideal selectivity and the performance of selectrodialysis by using TFC ion exchange membranes. J. Memb. Sci. 2019, 582, 236–245. [Google Scholar] [CrossRef]
- Tanaka, Y. Concentration polarization in ion-exchange membrane electrodialysis: The events arising in an unforced flowing solution in a desalting cell. J. Memb. Sci. 2004, 244, 1–16. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Nikonenko, V.V.; Belova, E.I.; Lopatkova, G.Y.; Sistat, P.; Pourcelly, G.; Larshe, K. Coupled convection of solution near the surface of ion-exchange membranes in intensive current regimes. Russ. J. Electrochem. 2007, 43, 307–327. [Google Scholar] [CrossRef]
- Kontturi, K.; Forssell, P.; Sipilä, A.H. Comparison of Nernst–Planck equations and Miller’s LN approximations for countercurrent electrolysis in a thin porous membrane. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1982, 78, 3613. [Google Scholar] [CrossRef]
- Apel, P. Track etching technique in membrane technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Nichka, V.S.; Mareev, S.A.; Apel, P.Y.; Sabbatovskiy, K.G.; Sobolev, V.D.; Nikonenko, V.V. Modeling the Conductivity and Diffusion Permeability of a Track-Etched Membrane Taking into Account a Loose Layer. Membranes 2022, 12, 1283. [Google Scholar] [CrossRef]
- Sabbatovsky, K.G.; Vilensky, A.I.; Sobolev, V.D.; McHedlishvili, B.V. Influence of thermal treatment on electrosurface and structural properties of poly(ethylene terephthalate) track membranes. Pet. Chem. 2012, 52, 480–486. [Google Scholar] [CrossRef]
- Tang, C.; Yaroshchuk, A.; Bruening, M.L. Flow through negatively charged, nanoporous membranes separates Li + and K + due to induced electromigration. Chem. Commun. 2020, 56, 10954–10957. [Google Scholar] [CrossRef] [PubMed]
- Sprycha, R. Electrical double layer at alumina/electrolyte interface. J. Colloid Interface Sci. 1989, 127, 12–25. [Google Scholar] [CrossRef]
- Winkler, B. Modification of the surface characteristics of anodic alumina membranes using sol–gel precursor chemistry. J. Memb. Sci. 2003, 226, 75–84. [Google Scholar] [CrossRef]
- Poznyak, A.; Knörnschild, G.; Karoza, A.; Norek, M.; Pligovka, A. Peculiar Porous Aluminum Oxide Films Produced via Electrochemical Anodizing in Malonic Acid Solution with Arsenazo-I Additive. Materials 2021, 14, 5118. [Google Scholar] [CrossRef]
- Zou, D.; Qiu, M.; Chen, X.; Fan, Y. One-step preparation of high-performance bilayer α-alumina ultrafiltration membranes via co-sintering process. J. Memb. Sci. 2017, 524, 141–150. [Google Scholar] [CrossRef]
- Shi, W.; Hu, X.; Qiu, M.; Jin, Z.; Chen, X.; Fan, Y. Low temperature preparation of high-flux α-alumina tight ultrafiltration membrane by modified co-sintering process. Sep. Purif. Technol. 2023, 306, 122524. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus recovery from low phosphate-containing solution by electrodialysis. J. Memb. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Ye, Z.-L.; Ghyselbrecht, K.; Monballiu, A.; Pinoy, L.; Meesschaert, B. Fractionating various nutrient ions for resource recovery from swine wastewater using simultaneous anionic and cationic selective-electrodialysis. Water Res. 2019, 160, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xiao, L.; Hu, Z.; Zhan, X. Nutrient recovery from animal manure using bipolar membrane electrodialysis: Study on product purity and energy efficiency. Water Cycle 2020, 1, 54–62. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Wu, G.; Luo, J.; Wang, S. Development of a selective electrodialysis for nutrient recovery and desalination during secondary effluent treatment. Chem. Eng. J. 2017, 322, 224–233. [Google Scholar] [CrossRef]
- Tran, A.T.K.; Zhang, Y.; Lin, J.; Mondal, P.; Ye, W.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. Phosphate pre-concentration from municipal wastewater by selectrodialysis: Effect of competing components. Sep. Purif. Technol. 2015, 141, 38–47. [Google Scholar] [CrossRef]
- Ballet, G.T.; Hafiane, A.; Dhahbi, M. Influence of operating conditions on the retention of phosphate in water by nanofiltration. J. Memb. Sci. 2007, 290, 164–172. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 0-444-50236-X. [Google Scholar]
- Helfferich, F.G. Ion Exchange; McGraw-Hill; Dover Publications: London, UK; New York, NY, USA, 1962; Volume 138, ISBN 0486687848. [Google Scholar]
- Gorobchenko, A.; Mareev, S.; Nikonenko, V. Mathematical Modeling of Monovalent Permselectivity of a Bilayer Ion-Exchange Membrane as a Function of Current Density. Int. J. Mol. Sci. 2022, 23, 4711. [Google Scholar] [CrossRef] [PubMed]


| Ion | Symbol | Diffusion Coefficient, 10−9 m2/s | Stokes Radius, Å |
|---|---|---|---|
| Sodium | Na+ | 1.33 | 1.84 |
| Dihydrogen phosphate | H2PO4− | 0.88 | 2.79 |
| Chloride | Cl− | 2.03 | 1.21 |
| Parameters | TEM #811 | PAAM |
|---|---|---|
| Thickness | 10 μm | 80 μm |
| Pore density * | 5.0 × 109 pores/cm2 | 1.0 × 1010 pores/cm2 |
| Pore diameter * | 35 ± 3.0 nm | 44 ± 2.0 nm |
| Surface porosity | 5.3 ± 1.0% | 16.7 ± 1.5% |
| Hydraulic permeability | 0.10 ± 0.02 cm3/(cm2·min·bar) | 0.06 ± 0.02 cm3/(cm2·min·bar) |
| Functional groups | Hydroxyl and carboxyl groups [44] | Alumina polyhydroxocomplexes [45] |
| Method | Membrane | Feed Solution | Experiment Details | Competing Anion, A− | |||
|---|---|---|---|---|---|---|---|
| Conventional Electrodialysis Ref. [63] | 2 cell pair with HDX100 CEM and HDX200 AEM (Iontech, Hangzhou, China) | 3.4 × 10−3 M Na2SO4, | 7 A/m2 | 4.2 × 10−3 | SO42− | 0.137 | 0.64 |
| 8.2 × 10−5 M Na2HPO4·7H2O, | |||||||
| 8.0 × 10−5 M NaH2PO4·H2O | |||||||
| (pH ≈ 7.0) | |||||||
| Selective Electrodialysis Ref. [64] | 5 cell pair with monovalent-selective PC-MVK and PC-MVA membranes, as well as PC-SA, PC-SK, PC-SC (PCA GmbH, Heusweiler, Germany) | 1.3 × 10−3 M NaH2PO4·H2O, | 8 V (~45 A/m2) | 6.4 × 10−3 | SO42− | 0.017 | 0.29 |
| 0.036 M NH4Cl, | |||||||
| 1 × 10−3 M Na2SO4, | |||||||
| 0.01 M KCl, | |||||||
| 2.5 × 10−3 M MgCl2, | |||||||
| 2.5 × 10−3 M CaCl2 | |||||||
| (pH = 4.9) | |||||||
| Bipolar Membrane Electrodialysis Ref. [65] | 5 cell pair with commercial heterogeneous CEM, AEM, and BPM (MemBrain®, Stráž pod Ralskem, Czech Republic) | 0.08 M NH4Cl, | ~167 A/m2, <60 V | 0.144 | Cl− CH3COO− | 0.46 0.35 | 1.2 1.3 |
| 0.075 M (NH4)2SO4, | |||||||
| 0.022 M NaH2PO4, | |||||||
| 0.07 M CH3COONH4, | |||||||
| 0.014 M H3PO4, | |||||||
| 2.64 mL/L butyric acid, | |||||||
| 2.04 mL/L valeric acid | |||||||
| (pH = 6.0) | |||||||
| Selective Electrodialysis Ref. [66] | 3 cell pair with monovalent-selective Neosepta ACS, as well as Neosepta CMX and Neosepta AMX (Astom Co., Shunan, Japan) | 0.01 M NaCl, | 9 V | 1.2 × 10−4 | Cl− NO3− | 0.043 4.5 × 10−3 | 0.09 0.175 |
| 2.1 × 10−3 M NaNO3, | |||||||
| 3.2 × 10−4 M Na2HPO4, | |||||||
| (pH = 7.0) | |||||||
| Conventional Electrodialysis and Selective Electrodialysis Ref. [67] | 3 cell pair with monovalent-selective PC-MVA, as well as PC-SA and PC-SK (PCA GmbH, Heusweiler, Germany) | 0.023 M Cl−, | 62.5 A/m2 <12 V | 0.016 over the PC-MVA membrane, S-ED 0.036 over the PC-SA membrane, ED | Cl− | 1.32 1.76 | 0.28 0.47 |
| 1 × 10−3 M HxPO4(3-x)−, | |||||||
| 2 × 10−3 M NO3−, | |||||||
| 2 × 10−3 M HCO3−, | |||||||
| 2 × 10−3 M SO42−, | |||||||
| 2 × 10−3 M Ca2+, | |||||||
| 2 × 10−3 M Mg2+ | |||||||
| (pH = 5.5) | |||||||
| Nanofiltration Ref. [22] | NF270 membrane (Dupont, New York, NY, USA) | 0.01 M NaCl, | 20 bar | ~0.9 × 10−3 for HPO42− | Cl− | ~0.57 | ~0.04- 0.07 |
| 2 × 10−3 M K2HPO4 | ~4.3 × 10−3 for HxPO4− | ||||||
| (pH = 8.9) | |||||||
| 0.01 M Na2SO4, | ~0.3 × 10−3 for HPO42− | SO42− | ~3.2 × 10−3 | 0.7−10 | |||
| 0.5 × 10−3 M K2HPO4 | ~1.5 × 10−3 for HxPO4− | ||||||
| (pH = 7.2) | |||||||
| Nanofiltration Ref. [68] | NF200 (Dow, Midland, MI, USA) | 1 × 10−3 M NaCl, | 5 bar | 2.8 × 10−3 | Cl− NO3− | 9.5 × 10−3 0.036 | 0.5 0.25 |
| 1 × 10−3 M NaNO3, | |||||||
| 1 × 10−3 M NaH2PO4 | |||||||
| (pH = 5.2–5.4) | |||||||
| Hybrid Electrobaromembrane (EBM) method [this work] | 1 cell pair with TEM #811 track-etched membrane | 0.05 M NaCl, 0.05 M NaH2PO4 (pH = 3.8–3.9) | 0.3 bar, 50 A/m2 | −0.285 | Cl− | −0.022 | 12.5 |
| 0.3 bar, 100 A/m2 | −0.055 | 0.402 | −0.13 | ||||
| or porous anodic alumina membrane (PAAM) | 0.3 bar, 50 A/m2 | −0.005 | Cl− | 0.330 | −0.015 | ||
| Both porous membranes were supplemented by two MK-40 (JCC Shchekinoazot, Russia) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butylskii, D.; Troitskiy, V.; Chuprynina, D.; Kharchenko, I.; Ryzhkov, I.; Apel, P.; Pismenskaya, N.; Nikonenko, V. Selective Separation of Singly Charged Chloride and Dihydrogen Phosphate Anions by Electrobaromembrane Method with Nanoporous Membranes. Membranes 2023, 13, 455. https://doi.org/10.3390/membranes13050455
Butylskii D, Troitskiy V, Chuprynina D, Kharchenko I, Ryzhkov I, Apel P, Pismenskaya N, Nikonenko V. Selective Separation of Singly Charged Chloride and Dihydrogen Phosphate Anions by Electrobaromembrane Method with Nanoporous Membranes. Membranes. 2023; 13(5):455. https://doi.org/10.3390/membranes13050455
Chicago/Turabian StyleButylskii, Dmitrii, Vasiliy Troitskiy, Daria Chuprynina, Ivan Kharchenko, Ilya Ryzhkov, Pavel Apel, Natalia Pismenskaya, and Victor Nikonenko. 2023. "Selective Separation of Singly Charged Chloride and Dihydrogen Phosphate Anions by Electrobaromembrane Method with Nanoporous Membranes" Membranes 13, no. 5: 455. https://doi.org/10.3390/membranes13050455
APA StyleButylskii, D., Troitskiy, V., Chuprynina, D., Kharchenko, I., Ryzhkov, I., Apel, P., Pismenskaya, N., & Nikonenko, V. (2023). Selective Separation of Singly Charged Chloride and Dihydrogen Phosphate Anions by Electrobaromembrane Method with Nanoporous Membranes. Membranes, 13(5), 455. https://doi.org/10.3390/membranes13050455












