Novel Approach to Landfill Wastewater Treatment Fouling Mitigation: Air Gap Membrane Distillation with Tin Sulfide-Coated PTFE Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedwater Samples and Chemicals
2.2. PTFE Membrane Specifications
2.3. TS-PFTE Membrane Coating
2.4. AGMD Module Setup
3. Experimental Methodology
3.1. Membrane Characterizations by FE-SEM and EDX Analysis
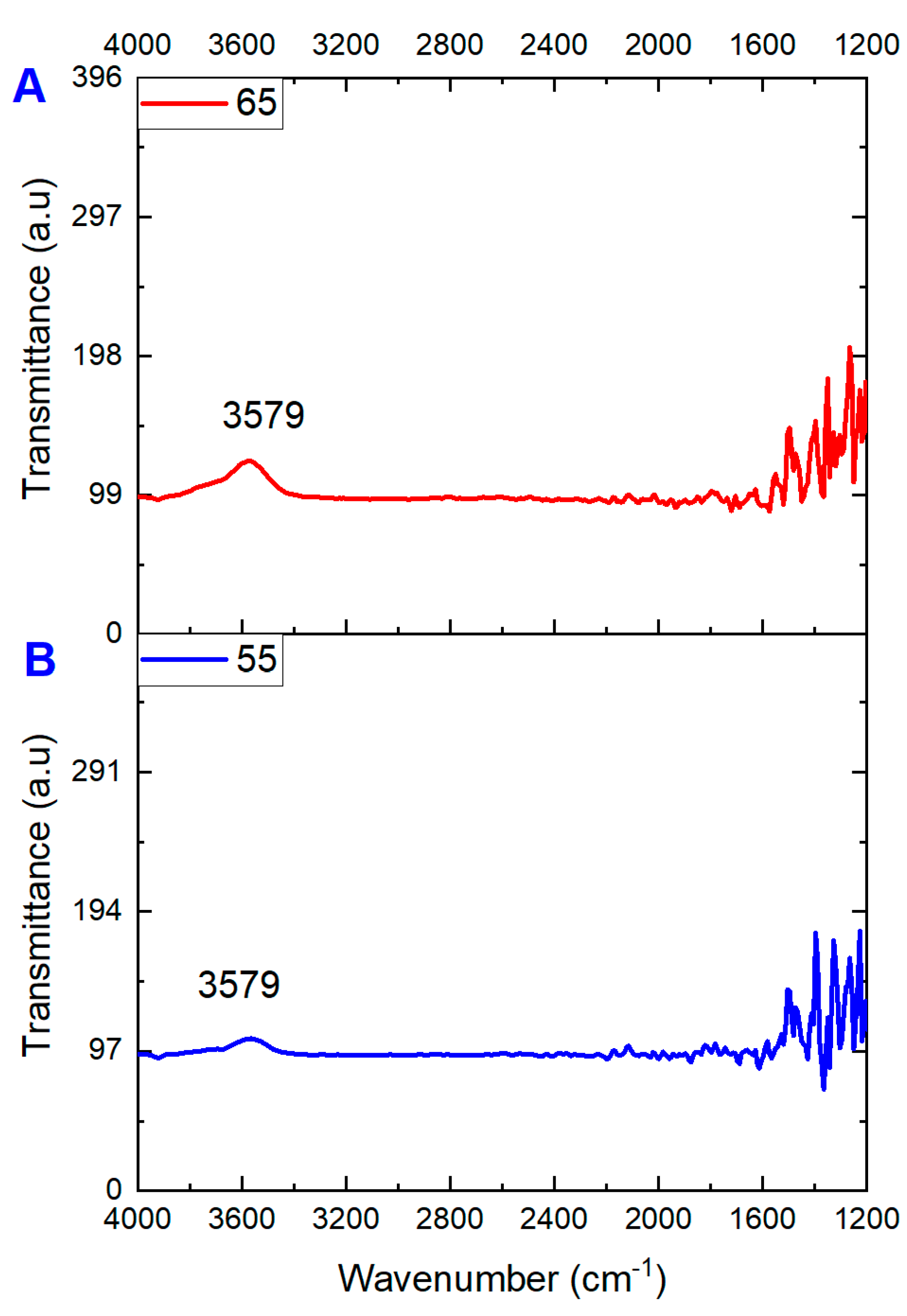
3.2. Pore Size, FT-IR Analysis, and Contact Angle Analysis
4. Results and Discussions
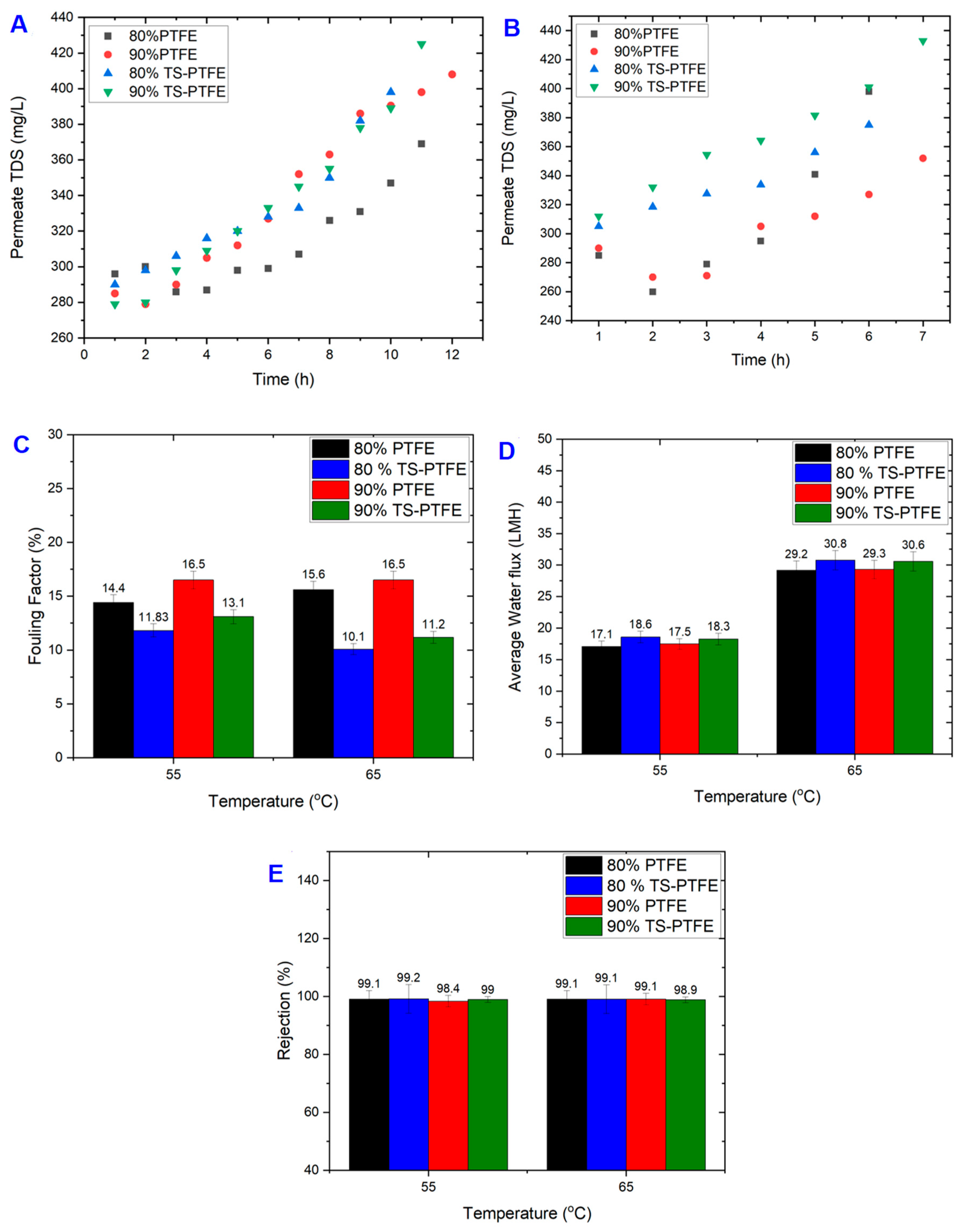
4.1. Impact of Temperature and Recovery Rate on TS-PTFE Membranes
4.2. TS-PTFE Membrane Fouling and Cleaning
4.3. Comparison of PTFE and TS-PTFE Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrar, I.; Yadav, S.; Altaee, A.; Samal, A.K.; Zhou, J.L.; Nguyen, T.V.; Ganbat, N. Treatment of biologically treated landfill leachate with forward osmosis: Investigating membrane performance and cleaning protocols. Sci. Total Environ. 2020, 744, 140901. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Ibrar, I.; Altaee, A.; Samal, A.K.; Ghobadi, R.; Zhou, J. Feasibility of brackish water and landfill leachate treatment by GO/MoS2-PVA composite membranes. Sci. Total Environ. 2020, 745, 141088. [Google Scholar] [CrossRef] [PubMed]
- Tałałaj, I.A.; Bartkowska, I.; Biedka, P. Treatment of young and stabilized landfill leachate by integrated sequencing batch reactor (SBR) and reverse osmosis (RO) process. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100502. [Google Scholar]
- Guo, Y.-F.; Sun, P.-C.; Wei, J.-F. New insight into the fouling behavior of hydrophobic and hydrophilic polypropylene membranes in integrated membrane bioreactors. Environ. Technol. 2018, 39, 3159–3168. [Google Scholar] [CrossRef] [PubMed]
- Morello, L.; Cossu, R.; Raga, R.; Pivato, A.; Lavagnolo, M.C. Recirculation of reverse osmosis concentrate in lab-scale anaerobic and aerobic landfill simulation reactors. Waste Manag. 2016, 56, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fan, Z.; Wu, D.; Qin, L.; Zhang, G.; Gao, C.; Meng, Q. Anoxic/aerobic granular active carbon assisted MBR integrated with nanofiltration and reverse osmosis for advanced treatment of municipal landfill leachate. Desalination 2014, 349, 136–144. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Tian, B.-H.; Zhang, X.; Ghulam, A.; Fang, C.-R.; He, R. Investigation on characteristics of leachate and concentrated leachate in three landfill leachate treatment plants. Waste Manag. 2013, 33, 2277–2286. [Google Scholar] [CrossRef]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and long-term composition of MSW landfill leachate: A review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Šír, M.; Podhola, M.; Patočka, T.; Honzajková, Z.; Kocurek, P.; Kubal, M.; Kuraš, M. The effect of humic acids on the reverse osmosis treatment of hazardous landfill leachate. J. Hazard. Mater. 2012, 207, 86–90. [Google Scholar] [CrossRef]
- Li, F.; Wichmann, K.; Heine, W. Treatment of the methanogenic landfill leachate with thin open channel reverse osmosis membrane modules. Waste Manag. 2009, 29, 960–964. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.-N.; Li, X.; Sun, Y.; Wu, H.; Chen, D. Removal of humic substances from reverse osmosis (RO) and nanofiltration (NF) concentrated leachate using continuously ozone generation-reaction treatment equipment. Waste Manag. 2016, 56, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, I.; Yadav, S.; Altaee, A.; Hawari, A.; Nguyen, V.; Zhou, J. A novel empirical method for predicting concentration polarization in forward osmosis for single and multicomponent draw solutions. Desalination 2020, 494, 114668. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Altaee, A.; Safaei, J.; Samal, A.K.; Subbiah, S.; Millar, G.; Deka, P.; Zhou, J. Sodium docusate as a cleaning agent for forward osmosis membranes fouled by landfill leachate wastewater. Chemosphere 2022, 308, 136237. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, I.; Yadav, S.; Naji, O.; Alanezi, A.A.; Ghaffour, N.; Déon, S.; Subbiah, S.; Altaee, A. Development in forward Osmosis-Membrane distillation hybrid system for wastewater treatment. Sep. Purif. Technol. 2022, 286, 120498. [Google Scholar] [CrossRef]
- Linde, K.; Jönsson, A.-S.; Wimmerstedt, R. Treatment of three types of landfill leachate with reverse osmosis. Desalination 1995, 101, 21–30. [Google Scholar] [CrossRef]
- Rukapan, W.; Khananthai, B.; Chiemchaisri, C.; Chiemchaisri, W.; Srisukphun, T. Short-and long-term fouling characteristics of reverse osmosis membrane at full scale leachate treatment plant. Water Sci. Technol. 2012, 65, 127–134. [Google Scholar] [CrossRef]
- Trebouet, D.; Schlumpf, J.; Jaouen, P.; Quemeneur, F. Stabilized landfill leachate treatment by combined physicochemical–nanofiltration processes. Water Res. 2001, 35, 2935–2942. [Google Scholar] [CrossRef]
- de Almeida, R.; Bila, D.M.; Quintaes, B.R.; Campos, J.C. Cost estimation of landfill leachate treatment by reverse osmosis in a Brazilian landfill. Waste Manag. Res. 2020, 38, 1087–1092. [Google Scholar] [CrossRef]
- Tow, E.W.; McGovern, R.K. Raising forward osmosis brine concentration efficiency through flow rate optimization. Desalination 2015, 366, 71–79. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, Y.; Chen, X.; Lu, Z.; Wei, Z.; Fan, G.; Liang, H.; Qu, F. Evaluation of applying membrane distillation for landfill leachate treatment. Desalination 2021, 520, 115358. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Xu, Z.; Liu, Y.; Cheng, F. Simultaneous anti-fouling and flux-enhanced membrane distillation via incorporating graphene oxide on PTFE membrane for coking wastewater treatment. Appl. Surf. Sci. 2020, 531, 147349. [Google Scholar] [CrossRef]
- Yan, Z.; Lu, Z.; Chen, X.; Jiang, Y.; Huang, Z.; Liu, L.; Fan, G.; Chang, H.; Qu, F.; Liang, H. Membrane distillation treatment of landfill leachate: Characteristics and mechanism of membrane fouling. Sep. Purif. Technol. 2022, 289, 120787. [Google Scholar] [CrossRef]
- Hu, R.; He, Y.; Huang, M.; Zhao, G.; Zhu, H. Strong adhesion of graphene oxide coating on polymer separation membranes. Langmuir 2018, 34, 10569–10579. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Choi, J.; Bang, J.; Lee, J.-H. Layer-by-layer assembly of graphene oxide nanosheets on polyamide membranes for durable reverse-osmosis applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Wu, H.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Thampi, S.; Nandkumar, A.M.; Muthuvijayan, V.; Parameswaran, R. Differential adhesive and bioactive properties of the polymeric surface coated with graphene oxide thin film. ACS Appl. Mater. Interfaces 2017, 9, 4498–4508. [Google Scholar] [CrossRef]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–95. [Google Scholar]
- Ibrar, I.; Yadav, S.; Braytee, A.; Altaee, A.; HosseinZadeh, A.; Samal, A.K.; Zhou, J.L.; Khan, J.A.; Bartocci, P.; Fantozzi, F. Evaluation of machine learning algorithms to predict internal concentration polarization in forward osmosis. J. Membr. Sci. 2022, 646, 120257. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Gong, B.; Yang, H.; Wu, S.; Xiong, G.; Yan, J.; Cen, K.; Bo, Z.; Ostrikov, K. Graphene Array-Based Anti-fouling Solar Vapour Gap Membrane Distillation with High Energy Efficiency. Nano-Micro Lett. 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.C.; Kim, Y.; Shim, W.-G.; Tijing, L.D.; Yao, M.; Nghiem, L.D.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Graphene/PVDF flat-sheet membrane for the treatment of RO brine from coal seam gas produced water by air gap membrane distillation. J. Membr. Sci. 2016, 513, 74–84. [Google Scholar] [CrossRef]
- Gontarek-Castro, E.; Di Luca, G.; Lieder, M.; Gugliuzza, A. Graphene-Coated PVDF Membranes: Effects of Multi-Scale Rough Structure on Membrane Distillation Performance. Membranes 2022, 12, 511. [Google Scholar] [CrossRef]
- Zahirifar, J.; Karimi-Sabet, J.; Moosavian, S.M.A.; Hadi, A.; Khadiv-Parsi, P. Fabrication of a novel octadecylamine functionalized graphene oxide/PVDF dual-layer flat sheet membrane for desalination via air gap membrane distillation. Desalination 2018, 428, 227–239. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. Desalination across a graphene oxide membrane via direct contact membrane distillation. Desalination 2016, 378, 37–43. [Google Scholar] [CrossRef]
- Koenig, S.P.; Boddeti, N.G.; Dunn, M.L.; Bunch, J.S. Ultrastrong adhesion of graphene membranes. Nat. Nanotechnol. 2011, 6, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Aljumaily, M.M.; Alayan, H.M.; Mohammed, A.A.; Alsaadi, M.A.; Alsalhy, Q.F.; Figoli, A.; Criscuoli, A. The influence of coating super-hydrophobic carbon nanomaterials on the performance of membrane distillation. Appl. Water Sci. 2022, 12, 28. [Google Scholar] [CrossRef]
- Chin, J.Y.; Teoh, G.H.; Ahmad, A.L.; Low, S.C. Superhydrophobic surface coating on electrospun polypropylene membrane to treat high salinity water in membrane distillation. Water Sci. Technol. 2020, 82, 2948–2961. [Google Scholar] [CrossRef]
- Khan, A.; Yadav, S.; Ibrar, I.; Al Juboori, R.A.; Razzak, S.A.; Deka, P.; Subbiah, S.; Shah, S. Fouling and Performance Investigation of Membrane Distillation at Elevated Recoveries for Seawater Desalination and Wastewater Reclamation. Membranes 2022, 12, 951. [Google Scholar] [CrossRef]
- Walter, W.G. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1961. [Google Scholar]
- Chang, Y.; Ruan, M.; Li, F.; Zheng, Z.; Chen, Y.; Ge, Z.; Fan, P. Synthesis process and thermoelectric properties of the layered crystal structure SnS2. J. Mater. Sci. Mater. Electron. 2020, 31, 5425–5433. [Google Scholar] [CrossRef]
- Qu, B.; Ma, C.; Ji, G.; Xu, C.; Xu, J.; Meng, Y.S.; Wang, T.; Lee, J.Y. Layered SnS2-reduced graphene oxide composite-A high-capacity, high-rate, and long-cycle life sodium-ion battery anode material. Adv. Mater. 2014, 26, 3854–3859. [Google Scholar] [CrossRef]
- Ge, J.; Peng, Y.; Li, Z.; Chen, P.; Wang, S. Membrane fouling and wetting in a DCMD process for RO brine concentration. Desalination 2014, 344, 97–107. [Google Scholar] [CrossRef]
- Gryta, M. Alkaline scaling in the membrane distillation process. Desalination 2008, 228, 128–134. [Google Scholar] [CrossRef]
- Luo, L.-W.; Wu, Y.-H.; Wang, Y.-H.; Tong, X.; Bai, Y.; Chen, G.-Q.; Wang, H.-B.; Ikuno, N.; Hu, H.-Y. Aggravated biofouling caused by chlorine disinfection in a pilot-scale reverse osmosis treatment system of municipal wastewater. J. Water Reuse Desalination 2021, 11, 201–211. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Ganbat, N.; Samal, A.K.; Altaee, A.; Zhou, J.L.; Nguyen, T.V. Feasibility of H2O2 cleaning for forward osmosis membrane treating landfill leachate. J. Environ. Manag. 2021, 294, 113024. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Andrés-Mañas, J.A.; Zaragoza, G. Evaluation of permeate quality in pilot scale membrane distillation systems. Membranes 2019, 9, 69. [Google Scholar] [CrossRef]
- Asif, M.B.; Ji, B.; Maqbool, T.; Zhang, Z. Algogenic organic matter fouling alleviation in membrane distillation by peroxymonosulfate (PMS): Role of PMS concentration and activation temperature. Desalination 2021, 516, 115225. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Samal, A.K.; Altaee, A.; Déon, S.; Zhou, J.; Ghaffour, N. Preparation of fouling resistant and highly perm-selective novel PSf/GO-vanillin nanofiltration membrane for efficient water purification. J. Hazard. Mater. 2022, 421, 126744. [Google Scholar] [CrossRef] [PubMed]
- Alnajjar, H.; Tabatabai, A.; Alpatova, A.; Leiknes, T.; Ghaffour, N. Organic fouling control in reverse osmosis (RO) by effective membrane cleaning using saturated CO2 solution. Sep. Purif. Technol. 2021, 264, 118410. [Google Scholar] [CrossRef]
- Gulied, M.; Al Momani, F.; Khraisheh, M.; Bhosale, R.; AlNouss, A. Influence of draw solution type and properties on the performance of forward osmosis process: Energy consumption and sustainable water reuse. Chemosphere 2019, 233, 234–244. [Google Scholar] [CrossRef]
- Ocakoglu, K.; Dizge, N.; Colak, S.G.; Ozay, Y.; Bilici, Z.; Yalcin, M.S.; Ozdemir, S.; Yatmaz, H.C. Polyethersulfone membranes modified with CZTS nanoparticles for protein and dye separation: Improvement of antifouling and self-cleaning performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126230. [Google Scholar] [CrossRef]



| Parameter | Concentration | Measuring Instrument |
|---|---|---|
| Color | Brown yellowish | - |
| pH | 8.01 | HQ40d multi |
| Turbidity, NTU | 35 | 2100P Turbidimeter |
| Conductivity, ms/cm | 12.10 | HQ14d Conductivity |
| TDS, mg/L | 4500 | |
| Total organic carbon, mg/L | 145.1 ± 5 | TOC analyzer (Shimadzu Corporation, Japan) |
| TSS, mg/L | 27–117 | 7900 ICP-MS |
| Total irons, mg/L | 3.5–52 | 7900 ICP-MS |
| Ammonia, mg/L | <0.5 | 5051—Ammonium Flow Plus ISE |
| Ca2+, mg/L | 126 ± 5 | 7900 ICP-MS |
| Mg2+, mg/L | 95.3 ± 5 | 7900 ICP-MS |
| K+, mg/L | 47.87 | 7900 ICP-MS |
| Membrane Type | PTFE |
|---|---|
| Wettability | Hydrophobic |
| Nominal pore size, µm | 0.45 |
| Thickness, µm | 146–223 |
| Bubble point, psi | 11.60–13.05 |
| Flow rate, mL/min/cm2 | 43–52 |
| Contact angle | 129 ± 3° |
| TS-PTFE | 131.6 ± 3° |
| Membrane Type | Smallest Pore Diameter (µm) | Largest Pore Diameter (µm) | Mean Pore Diameter (µm) | Water Contact Angle (°) |
|---|---|---|---|---|
| PTFE pristine | 0.213 ± 0.01 | 0.296 ± 0.01 | 0.248 ± 0.01 | 129 ± 2 |
| TS-PTFE pristine | 0.167 ± 0.01 | 0.248 ± 0.01 | 0.174 ± 0.01 | 131.4 ± 3 |
| PTFE fouled 15 h | 0.182 ± 0.08 | 0.296 ± 0.07 | 0.194 ± 0.07 | 102 ± 3 |
| PTFE fouled 30 h | 0.166 ± 0.06 | 0.244 ± 0.07 | 0.170 ± 0.08 | 93 ± 3 |
| TS-PTFE fouled 20 h | 0.151 ± 0.01 | 0.213 ± 0.01 | 0.154 ± 0.01 | 87.8 ± 3 |
| TS-PTFE fouled 28 h | 0.11 ± 0.01 | 0.248 ± 0.01 | 0.155 ± 0.01 | 85.6 ± 3 |
| Element | Pristine PTFE | Pristine TS-PTFE | TS-PTFE 20 h Fouled | TS-PTFE 28 h Fouled | PTFE 15 h Fouled | PTFE 30 h Fouled |
|---|---|---|---|---|---|---|
| C | 56 | 71.9 | 66.3 | 71.6 | 70.42 | 44.01 |
| Mg | 0.3 | 0.3 | 0.9 | 0.62 | 0.90 | 1.42 |
| Cl | 0.60 | - | - | - | 0.19 | 0.29 |
| K | 0 | - | 0.04 | - | 0.20 | 0.29 |
| Ca | 0.1 | 0.041 | 0.035 | 0.03 | 0.20 | 0.39 |
| Fe | 1.7 | 0.03 | 0.034 | 0.07 | 0.66 | 2.06 |
| S | - | 4.2 | 5.3 | 4.1 | - | - |
| Sn | - | 8.2 | 9.2 | 8.2 | - | - |
| Na | - | 0 | 0.91 | 0.7 | - | - |
| O | - | 15.6 | 17.2 | 14.7 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Ibrar, I.; Mirdad, A.; Al-Juboori, R.A.; Deka, P.; Subbiah, S.; Altaee, A. Novel Approach to Landfill Wastewater Treatment Fouling Mitigation: Air Gap Membrane Distillation with Tin Sulfide-Coated PTFE Membrane. Membranes 2023, 13, 483. https://doi.org/10.3390/membranes13050483
Khan A, Ibrar I, Mirdad A, Al-Juboori RA, Deka P, Subbiah S, Altaee A. Novel Approach to Landfill Wastewater Treatment Fouling Mitigation: Air Gap Membrane Distillation with Tin Sulfide-Coated PTFE Membrane. Membranes. 2023; 13(5):483. https://doi.org/10.3390/membranes13050483
Chicago/Turabian StyleKhan, Abdulaziz, Ibrar Ibrar, Abeer Mirdad, Raed A. Al-Juboori, Priyamjeet Deka, Senthilmurugan Subbiah, and Ali Altaee. 2023. "Novel Approach to Landfill Wastewater Treatment Fouling Mitigation: Air Gap Membrane Distillation with Tin Sulfide-Coated PTFE Membrane" Membranes 13, no. 5: 483. https://doi.org/10.3390/membranes13050483
APA StyleKhan, A., Ibrar, I., Mirdad, A., Al-Juboori, R. A., Deka, P., Subbiah, S., & Altaee, A. (2023). Novel Approach to Landfill Wastewater Treatment Fouling Mitigation: Air Gap Membrane Distillation with Tin Sulfide-Coated PTFE Membrane. Membranes, 13(5), 483. https://doi.org/10.3390/membranes13050483








