The Potassium Efflux System Kef: Bacterial Protection against Toxic Electrophilic Compounds
Abstract
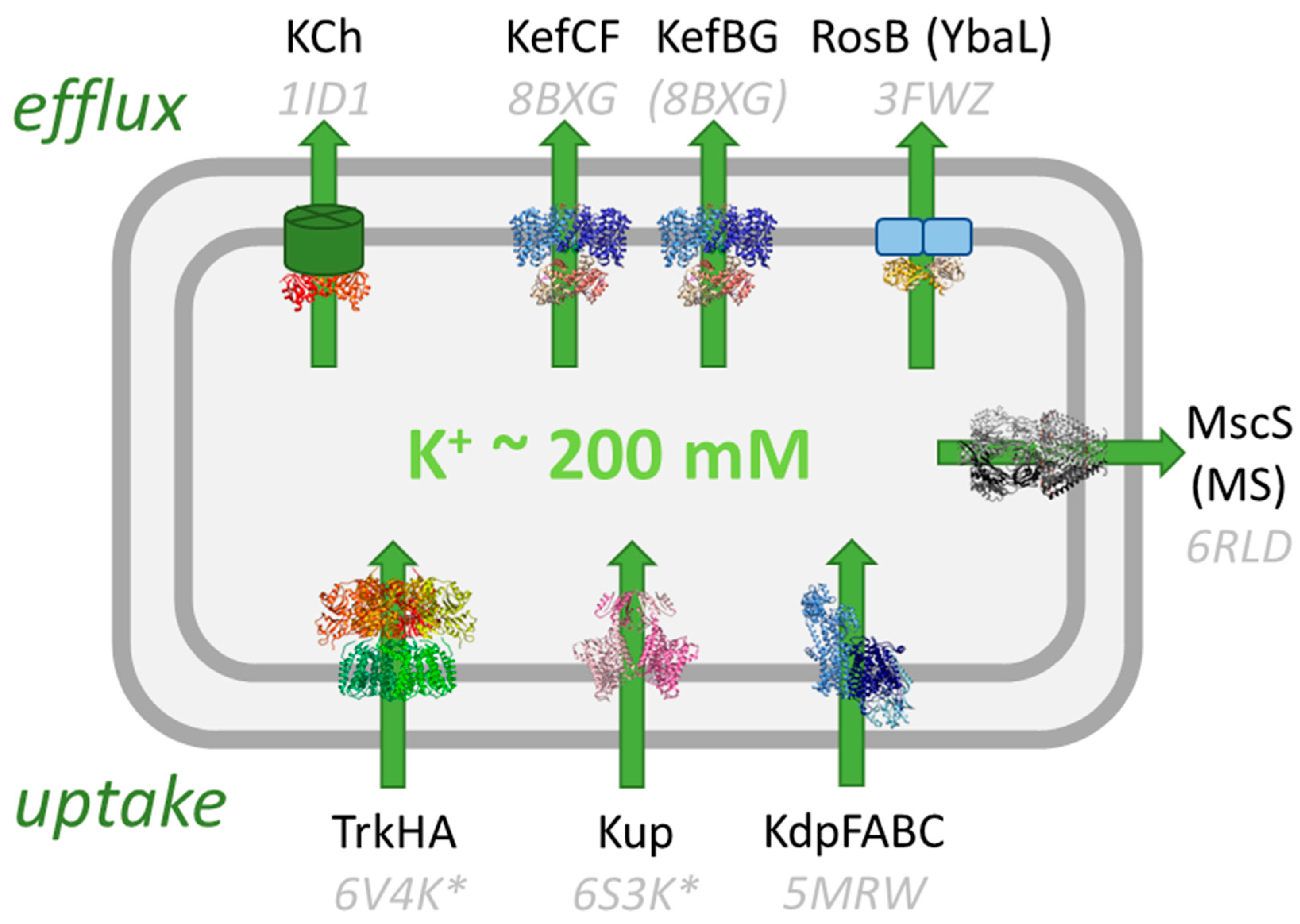
:1. Introduction
2. Natural Sources of Electrophiles

3. Activation and Specificity of Kef
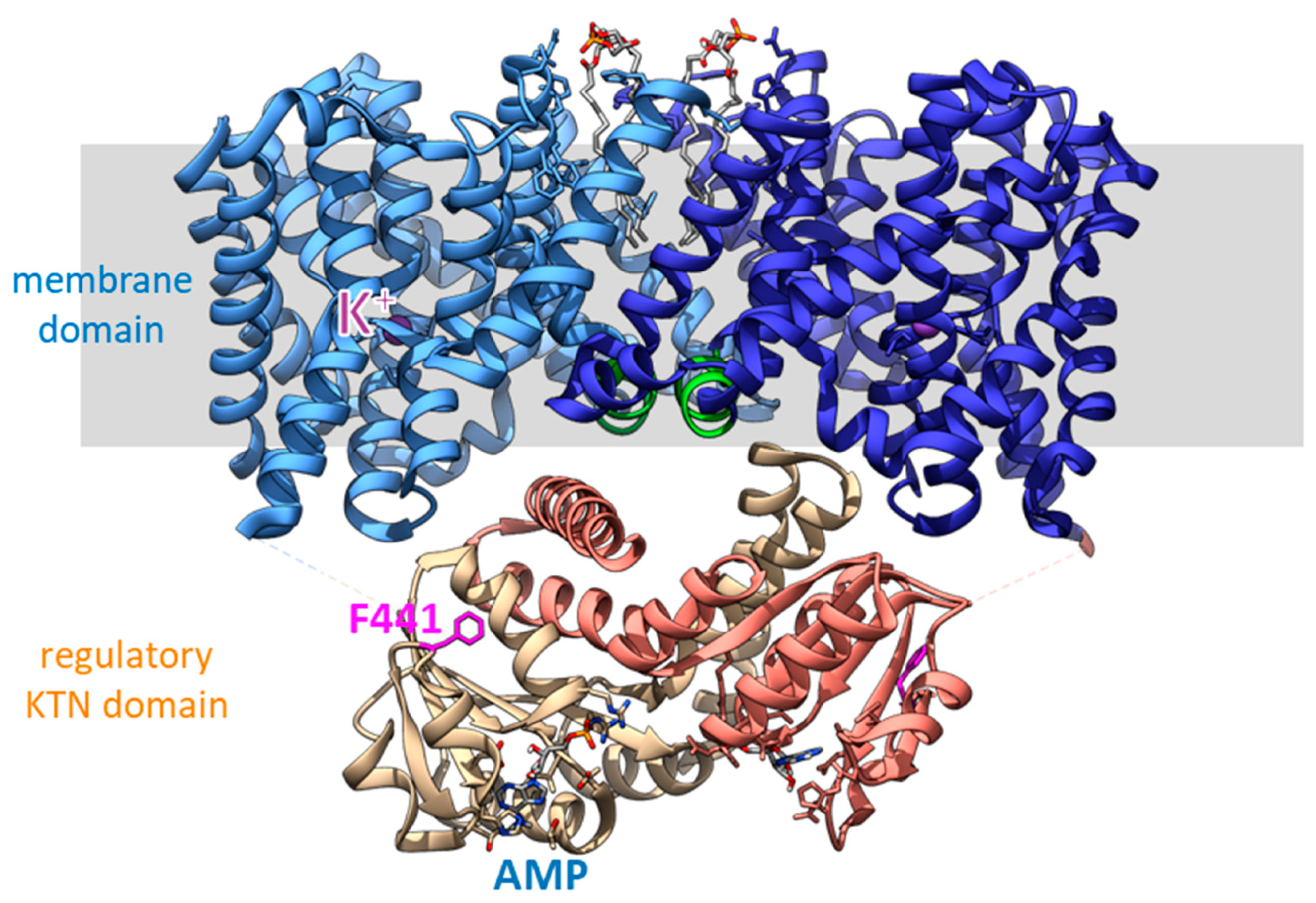
4. Structural Studies of Kef Revealed Details of Regulation
5. The Ancillary Subunit of Kef Is an Oxidoreductase
6. Homologs of Kef
7. Conclusions and Outlook
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epstein, W. The Roles and Regulation of Potassium in Bacteria. In Progress in Nucleic Acid Research and Molecular Biology; Academic Press: New York, NY, USA, 2003; Volume 75, pp. 293–320. ISBN 0125400756. [Google Scholar]
- Stautz, J.; Hellmich, Y.; Fuss, M.F.; Silberberg, J.M.; Devlin, J.R.; Stockbridge, R.B.; Hänelt, I. Molecular Mechanisms for Bacterial Potassium Homeostasis. J. Mol. Biol. 2021, 433, 166968. [Google Scholar] [CrossRef] [PubMed]
- Shabala, L.; Bowman, J.; Brown, J.; Ross, T.; McMeekin, T.; Shabala, S. Ion Transport and Osmotic Adjustment in Escherichia coli in Response to Ionic and Non-Ionic Osmotica. Environ. Microbiol. 2009, 11, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Booth, I. Bacterial Mechanosensitive Channels: Progress towards an Understanding of Their Roles in Cell Physiology. Curr. Opin. Microbiol. 2014, 18, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Blount, P.; Iscla, I. Life with Bacterial Mechanosensitive Channels, from Discovery to Physiology to Pharmacological Target. Microbiol. Mol. Biol. Rev. 2020, 84, e00055-19. [Google Scholar] [CrossRef] [PubMed]
- Ungar, D.; Barth, A.; Haase, W.; Kaunzinger, A.; Lewitzki, E.; Ruiz, T.; Reiländer, H.; Michel, H. Analysis of a Putative Voltage-Gated Prokaryotic Potassium Channel. Eur. J. Biochem. 2001, 268, 5386–5396. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; Hu, L.; Hudson, M.A.; Hofstetter, K.S.; Xu, Z.; Rong, M.; Wang, Z.; Prasad, B.V.V.; Lockless, S.W.; et al. TrkA Undergoes a Tetramer-to-Dimer Conversion to Open TrkH Which Enables Changes in Membrane Potential. Nat. Commun. 2020, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Tascón, I.; Sousa, J.S.; Corey, R.A.; Mills, D.J.; Griwatz, D.; Aumüller, N.; Mikusevic, V.; Stansfeld, P.J.; Vonck, J.; Hänelt, I. Structural Basis of Proton-Coupled Potassium Transport in the KUP Family. Nat. Commun. 2020, 11, 626. [Google Scholar] [CrossRef]
- Huang, C.S.; Pedersen, B.P.; Stokes, D.L. Crystal Structure of the Potassium-Importing KdpFABC Membrane Complex. Nature 2017, 546, 681–685. [Google Scholar] [CrossRef]
- Rasmussen, T.; Flegler, V.J.; Rasmussen, A.; Böttcher, B. Structure of the Mechanosensitive Channel MscS Embedded in the Membrane Bilayer. J. Mol. Biol. 2019, 431, 3081–3090. [Google Scholar] [CrossRef]
- Gulati, A.; Kokane, S.; Boerema, A.; Alleva, C.; Meier, P.; Matsuoka, R.; Drew, D. Structure and Mechanism of the K+/H+ Exchanger KefC. Res. Sq. 2023, 1–48, in preprint. [Google Scholar] [CrossRef]
- Jiang, Y.; Pico, A.; Cadene, M.; Chait, B.T.; MacKinnon, R. Structure of the RCK Domain from the E. coli K+ Channel and Demonstration of Its Presence in the Human BK Channel. Neuron 2001, 29, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D.C. Protein Damage by Reactive Electrophiles: Targets and Consequences. Chem. Res. Toxicol. 2008, 21, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.E. Preformed Antimicrobial Compounds and Plant Defense against Fungal Attack. Plant Cell 1996, 8, 1821–1831. [Google Scholar] [CrossRef]
- Cowan, M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Tierens, K.F.; Thomma, B.P.; Brouwer, M.; Schmidt, J.; Kistner, K.; Porzel, A.; Mauch-Mani, B.; Cammue, B.P.; Broekaert, W.F. Study of the Role of Antimicrobial Glucosinolate-Derived Isothiocyanates in Resistance of Arabidopsis to Microbial Pathogens. Plant Physiol. 2001, 125, 1688–1699. [Google Scholar] [CrossRef]
- Brigham, L.; Michaels, P.; Flores, H. Cell-Specific Production and Antimicrobial Activity of Naphthoquinones in Roots of Lithospermum erythrorhizon. Plant Physiol. 1999, 119, 417–428. [Google Scholar] [CrossRef]
- Duffey Sean, S.; Felton Gary, W. Enzymatic Antinutritive Defenses of the Tomato Plant Against Insects. In Naturally Occurring Pest Bioregulators; Hedin, P.A., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1991; Volume 449, pp. 166–197. ISBN 0-8412-1897-8. [Google Scholar]
- Gersch, M.; Kreuzer, J.; Sieber, S. Electrophilic Natural Products and Their Biological Targets. Nat. Prod. Rep. 2012, 29, 659. [Google Scholar] [CrossRef] [PubMed]
- Blum, M. Chemical Defenses of Arthropods, 1st ed.; Academic Press: New York, NY, USA, 1981; ISBN 0323145558/9780323145558. [Google Scholar]
- Gehrtz, P.; London, N. Electrophilic Natural Products as Drug Discovery Tools. Trends Pharmacol. Sci. 2021, 42, 434–447. [Google Scholar] [CrossRef]
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous Generation of Reactive Oxidants and Electrophiles and Their Reactions with DNA and Protein. J. Clin. Investig. 2003, 111, 583–593. [Google Scholar] [CrossRef]
- Halliwell, B. Phagocyte-Derived Reactive Species: Salvation or Suicide? Trends Biochem. Sci. 2006, 31, 509–515. [Google Scholar] [CrossRef]
- Lindblad, B.; Lindstedt, S.; Steen, G. On the Enzymic Defects in Hereditary Tyrosinemia. Proc. Natl. Acad. Sci. USA 1977, 74, 4641–4645. [Google Scholar] [CrossRef] [PubMed]
- Zimniak, P. Relationship of Electrophilic Stress to Aging. Free Radic. Biol. Med. 2011, 51, 1087–1105. [Google Scholar] [CrossRef]
- Totemeyer, S.; Booth, N.A.; Nichols, W.W.; Dunbar, B.; Booth, I.R. From Famine to Feast: The Role of Methylglyoxal Production in Escherichia coli. Mol. Microbiol. 1998, 27, 553–562. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Tötemeyer, S.; MacLean, M.J.; Booth, I.R. Methylglyoxal Production in Bacteria: Suicide or Survival? Arch. Microbiol. 1998, 170, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.; Ekkerman, S.; Pliotas, C.; Richard, M.; Bartlett, W.; Grayer, S.C.; Morris, G.M.; Miller, S.; Booth, I.R.; Conway, S.J.; et al. Understanding the Structural Requirements for Activators of the Kef Bacterial Potassium Efflux System. Biochemistry 2014, 53, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Roosild, T.P.; Castronovo, S.; Healy, J.; Miller, S.; Pliotas, C.; Rasmussen, T.; Bartlett, W.; Conway, S.J.; Booth, I.R. Mechanism of Ligand-Gated Potassium Efflux in Bacterial Pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 19784–19789. [Google Scholar] [CrossRef]
- Miller, S.; Ness, L.S.; Wood, C.M.; Fox, B.C.; Booth, I.R. Identification of an Ancillary Protein, YabF, Required for Activity of the KefC Glutathione-Gated Potassium Efflux System in Escherichia coli. J. Bacteriol. 2000, 182, 6536–6540. [Google Scholar] [CrossRef]
- Lyngberg, L.; Healy, J.; Bartlett, W.; Miller, S.; Conway, S.J.; Booth, I.R.; Rasmussen, T. KefF, the Regulatory Subunit of the Potassium Efflux System KefC, Shows Quinone Oxidoreductase Activity. J. Bacteriol. 2011, 193, 4925–4932. [Google Scholar] [CrossRef]
- Meury, J.; Lebail, S.; Kepes, A. Opening of Potassium Channels in Escherichia coli Membranes by Thiol Reagents and Recovery of Potassium Tightness. Eur. J. Biochem. 1980, 113, 33–38. [Google Scholar] [CrossRef]
- Bakker, E.P.; Mangerich, W.E. N-Ethylmaleimide Induces K+ -H+ Antiport Activity in Escherichia coli K-12. FEBS Lett. 1982, 140, 177–180. [Google Scholar] [CrossRef]
- Nakamura, T.; Tokuda, H.; Unemoto, T. N-Ethylmaleimide Desensitizes PH-Dependence of K+ H+ Antiporter in a Marine Bacterium, Vibrio alginolyticus. Biochem. Biophys. Res. Commun. 1986, 136, 1030–1035. [Google Scholar] [CrossRef]
- Epstein, W.; Kim, B.S. Potassium Transport Loci in Escherichia coli K-12. J. Bacteriol. 1971, 108, 639–644. [Google Scholar] [CrossRef]
- Booth, I.R.; Epstein, W.; Giffard, P.M.; Rowland, G.C. Roles of the TrkB and TrkC Gene Products of Escherichia coli in K+ Transport. Biochimie 1985, 67, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.P.; Booth, I.R.; Dinnbier, U.; Epstein, W.; Gajewska, A. Evidence for Multiple K+ Export Systems in Escherichia coli. J. Bacteriol. 1987, 169, 3743–3749. [Google Scholar] [CrossRef]
- Meury, J.; Kepes, A. Glutathione and the Gated Potassium Channels of Escherichia coli. EMBO J. 1982, 1, 339–343. [Google Scholar] [CrossRef]
- Elmore, M.J.; Lamb, A.J.; Ritchie, G.Y.; Douglas, R.M.; Munro, A.; Gajewska, A.; Booth, I.R. Activation of Potassium Efflux from Escherichia coli by Glutathione Metabolites. Mol. Microbiol. 1990, 4, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.P.; Munro, A.W.; Douglas, R.M.; McLaggan, D.; Booth, I.R. Activation of Potassium Channels during Metabolite Detoxification in Escherichia coli. Mol. Microbiol. 1993, 9, 1297–1303. [Google Scholar] [CrossRef]
- Cooper, R.A. Metabolism of Methylglyoxal in Microorganisms. Annu. Rev. Microbiol. 1984, 38, 49–68. [Google Scholar] [CrossRef] [PubMed]
- MacLean, M.J.; Ness, L.S.; Ferguson, G.P.; Booth, I.R. The Role of Glyoxalase I in the Detoxification of Methylglyoxal and in the Activation of the KefB K+ Efflux System in Escherichia coli. Mol. Microbiol. 1998, 27, 563–571. [Google Scholar] [CrossRef]
- Ozyamak, E.; Black, S.S.; Walker, C.A.; Maclean, M.J.; Bartlett, W.; Miller, S.; Booth, I.R. The Critical Role of S-Lactoylglutathione Formation during Methylglyoxal Detoxification in Escherichia coli. Mol. Microbiol. 2010, 78, 1577–1590. [Google Scholar] [CrossRef]
- Ferguson, G.P.; McLaggan, D.; Booth, I.R. Potassium Channel Activation by Glutathione-S-Conjugates in Escherichia coli: Protection against Methylglyoxal Is Mediated by Cytoplasmic Acidification. Mol. Microbiol. 1995, 17, 1025–1033. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Nikolaev, Y.; McLaggan, D.; Maclean, M.; Booth, I.R. Survival during Exposure to the Electrophilic Reagent N-Ethylmaleimide in Escherichia coli: Role of KefB and KefC Potassium Channels. J. Bacteriol. 1997, 179, 1007–1012. [Google Scholar] [CrossRef]
- Ness, L.; Ferguson, G.; Nikolaev, Y.; Booth, I. Survival of Escherichia coli Cells Exposed to Iodoacetate and Chlorodinitrobenzene Is Independent of the Glutathione-Gated K+ Efflux Systems KefB and KefC. Appl. Environ. Microbiol. 1997, 63, 4083–4086. [Google Scholar] [CrossRef]
- Bott, C.; Love, N. Investigating a Mechanistic Cause for Activated-Sludge Deflocculation in Response to Shock Loads of Toxic Electrophilic Chemicals. Water Environ. Res. 2002, 74, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Gillam, D.; Bishop, P.; Love, N. A Study of Glutathione-Gated Potassium Efflux in Biofilms Using Potassium Microelectrodes. Environ. Eng. Sci. 2005, 22, 489–495. [Google Scholar] [CrossRef]
- Bott, C.B.; Love, N.G. Implicating the Glutathione-Gated Potassium Efflux System as a Cause of Electrophile-Induced Activated Sludge Deflocculation. Appl. Environ. Microbiol. 2004, 70, 5569–5578. [Google Scholar] [CrossRef]
- Mojica, E.-R.E.; Kim, S.; Aga, D.S. Formation of N-Ethylmaleimide (NEM)-Glutathione Conjugate and N-Ethylmaleamic Acid Revealed by Mass Spectral Characterization of Intracellular and Extracellular Microbial Metabolites of NEM. Appl. Environ. Microbiol. 2008, 74, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Henriques, I.D.S.; Kelly, R.T.; Love, N.G. Deflocculation Effects Due to Chemical Perturbations in Sequencing Batch Reactors. Water Sci. Technol. 2004, 50, 287–294. [Google Scholar] [CrossRef]
- Zhang, W.; McLamore, E.S.; Wu, R.; Stensberg, M.; Porterfield, D.M.; Banks, M.K. Glutathione-Gated Potassium Efflux as a Mechanism of Active Biofilm Detachment. Water Environ. Res. 2014, 86, 462–469. [Google Scholar] [CrossRef]
- Munro, A.W.; Ritchie, G.Y.; Lamb, A.J.; Douglas, R.M.; Booth, I.R. The Cloning and DNA Sequence of the Gene for the Glutathione-Regulated Potassium-Efflux System KefC of Escherichia coli. Mol. Microbiol. 1991, 5, 607–616. [Google Scholar] [CrossRef]
- Wootton, J.C.; Drummond, M.H. The Q-Linker: A Class of Interdomain Sequences Found in Bacterial Multidomain Regulatory Proteins. Protein Eng. Des. Sel. 1989, 2, 535–543. [Google Scholar] [CrossRef]
- Douglas, R.M.; Ritchie, G.Y.; Munro, A.W.; McLaggan, D.; Booth, I.R. The K(+)-Efflux System, KefC, in Escherichia coli: Genetic Evidence for Oligomeric Structure. Mol. Membr. Biol. 1994, 11, 55–61. [Google Scholar] [CrossRef]
- Miller, S.; Douglas, R.M.; Carter, P.; Booth, I.R. Mutations in the Glutathione-Gated KefC K+ Efflux System of Escherichia Coli That Cause Constitutive Activation. J. Biol. Chem. 1997, 272, 24942–24947. [Google Scholar] [CrossRef] [PubMed]
- Ness, L.S.; Booth, I.R. Different Foci for the Regulation of the Activity of the KefB and KefC Glutathione-Gated K+ Efflux Systems. J. Biol. Chem. 1999, 274, 9524–9530. [Google Scholar] [CrossRef] [PubMed]
- Roosild, T.P.; Castronovo, S.; Miller, S.; Li, C.; Rasmussen, T.; Bartlett, W.; Gunasekera, B.; Choe, S.; Booth, I.R. KTN (RCK) Domains Regulate K+ Channels and Transporters by Controlling the Dimer-Hinge Conformation. Structure 2009, 17, 893–903. [Google Scholar] [CrossRef]
- Pliotas, C.; Grayer, S.C.; Ekkerman, S.; Chan, A.K.N.; Healy, J.; Marius, P.; Bartlett, W.; Khan, A.; Cortopassi, W.A.; Chandler, S.A.; et al. Adenosine Monophosphate Binding Stabilizes the KTN Domain of the Shewanella denitrificans Kef Potassium Efflux System. Biochemistry 2017, 56, 4219–4234. [Google Scholar] [CrossRef]
- Roosild, T.P.; Miller, S.; Booth, I.R.; Choe, S. A Mechanism of Regulating Transmembrane Potassium Flux through a Ligand-Mediated Conformational Switch. Cell 2002, 109, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Schrecker, M.; Wunnicke, D.; Hänelt, I. How RCK Domains Regulate Gating of K+ Channels. Biol. Chem. 2019, 400, 1303–1322. [Google Scholar] [CrossRef]
- Fujisawa, M.; Ito, M.; Krulwich, T.A. Three Two-Component Transporters with Channel-like Properties Have Monovalent Cation/Proton Antiport Activity. Proc. Natl. Acad. Sci. USA 2007, 104, 13289–13294. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- McLaggan, D.; Rufino, H.; Jaspars, M.; Booth, I.R. Glutathione-Dependent Conversion of N-Ethylmaleimide to the Maleamic Acid by Escherichia coli: An Intracellular Detoxification Process. Appl. Environ. Microbiol. 2000, 66, 1393–1399. [Google Scholar] [CrossRef]
- Misra, K.; Banerjee, A.B.; Ray, S.; Ray, M. Glyoxalase III from Escherichia coli: A Single Novel Enzyme for the Conversion of Methylglyoxal into D-Lactate without Reduced Glutathione. Biochem. J. 1995, 305, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tomioka, Y.; Suzuki, H.; Yonezawa, M.; Hishinuma, T.; Mizugaki, M. Molecular Cloning of the NemA Gene Encoding N-Ethylmaleimide Reductase from Escherichia coli. Biol. Pharm. Bull. 1997, 20, 110–112. [Google Scholar] [CrossRef]
- Masrati, G.; Dwivedi, M.; Rimon, A.; Gluck-Margolin, Y.; Kessel, A.; Ashkenazy, H.; Mayrose, I.; Padan, E.; Ben-Tal, N. Broad Phylogenetic Analysis of Cation/Proton Antiporters Reveals Transport Determinants. Nat. Commun. 2018, 9, 4205. [Google Scholar] [CrossRef]
- Saier, M.H.; Eng, B.H.; Fard, S.; Garg, J.; Haggerty, D.A.; Hutchinson, W.J.; Jack, D.L.; Lai, E.C.; Liu, H.J.; Nusinew, D.P.; et al. Phylogenetic Characterization of Novel Transport Protein Families Revealed by Genome Analyses. Biochim. Biophys. Acta 1999, 1422, 1–56. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A Novel Method for Fast and Accurate Multiple Sequence Alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.M.; Taly, J.F.; Notredame, C. T-Coffee: A Web Server for the Multiple Sequence Alignment of Protein and RNA Sequences Using Structural Information and Homology Extension. Nucleic Acids Res. 2011, 39, W13–W17. [Google Scholar] [CrossRef]
- Douglas, R.M.; Roberts, J.A.; Munro, A.W.; Ritchie, G.Y.; Lamb, A.J.; Booth, I.R. The Distribution of Homologues of the Escherichia coli KefC K+-Efflux System in Other Bacterial Species. J. Gen. Microbiol. 1991, 137, 1999–2005. [Google Scholar] [CrossRef]
- Fahey, R.C.; Brown, W.C.; Adams, W.B.; Worsham, M.B. Occurrence of Glutathione in Bacteria. J. Bacteriol. 1978, 133, 1126–1129. [Google Scholar] [CrossRef]
- Newton, G.L.; Arnold, K.; Price, M.S.; Sherrill, C.; Delcardayre, S.B.; Aharonowitz, Y.; Cohen, G.; Davies, J.; Fahey, R.C.; Davis, C. Distribution of Thiols in Microorganisms: Mycothiol Is a Major Thiol in Most Actinomycetes. J. Bacteriol. 1996, 178, 1990–1995. [Google Scholar] [CrossRef]
- Fahey, R. Novel Thiols of Prokaryotes. Annu. Rev. Microbiol. 2001, 55, 333–356. [Google Scholar] [CrossRef]
- Fahey, R.C. Glutathione Analogs in Prokaryotes. Biochim. Biophys. Acta 2012, 1830, 3182–3198. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Skurnik, M. Temperature-Regulated Efflux Pump/Potassium Antiporter System Mediates Resistance to Cationic Antimicrobial Peptides in Yersinia. Mol. Microbiol. 2000, 37, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Zhang, L.; Toivanen, P.; Skurnik, M. Regulatory Network of Lipopolysaccharide O-Antigen Biosynthesis in Yersinia enterocolitica Includes Cell Envelope-Dependent Signals. Mol. Microbiol. 2002, 44, 1045–1062. [Google Scholar] [CrossRef]
- Skurnik, M.; Bengoechea, J.A. The Biosynthesis and Biological Role of Lipopolysaccharide O-Antigens of Pathogenic Yersiniae. Carbohydr. Res. 2003, 338, 2521–2529. [Google Scholar] [CrossRef]
- Booth, I.R.; Edwards, M.D.; Gunasekera, B.; Li, C.; Miller, S. The Ktn Domain and Its Role as a Channel and Transporter Regulator. In Bacterial Ion Channels and Their Eukaryotic Homologs; Kubalski, A., Martinac, B., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 21–40. ISBN 978-1-683-67195-4. [Google Scholar]
- Lambert, C.; Ivanov, P.; Sockett, R.E. A Transcriptional “Scream” Early Response of E. coli Prey to Predatory Invasion by Bdellovibrio. Curr. Microbiol. 2010, 60, 419–427. [Google Scholar] [CrossRef]
- Tenaillon, O.; Rodríguez-Verdugo, A.; Gaut, R.L.; McDonald, P.; Bennett, A.F.; Long, A.D.; Gaut, B.S. The Molecular Diversity of Adaptive Convergence. Science 2012, 335, 457–461. [Google Scholar] [CrossRef]
- Kishimoto, T.; Iijima, L.; Tatsumi, M.; Ono, N.; Oyake, A.; Hashimoto, T.; Matsuo, M.; Okubo, M.; Suzuki, S.; Mori, K.; et al. Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution. PLoS Genet. 2010, 6, e1001164. [Google Scholar] [CrossRef]
- Deatherage, D.E.; Kepner, J.L.; Bennett, A.F.; Lenski, R.E.; Barrick, J.E. Specificity of Genome Evolution in Experimental Populations of Escherichia coli Evolved at Different Temperatures. Proc. Natl. Acad. Sci. USA 2017, 114, E1904–E1912. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Kanwar, P. Role of K+ Efflux Antiporter (KEA) in Plants. In Cation Transporters in Plants; Academic Press: New York, NY, USA, 2021; pp. 115–128. ISBN 9780323857901. [Google Scholar]
- Nestrerenko, E.O.; Krasnoperova, O.E.; Isayenkov, S.V. Potassium Transport Systems and Their Role in Stress Response, Plant Growth, and Development. Cytol. Genet. 2021, 55, 63–79. [Google Scholar] [CrossRef]
- Chanroj, S.; Wang, G.; Venema, K.; Zhang, M.W.; Delwiche, C.F.; Sze, H. Conserved and Diversified Gene Families of Monovalent Cation/H+ Antiporters from Algae to Flowering Plants. Front. Plant Sci. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, M.; Kera, K.; Hamamoto, S.; Kuromori, T.; Shikanai, T.; Uozumi, N. Evidence for Potassium Transport Activity of Arabidopsis KEA1-KEA6. Sci. Rep. 2019, 9, 10040. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Sicilia, M.N.; Cagnac, O.; Chanroj, S.; Sze, H.; Rodríguez-Rosales, M.P.; Venema, K. Arabidopsis KEA2, a Homolog of Bacterial KefC, Encodes a K(+)/H(+) Antiporter with a Chloroplast Transit Peptide. Biochim. Biophys. Acta 2012, 1818, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Pan, T.; Fan, L.; Qiu, Q.S. A Novel AtKEA Gene Family, Homolog of Bacterial K+/H+ Antiporters, Plays Potential Roles in K+ Homeostasis and Osmotic Adjustment in Arabidopsis. PLoS ONE 2013, 8, e81463. [Google Scholar] [CrossRef]
- Kunz, H.H.; Gierth, M.; Herdean, A.; Satoh-Cruz, M.; Kramer, D.M.; Spetea, C.; Schroeder, J.I. Plastidial Transporters KEA1, -2, and -3 Are Essential for Chloroplast Osmoregulation, Integrity, and PH Regulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 7480–7485. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Salvi, D.; Brugière, S.; Miras, S.; Kowalski, S.; Louwagie, M.; Garin, J.; Joyard, J.; Rolland, N. Proteomics of the Chloroplast Envelope Membranes from Arabidopsis Thaliana. Mol. Cell. Proteom. 2003, 2, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Zybailov, B.; Rutschow, H.; Friso, G.; Rudella, A.; Emanuelsson, O.; Sun, Q.; van Wijk, K.J. Sorting Signals, N-Terminal Modifications and Abundance of the Chloroplast Proteome. PLoS ONE 2008, 3, e1994. [Google Scholar] [CrossRef]
- Aranda-Sicilia, M.N.; Aboukila, A.; Armbruster, U.; Cagnac, O.; Schumann, T.; Kunz, H.H.; Jahns, P.; Rodríguez-Rosales, M.P.; Sze, H.; Venema, K. Envelope K+/H+ Antiporters AtKEA1 and AtKEA2 Function in Plastid Development. Plant Physiol. 2016, 172, 441–449. [Google Scholar] [CrossRef]
- Aranda Sicilia, M.N.; Sánchez Romero, M.E.; Rodríguez Rosales, M.P.; Venema, K. Plastidial Transporters KEA1 and KEA2 at the Inner Envelope Membrane Adjust Stromal PH in the Dark. New Phytol. 2021, 229, 2080–2090. [Google Scholar] [CrossRef]
- Bölter, B.; Mitterreiter, M.J.; Schwenkert, S.; Finkemeier, I.; Kunz, H.H. The Topology of Plastid Inner Envelope Potassium Cation Efflux Antiporter KEA1 Provides New Insights into Its Regulatory Features. Photosynth. Res. 2020, 145, 43–54. [Google Scholar] [CrossRef]
- Sánchez-McSweeney, A.; González-Gordo, S.; Aranda-Sicilia, M.N.; Rodríguez-Rosales, M.P.; Venema, K.; Palma, J.M.; Corpas, F.J. Loss of Function of the Chloroplast Membrane K+/H+ Antiporters AtKEA1 and AtKEA2 Alters the ROS and NO Metabolism but Promotes Drought Stress Resilience. Plant Physiol. Biochem. 2021, 160, 106–119. [Google Scholar] [CrossRef]
- Armbruster, U.; Carrillo, L.R.; Venema, K.; Pavlovic, L.; Schmidtmann, E.; Kornfeld, A.; Jahns, P.; Berry, J.A.; Kramer, D.M.; Jonikas, M.C. Ion Antiport Accelerates Photosynthetic Acclimation in Fluctuating Light Environments. Nat. Commun. 2014, 5, 5439. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yamamoto, H.; Narumiya, F.; Munekage, Y.N.; Finazzi, G.; Szabo, I.; Shikanai, T. Fine-Tuned Regulation of the K+/H+ Antiporter KEA3 Is Required to Optimize Photosynthesis during Induction. Plant J. 2017, 89, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, U.; Leonelli, L.; Galvis, V.C.; Strand, D.; Quinn, E.H.; Jonikas, M.C.; Niyogi, K.K. Regulation and Levels of the Thylakoid K+/H+ Antiporter KEA3 Shape the Dynamic Response of Photosynthesis in Fluctuating Light. Plant Cell Physiol. 2016, 57, 1557–1567. [Google Scholar] [CrossRef]
- Wang, C.; Shikanai, T. Modification of Activity of the Thylakoid H+/K+ Antiporter Kea3 Disturbs ΔpH-Dependent Regulation of Photosynthesis. Plant Physiol. 2019, 181, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Pan, T.; Zhang, X.; Fan, L.; Quintero, F.J.; Zhao, H.; Su, X.; Li, X.; Villalta, I.; Mendoza, I.; et al. K + Efflux Antiporters 4, 5, and 6 Mediate PH and K + Homeostasis in Endomembrane Compartments. Plant Physiol. 2018, 178, 1657–1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, R.J.; Yang, X.; Zheng, X.; Shao, Q.; Tang, Q.L.; Fu, A.; Luan, S. Golgi-Localized Cation/Proton Exchangers Regulate Ionic Homeostasis and Skotomorphogenesis in Arabidopsis. Plant Cell Environ. 2019, 42, 673–687. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, T. The Potassium Efflux System Kef: Bacterial Protection against Toxic Electrophilic Compounds. Membranes 2023, 13, 465. https://doi.org/10.3390/membranes13050465
Rasmussen T. The Potassium Efflux System Kef: Bacterial Protection against Toxic Electrophilic Compounds. Membranes. 2023; 13(5):465. https://doi.org/10.3390/membranes13050465
Chicago/Turabian StyleRasmussen, Tim. 2023. "The Potassium Efflux System Kef: Bacterial Protection against Toxic Electrophilic Compounds" Membranes 13, no. 5: 465. https://doi.org/10.3390/membranes13050465
APA StyleRasmussen, T. (2023). The Potassium Efflux System Kef: Bacterial Protection against Toxic Electrophilic Compounds. Membranes, 13(5), 465. https://doi.org/10.3390/membranes13050465





