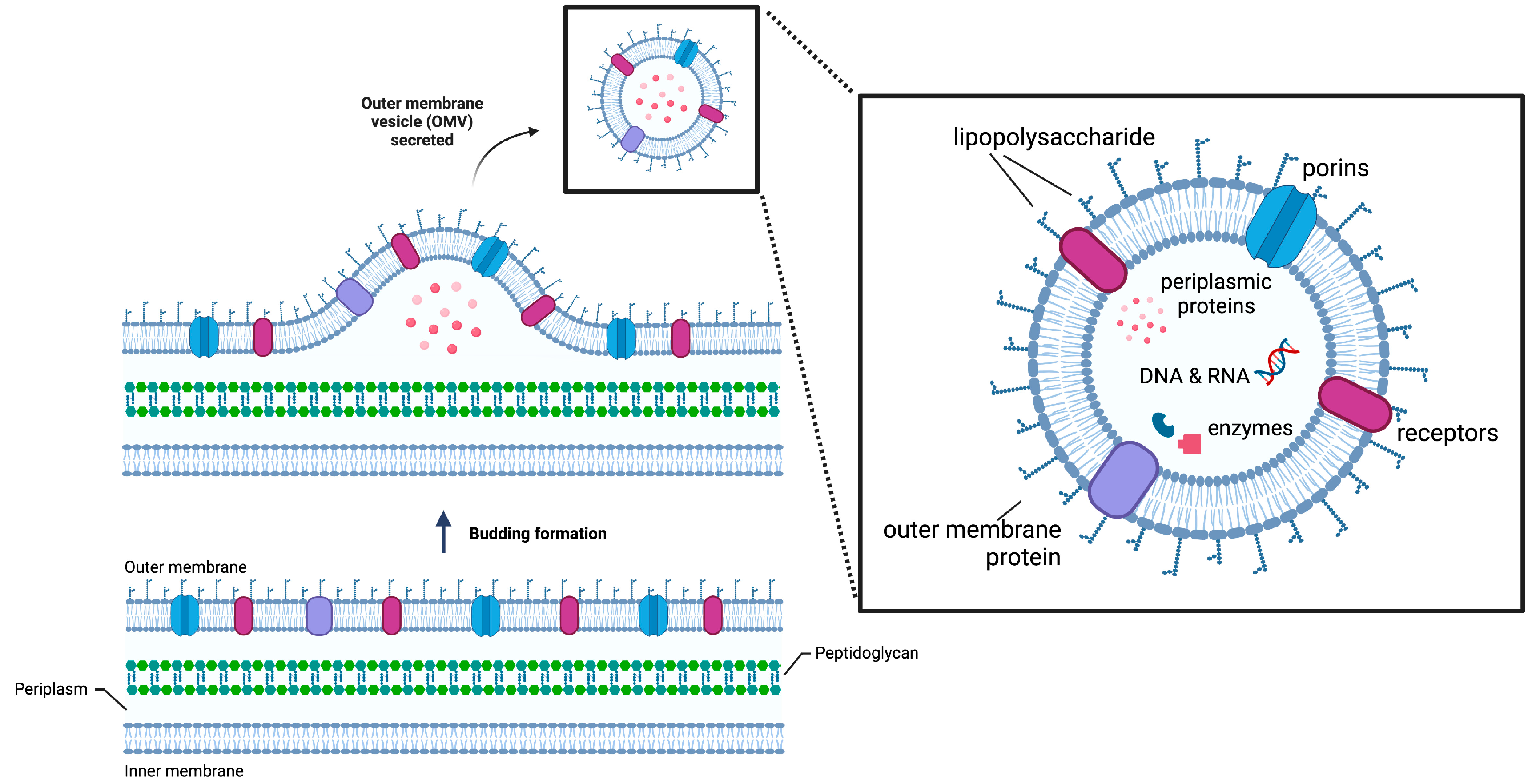
Functionalization of OMVs for Biocatalytic Applications
Abstract
:1. Introduction
2. OMVs Functionalization by Immobilization of Enzyme
2.1. Enzyme Display on OMV Surface
2.1.1. Fusion with ClyA
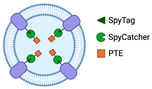
2.1.2. Bioconjugation with SpyTag/SpyCatcher
2.1.3. The Utilization of the Ice-Nucleation Protein
2.2. OMV-Mediated Encapsulation
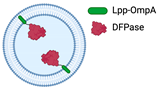
2.2.1. Physical Functionalization
2.2.2. Genetic Engineering Approach
3. The Role of OMVs as Biocatalysts
3.1. Bioconversion
3.2. Bioremediation
3.3. Biomass Degradation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Furuyama, N.; Sircili, M.P. Outer membrane vesicles (OMVs) produced by gram-negative bacteria: Structure, functions, biogenesis, and vaccine application. BioMed Res. Int. 2021, 2021, 1490732. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Sullivan, C.J.; Kuehn, M.J. Envelope control of outer membrane vesicle production in Gram-negative bacteria. Biochemistry 2013, 52, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K. Bacterial membrane vesicles (MVs): Novel tools as nature- and nano-carriers for immunogenic antigen, enzyme support, and drug delivery. Appl. Microbiol. Biotechnol. 2016, 100, 9837–9843. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.D.; Sirisaengtaksin, N.; O’Brien-Simpson, N.M.; Krachler, A.M. Outer Membrane Vesicle-Host Cell Interactions. Microbiol. Spectr. 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J. Structures of Gram-Negative Cell Walls and Their Derived Membrane. Vesicles 1999, 181, 4725–4733. [Google Scholar] [CrossRef]
- Wai, S.N.; Lindmark, B.; Söderblom, T.; Takade, A.; Westermark, M.; Oscarsson, J.; Jass, J.; Richter-Dahlfors, A.; Mizunoe, Y.; Uhlin, B.E. Vesicle-Mediated Export and Assembly of Pore-Forming Oligomers of the Enterobacterial ClyA Cytotoxin. Cell 2003, 115, 25–35. [Google Scholar] [CrossRef]
- Kim, J.Y.; Doody, A.M.; Chen, D.J.; Cremona, G.H.; Shuler, M.L.; Putnam, D.; DeLisa, M.P. Engineered Bacterial Outer Membrane Vesicles with Enhanced Functionality. J. Mol. Biol. 2008, 380, 51–66. [Google Scholar] [CrossRef]
- Yoon, H. Bacterial outer membrane vesicles as a delivery system for virulence regulation. J. Microbiol. Biotechnol. 2016, 26, 1343–1347. [Google Scholar] [CrossRef]
- Li, R.; Liu, Q. Engineered Bacterial Outer Membrane Vesicles as Multifunctional Delivery Platforms. Front. Mater. 2020, 7, 10. [Google Scholar] [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: An overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Vitse, J.; Devreese, B. The Contribution of Membrane Vesicles to Bacterial Pathogenicity in Cystic Fibrosis Infections and Healthcare Associated Pneumonia. Front. Microbiol. 2020, 11, 630. [Google Scholar] [CrossRef]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of gram-negative bacteria: A perspective update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Rutherford, N.; Mourez, M. Surface display of proteins by Gram-negative bacterial autotransporters. Microb. Cell Factories 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Bonnington, K.E.; Kuehn, M.J. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.J.; Turner, K.B.; Daniele, M.A.; Oh, E.; Medintz, I.L.; Walper, S.A. Bacterial Nanobioreactors-Directing Enzyme Packaging into Bacterial Outer Membrane Vesicles. ACS Appl. Mater. Interfaces 2015, 7, 24963–24972. [Google Scholar] [CrossRef]
- Alves, N.J.; Moore, M.; Johnson, B.J.; Dean, S.N.; Turner, K.B.; Medintz, I.L.; Walper, S.A. Environmental Decontamination of a Chemical Warfare Simulant Utilizing a Membrane Vesicle-Encapsulated Phosphotriesterase. ACS Appl. Mater. Interfaces 2018, 10, 15712–15719. [Google Scholar] [CrossRef]
- Alves, N.J.; Turner, K.B.; Medintz, I.L.; Walper, S.A. Protecting enzymatic function through directed packaging into bacterial outer membrane vesicles. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Thakur, M.; Dean, S.N.; Moore, M.; Spangler, J.R.; Johnson, B.J.; Medintz, I.L.; Walper, S.A. Packaging of Diisopropyl Fluorophosphatase (DFPase) in Bacterial Outer Membrane Vesicles Protects Its Activity at Extreme Temperature. ACS Biomater. Sci. Eng. 2022, 8, 493–501. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Woo, J.M.; Kim, M.Y.; Song, J.W.; Baeg, Y.; Jo, H.J.; Cha, S.S.; Park, J.B. Engineering of a bacterial outer membrane vesicle to a nano-scale reactor for the biodegradation of β-lactam antibiotics. J. Biotechnol. 2022, 356, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological Functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Sun, Q.; Liu, F.; DeLisa, M.P.; Chen, W. Positional assembly of enzymes on bacterial outer membrane vesicles for cascade reactions. PLoS ONE 2014, 9, e97103. [Google Scholar] [CrossRef] [PubMed]
- Su, F.H.; Tabañag, I.D.F.; Wu, C.Y.; Tsai, S.L. Decorating outer membrane vesicles with organophosphorus hydrolase and cellulose binding domain for organophosphate pesticide degradation. Chem. Eng. J. 2017, 308, 1–7. [Google Scholar] [CrossRef]
- Kesty, N.C.; Kuehn, M.J. Incorporation of Heterologous Outer Membrane and Periplasmic Proteins into Escherichia coli Outer Membrane Vesicles. J. Biol. Chem. 2004, 279, 2069–2076. [Google Scholar] [CrossRef]
- Song, J.W.; Baeg, Y.; Jeong, H.Y.; Lee, J.; Oh, D.K.; Hollmann, F.; Park, J.B. Bacterial Outer Membrane Vesicles as Nano-Scale Bioreactors: A Fatty Acid Conversion Case Study. ChemCatChem 2021, 13, 4080–4086. [Google Scholar] [CrossRef]
- Ludwig, A.; Tengel, C.; Bubert, S.B.A.; Benz, R.; Goebel, H.M.-J.W. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coil. Mol. Gen. Genet. MGG 1995, 249, 474–486. [Google Scholar] [CrossRef]
- Oscarsson, J.; Mizunoe, Y.; Bernt; Uhlin, E.; Haydon, D.J. Induction of haemolytic activity in Escherichia coli by the s/yA gene product. Mol. Microbiol. 1996, 20, 191–199. [Google Scholar] [CrossRef]
- Murase, K. Cytolysin A (ClyA): A Bacterial Virulence Factor with Potential Applications in Nanopore Technology, Vaccine Development, and Tumor Therapy. Toxins 2022, 14, 78. [Google Scholar] [CrossRef]
- Molloy, M.P.; Herbert, B.R.; Slade, M.B.; Rabilloud, T.; Nouwens, A.S.; Williams, K.L.; Gooley, A.A. Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 2000, 267, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Bang, J.Y.; Park, G.W.; Choi, D.S.; Kang, J.S.; Kim, H.J.; Park, K.S.; Lee, J.O.; Kim, Y.K.; Kwon, K.H.; et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Matsuyama, S.I. Sorting of lipoproteins to the outer membrane in E. coli. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1693, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Reddington, S.C.; Howarth, M. Secrets of a covalent interaction for biomaterials and biotechnology: SpyTag and SpyCatcher. Curr. Opin. Chem. Biol. 2015, 29, 94–99. [Google Scholar] [CrossRef]
- Amelung, S.; Nerlich, A.; Rohde, M.; Spellerberg, B.; Cole, J.N.; Nizet, V.; Chhatwal, G.S.; Talay, S.R. The fbab-type fibronectin-binding protein of streptococcus pyogenes promotes specific invasion into endothelial cells. Cell Microbiol. 2011, 13, 1200–1211. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- van den Berg van Saparoea, H.B.; Houben, D.; de Jonge, M.I.; Jong, W.S.P.; Luirink, J. Display of recombinant proteins on bacterial outer membrane vesicles by using protein ligation. Appl. Environ. Microbiol. 2018, 84, e02567-17. [Google Scholar] [CrossRef]
- Jiang, L.; Driedonks, T.A.; Jong, W.S.; Dhakal, S.; Bart van den Berg van Saparoea, H.; Sitaras, I.; Zhou, R.; Caputo, C.; Littlefield, K.; Lowman, M.; et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J. Extracell. Vesicles 2022, 11, e12192. [Google Scholar] [CrossRef]
- Hatlem, D.; Trunk, T.; Linke, D.; Leo, J.C. Catching a SPY: Using the SpyCatcher-SpyTag and related systems for labeling and localizing bacterial proteins. Int. J. Mol. Sci. 2019, 20, 2129. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Q.; Chen, J.; He, Y.; Wang, J.; Zhang, H.; Yu, Z.; Li, L. Molecular characterization of an ice nucleation protein variant (InaQ) from Pseudomonas syringae and the analysis of its transmembrane transport activity in Escherichia coli. Int. J. Biol. Sci. 2012, 8, 1097–1108. [Google Scholar] [CrossRef]
- Warren, G.; Corotto, L.; Wolber, P. Conserved repeats in diverged ice nucleation structural genes from two species of Pseudomonas. Nucleic Acids Res. 1986, 14, 8047–8060. [Google Scholar] [CrossRef] [PubMed]
- Deininger, C.A.; Mueller, G.M.; Wolber, P.K. Immunological Characterization of Ice Nucleation Proteins from Pseudomonas syringae, Pseudomonas fluorescens, and Erwinia herbicola. J. Bacteriol. 1988, 170, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Lorv, J.S.H.; Rose, D.R.; Glick, B.R. Bacterial Ice Crystal Controlling Proteins. Scientifica 2014, 2014, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Schinner, F.; Marx, J.C.; Gerday, C. Psychrophiles: From Biodiversity to Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Sarhan, M.A.A. Ice nucleation protein as a bacterial surface display protein. Arch. Biol. Sci. 2011, 63, 943–948. [Google Scholar] [CrossRef]
- Li, L.; Kang, D.G.; Cha, H.J. Functional Display of Foreign Protein on Surface of Escherichia coli Using N-Terminal Domain of Ice Nucleation Protein. Biotechnol. Bioeng. 2004, 85, 214–221. [Google Scholar] [CrossRef]
- Tsai, S.L.; Oh, J.; Singh, S.; Chen, R.; Chen, W. Functional assembly of minicellulosomes on the Saccharomyces cerevisiae cell surface for cellulose hydrolysis and ethanol production. Appl. Environ. Microbiol. 2009, 75, 6087–6093. [Google Scholar] [CrossRef]
- Kuerban, K.; Gao, X.; Zhang, H.; Liu, J.; Dong, M.; Wu, L.; Ye, R.; Feng, M.; Ye, L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm. Sin. B 2020, 10, 1534–1548. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Qiu, X.; Dong, S.; He, J.; Liu, J.; Xu, W.; Huang, S.; Hu, X.; Xiang, D.X. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact. Mater. 2023, 20, 548–560. [Google Scholar] [CrossRef]
- Huang, Y.; Nieh, M.P.; Chen, W.; Lei, Y. Outer membrane vesicles (OMVs) enabled bio-applications: A critical review. Biotechnol. Bioeng. 2022, 119, 34–47. [Google Scholar] [CrossRef]
- Ayed, Z.; Cuvillier, L.; Dobhal, G.; Goreham, R.V. Electroporation of outer membrane vesicles derived from Pseudomonas aeruginosa with gold nanoparticles. SN Appl. Sci. 2019, 1, 1600. [Google Scholar] [CrossRef]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Drug delivery application of extracellular vesicles; insight into production, drug loading, targeting, and pharmacokinetics. AIMS Bioeng 2017, 4, 73–92. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, H.; Wu, W.; Huang, G.; Li, Y.; Wu, M.; Tang, G.; Ping, Y. Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention. Nano Lett. 2020, 20, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Fonseca, S.; Miquel-Clopés, A.; Cross, K.; Kok, K.S.; Wegmann, U.; Gil-Cardoso, K.; Bentley, E.G.; Al Katy, S.H.; Coombes, J.L.; et al. Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J. Extracell. Vesicles 2019, 8, 1632100. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Antenucci, F.; Villumsen, K.R.; Bojesen, A.M. Bacterial outer membrane vesicles as a versatile tool in vaccine research and the fight against antimicrobial resistance. mBio 2021, 12, e01707-21. [Google Scholar] [CrossRef]
- Kim, O.Y.; Park, H.T.; Dinh, N.T.H.; Choi, S.J.; Lee, J.; Kim, J.H.; Lee, S.W.; Gho, Y.S. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat. Commun. 2017, 8, 626. [Google Scholar] [CrossRef]
- Gujrati, V.; Prakash, J.; Malekzadeh-Najafabadi, J.; Stiel, A.; Klemm, U.; Mettenleiter, G.; Aichler, M.; Walch, A.; Ntziachristos, V. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat. Commun. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Chen, W.; Nieh, M.P.; Lei, Y. Genetically engineered bio-nanoparticles with co-expressed enzyme reporter and recognition element for IgG immunoassay. Sens. Actuators Rep. 2019, 1, 100003. [Google Scholar] [CrossRef]
- Devriese, D.; Surmont, P.; Lynen, F.; Devreese, B. Display of the self-sufficient CYP102A1 on the surface of E. coli-derived Outer Membrane Vesicles. bioRxiv 2021, 3, 2021–2106. [Google Scholar] [CrossRef]
- Stentz, R.; Horn, N.; Cross, K.; Salt, L.; Brearley, C.; Livermore, D.M.; Carding, S.R. Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against β-lactam antibiotics. J. Antimicrob. Chemother. 2015, 70, 701–709. [Google Scholar] [CrossRef]
- Bos, M.P.; Poolman, J.; Tefsen, B.; Tommassen, J.P.M. Outer Membrane Vesicles and Uses Thereof. U.S. Patent US007628995B2, 8 December 2009. [Google Scholar]
- Van De Waterbeemd, B.; Van Pol, L.A. Process for Detergent-Free Production of Outer Membrane Vesicles of A Gram-Negative Bacterium. U.S. Patent US009707286B2, 18 July 2017. Available online: https://patentimages.storage.googleapis.com/3f/84/39/5cd3b6c5de698a/US9707286.pdf (accessed on 18 April 2023).
| Enzyme Immobilization | Superiority | Deficiency | Detail Method/Illustration | Application | Ref. |
|---|---|---|---|---|---|
| |||||
| Fusion with ClyA (and other OMVs’ anchoring motifs, such as OmpA) | Numerous outer membrane proteins can serve as anchoring motifs | Limited expression of proteins that are too large to be transported out of the cytoplasm | 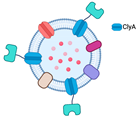 | Degradation of paraoxon and antibiotics | [7] |
| Bioconjugation with SpyTag/SpyCatcher | Capable of displaying functional proteins that are challenging to export from the cell | SpyTag’s and SpyCatcher’s covalent bond is irreversible. | 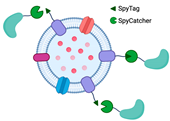 | Little is known regarding the use of Spy systems to display enzymes on OMVs for biocatalyst purposes | * |
| Utilization of ice-nucleation protein (INP) | Facilitating assemblies with trivalent scaffolds is highly promising for simultaneously expressing multiple enzymes for cascade reactions | The size of INP is relatively bigger than Spycatcher. | 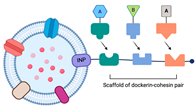 | Enhanced glucose yield from cellulose degradation | [24,25] |
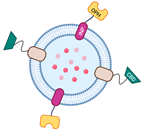 | Paraoxon degradation to para-nitrophenol | [25] | |||
| |||||
| Physical functionalization | Comparatively simple operation | Limited loading efficiency |
| There has been no report on the use of this technique for enzyme entrapment in the OMVs lumen for biocatalytic purposes | |
| Genetic engineering | Improved enzyme stability and offers high loading efficiency | Limited interaction with the surrounding substrate |
| Decontamination of CWA, including paraoxon | [16,17,18] |
| Hydrolyze DFP and paraoxon | [19] | |||
| GFP entrapment in OMVs lumen (there have been no data for enzyme entrapment utilizes Tat signal for functionalization of OMVs as biocatalysts) | [26] | |||
| Bioconversion of fatty acid | [27] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amalia, L.; Tsai, S.-L. Functionalization of OMVs for Biocatalytic Applications. Membranes 2023, 13, 459. https://doi.org/10.3390/membranes13050459
Amalia L, Tsai S-L. Functionalization of OMVs for Biocatalytic Applications. Membranes. 2023; 13(5):459. https://doi.org/10.3390/membranes13050459
Chicago/Turabian StyleAmalia, Lita, and Shen-Long Tsai. 2023. "Functionalization of OMVs for Biocatalytic Applications" Membranes 13, no. 5: 459. https://doi.org/10.3390/membranes13050459
APA StyleAmalia, L., & Tsai, S.-L. (2023). Functionalization of OMVs for Biocatalytic Applications. Membranes, 13(5), 459. https://doi.org/10.3390/membranes13050459