The Interaction of the Transmembrane Domain of SARS-CoV-2 E-Protein with Glycyrrhizic Acid in Lipid Bilayer
Abstract
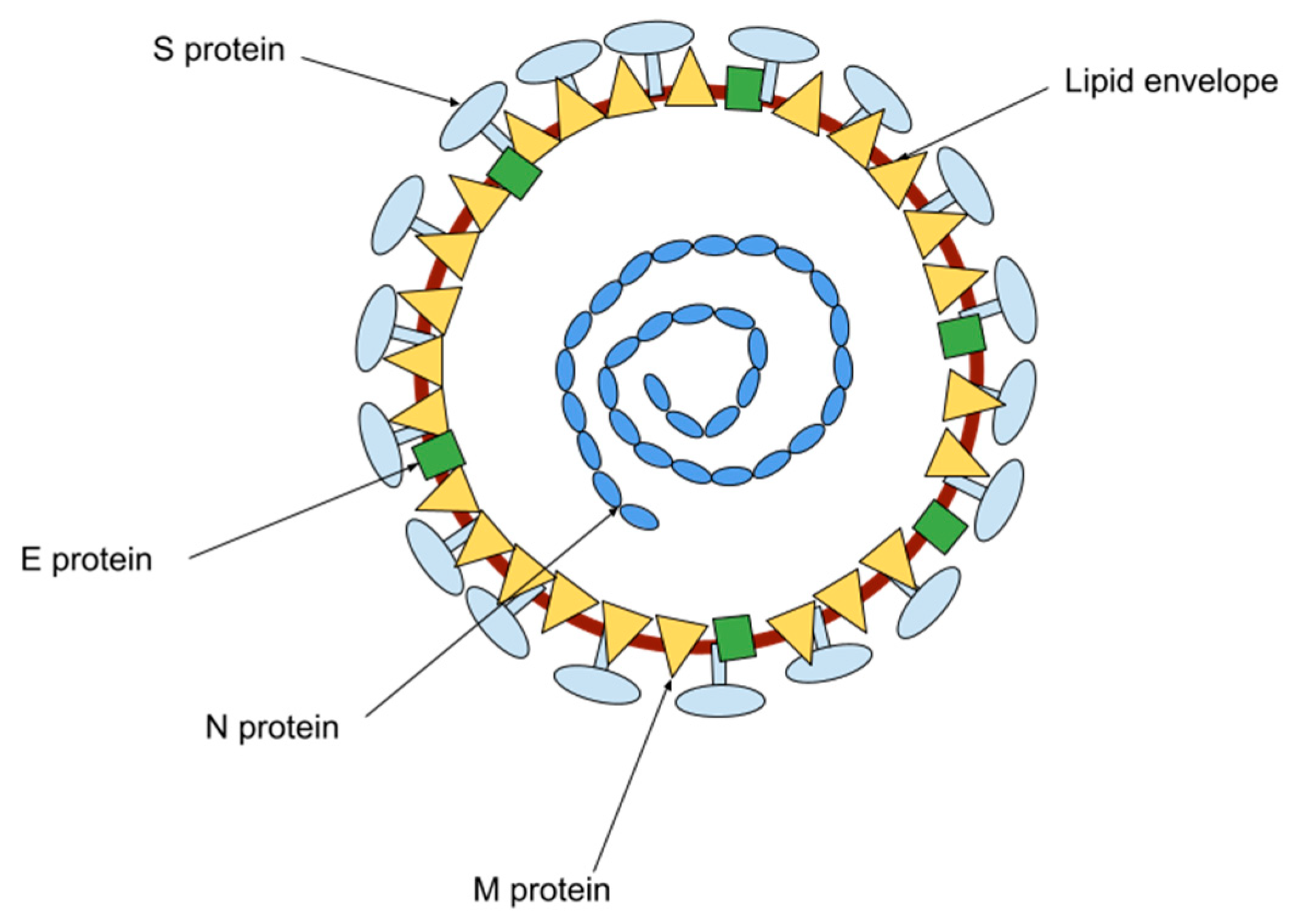
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NMR Study
3. Results and Discussion
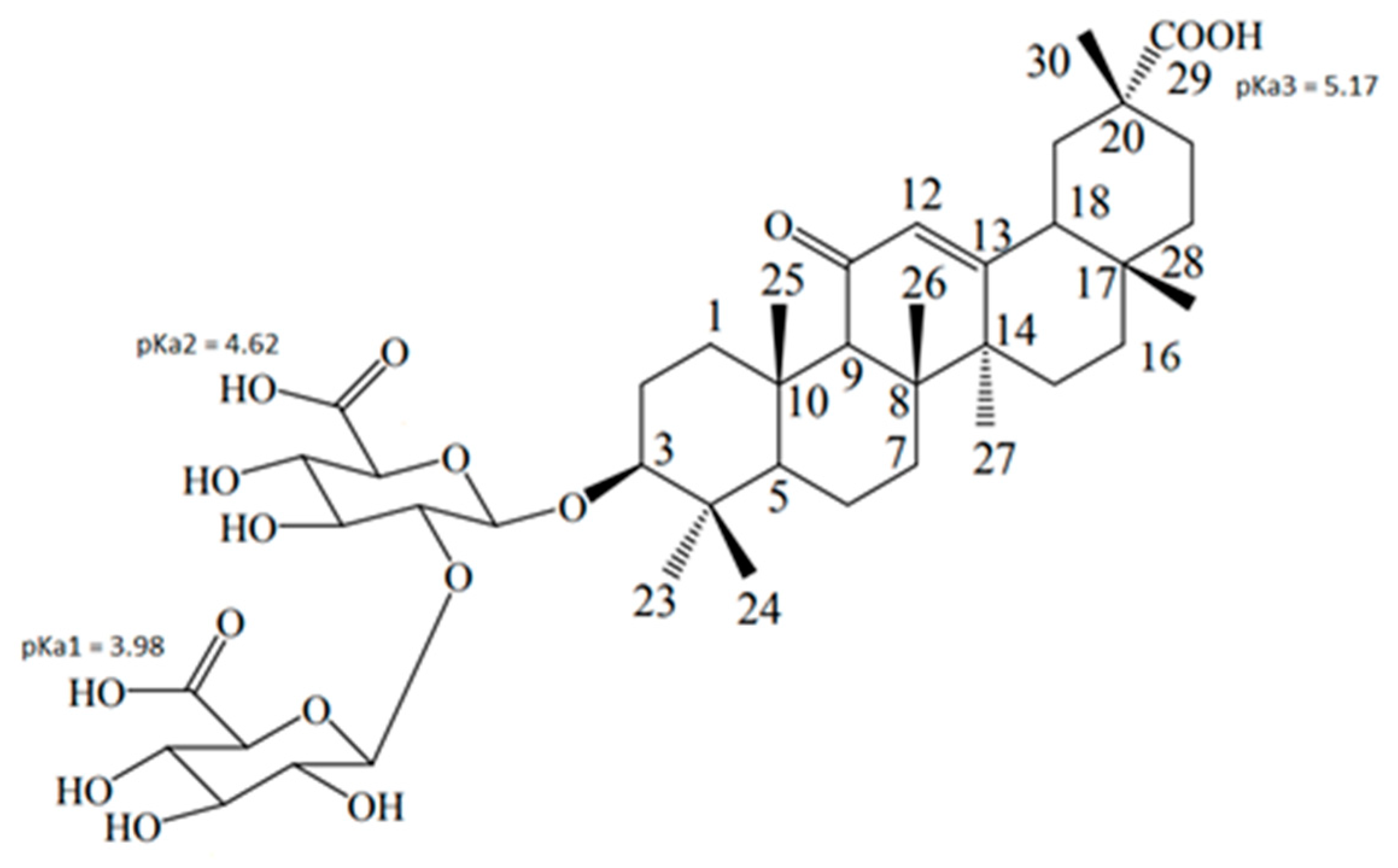
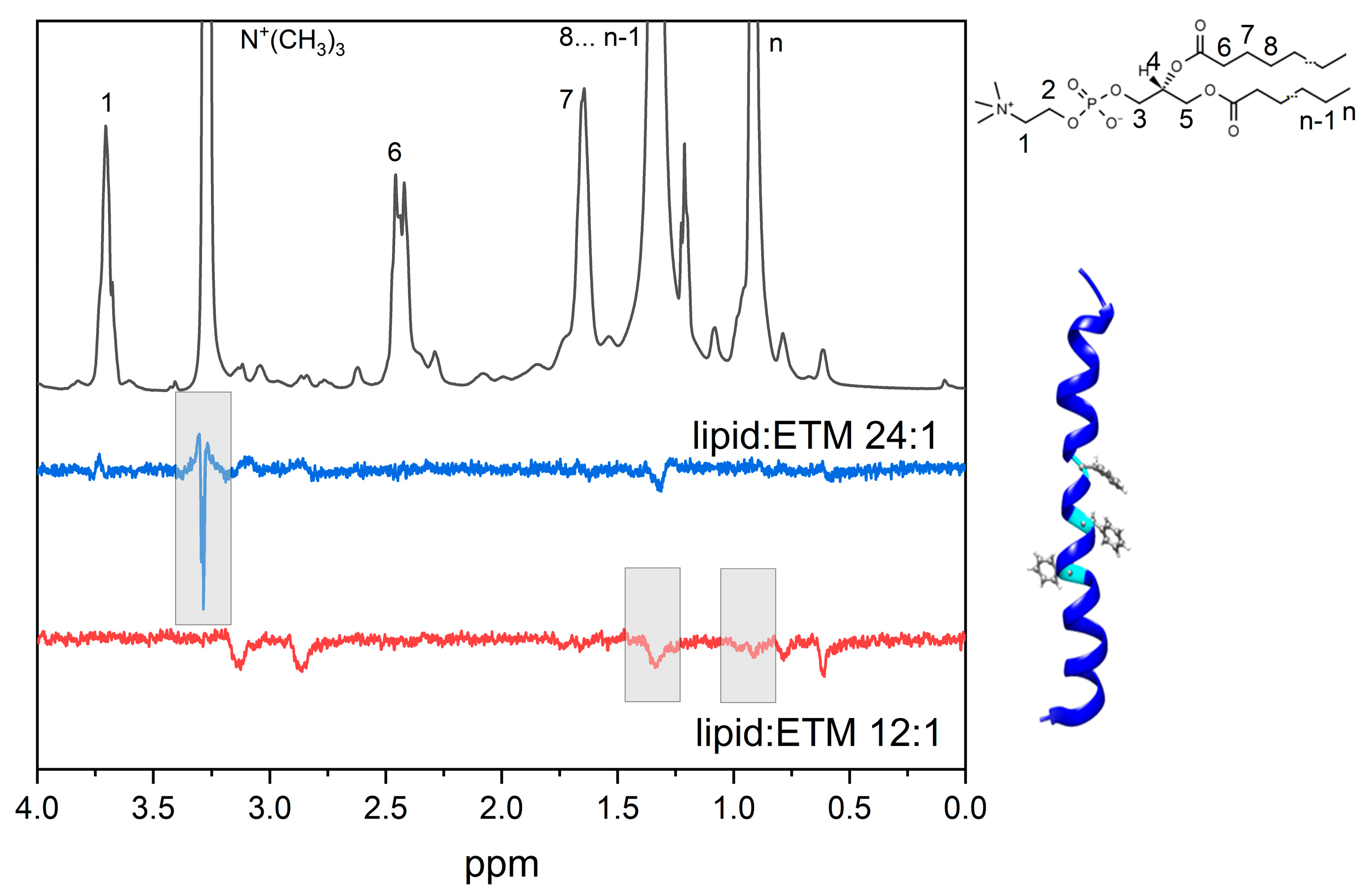
3.1. GA Interaction with Lipid Bilayer
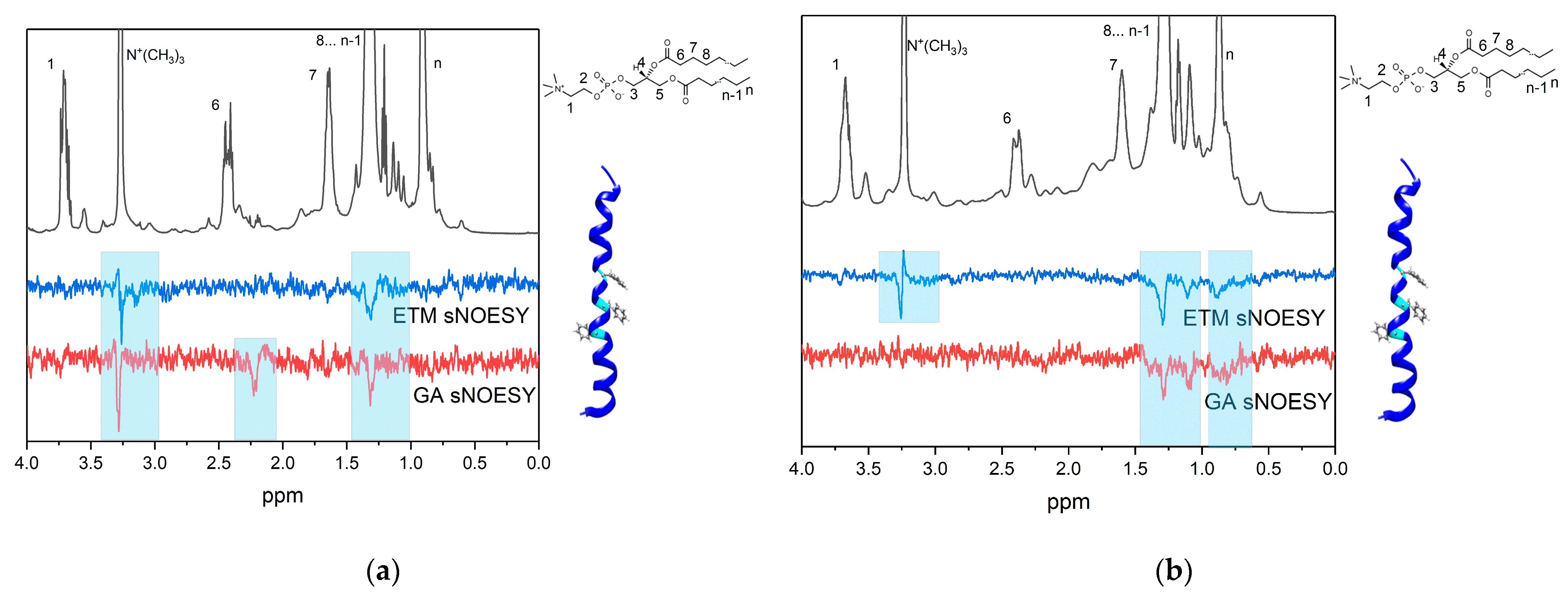
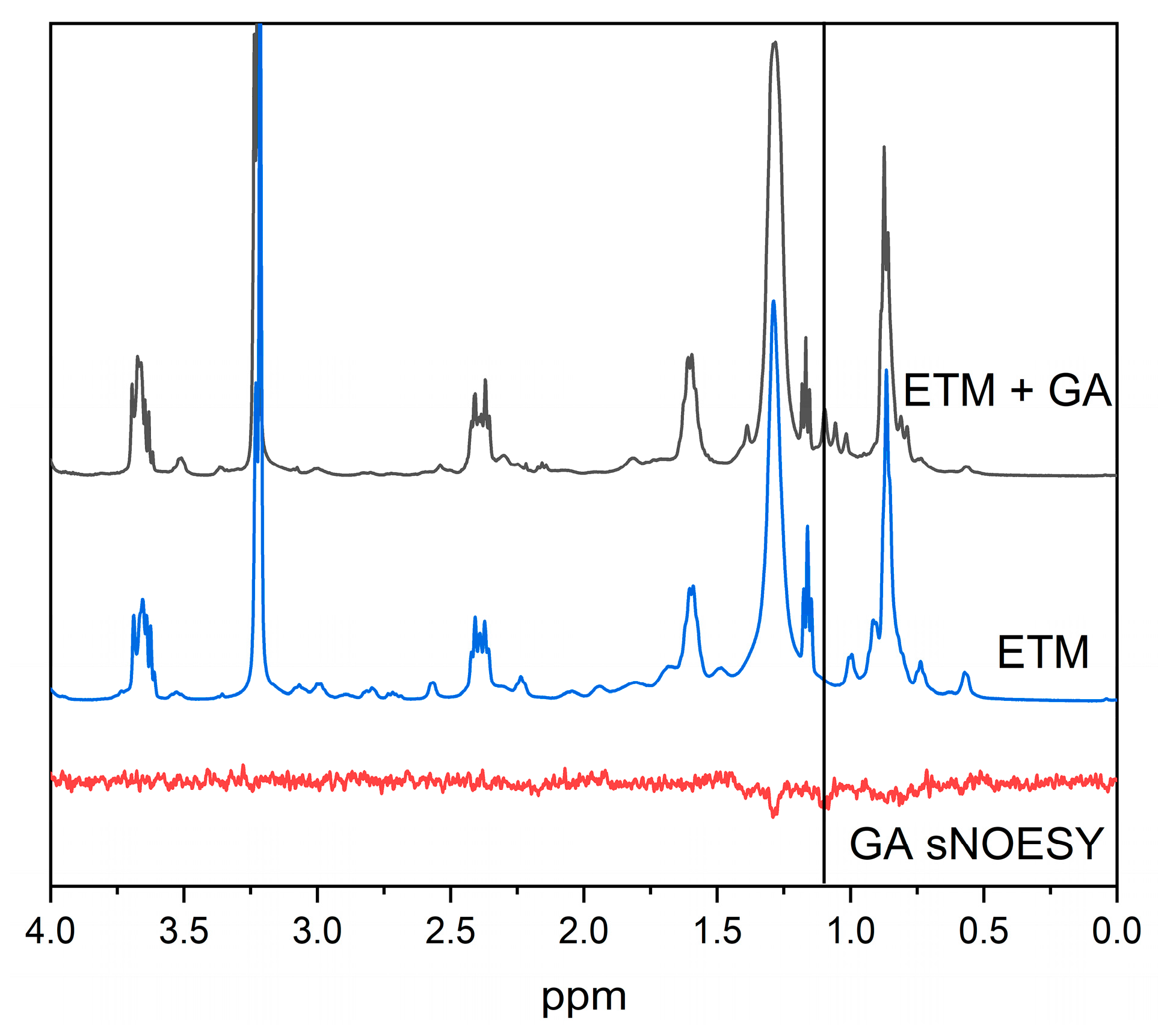
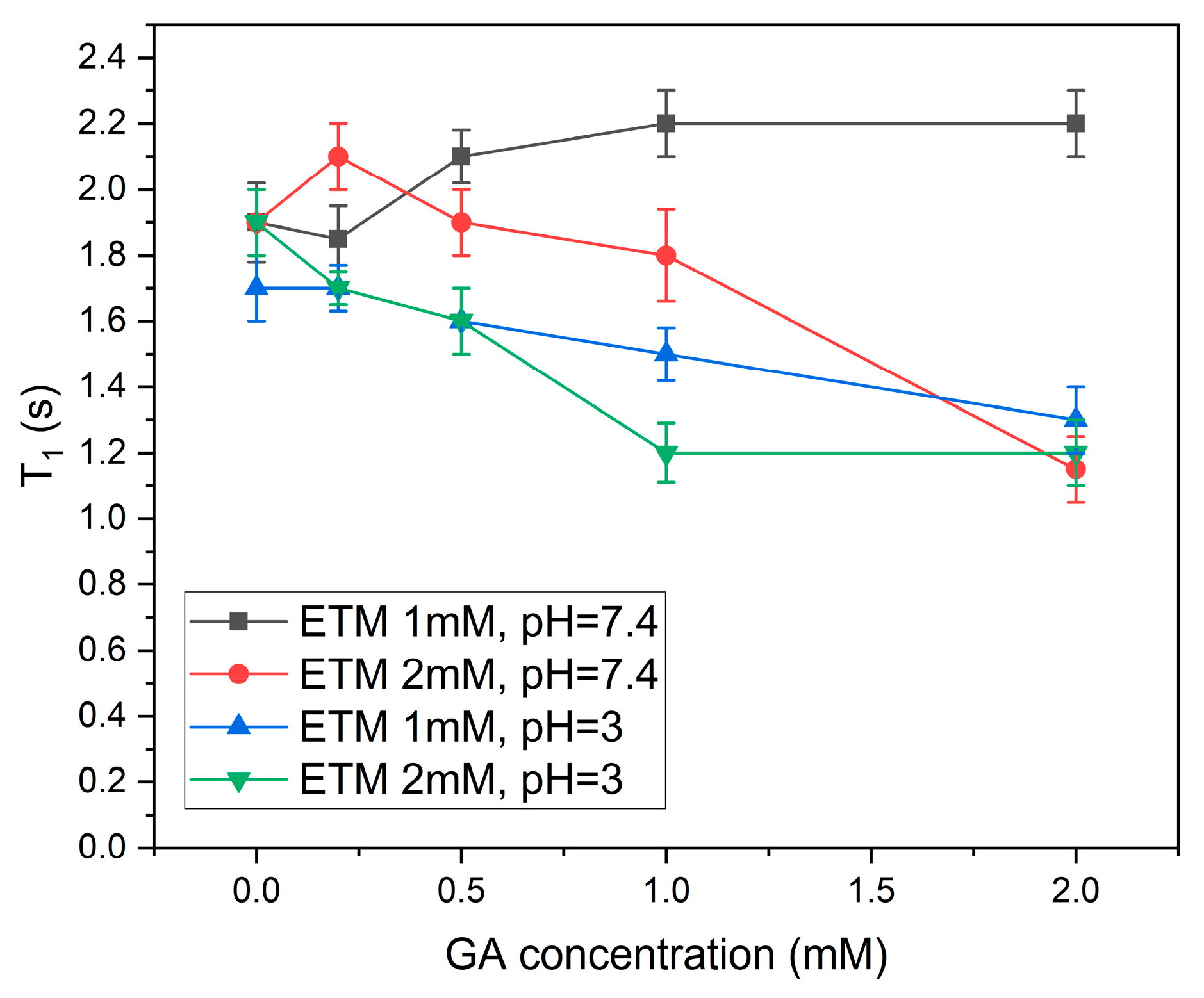
3.2. GA Interaction with ETM in Bicelles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brian, D.A.; Baric, R.S. Coronavirus Genome Structure and Replication. Curr. Top. Microbiol. Immunol. 2005, 287, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, R.; Lee, I.; Zhang, W.; Sun, J.; Wang, W.; Meng, X. Characterization of the SARS-CoV-2 E Protein: Sequence, Structure, Viroporin, and Inhibitors. Protein Sci. 2021, 30, 1114. [Google Scholar] [CrossRef] [PubMed]
- Arbely, E.; Khattari, Z.; Brotons, G.; Akkawi, M.; Salditt, T.; Arkin, I.T. A Highly Unusual Palindromic Transmembrane Helical Hairpin Formed by SARS Coronavirus E Protein. J. Mol. Biol. 2004, 341, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Baudoux, P.; Carrat, C.; Besnardeau, L.; Charley, B.; Laude, H. Coronavirus Pseudoparticles Formed with Recombinant M and E Proteins Induce Alpha Interferon Synthesis by Leukocytes. J. Virol. 1998, 72, 8636–8643. [Google Scholar] [CrossRef]
- Vennema, H.; Godeke, G.J.; Rossen, J.W.A.; Voorhout, W.F.; Horzinek, M.C.; Opstelten, D.J.E.; Rottier, P.J.M. Nucleocapsid-Independent Assembly of Coronavirus-like Particles by Co-Expression of Viral Envelope Protein Genes. EMBO J. 1996, 15, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Bos, E.C.W.; Luytjes, W.; Van Der Meulen, H.; Koerten, H.K.; Spaan, W.J.M. The Production of Recombinant Infectious DI-Particles of a Murine Coronavirus in the Absence of Helper Virus. Virology 1996, 218, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Torres, J.L.; DeDiego, M.L.; Álvarez, E.; Jiménez-Guardeño, J.M.; Regla-Nava, J.A.; Llorente, M.; Kremer, L.; Shuo, S.; Enjuanes, L. Subcellular Location and Topology of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein. Virology 2011, 415, 69–82. [Google Scholar] [CrossRef]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar]
- Surya, W.; Li, Y.; Verdià-Bàguena, C.; Aguilella, V.M.; Torres, J. MERS Coronavirus Envelope Protein Has a Single Transmembrane Domain That Forms Pentameric Ion Channels. Virus Res. 2015, 201, 61–66. [Google Scholar] [CrossRef]
- Madan, V.; García, M.D.J.; Sanz, M.A.; Carrasco, L. Viroporin Activity of Murine Hepatitis Virus E Protein. FEBS Lett. 2005, 579, 3607–3612. [Google Scholar] [CrossRef]
- Ye, Y.; Hogue, B.G. Role of the Coronavirus E Viroporin Protein Transmembrane Domain in Virus Assembly. J. Virol. 2007, 81, 3597. [Google Scholar] [CrossRef] [PubMed]
- Mandala, V.S.; McKay, M.J.; Shcherbakov, A.A.; Dregni, A.J.; Kolocouris, A.; Hong, M. Structure and Drug Binding of the SARS-CoV-2 Envelope Protein Transmembrane Domain in Lipid Bilayers. Nat. Struct. Mol. Biol. 2020, 27, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lv, P.; Li, M.; Chen, Z.; Xin, H.; Reilly, S.; Zhang, X. SARS-CoV-2 E Protein: Pathogenesis and Potential Therapeutic Development. Biomed. Pharmacother. 2023, 159, 114242. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.; Eisenhut, M.; Krausse, R.; Ragazzi, E.; Pellati, D.; Armanini, D.; Bielenberg, J. Antiviral Effects of Glycyrrhiza Species. Phytother. Res. 2008, 22, 141–148. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Zhao, T.-T.; Lu, N.; Yang, Y.-A.; Zhu, H.-L. Research Progress of Glycyrrhizic Acid on Antiviral Activity. Mini Rev. Med. Chem. 2019, 19, 826–832. [Google Scholar] [CrossRef]
- Pompei, R.; Pani, A.; Flore, O.; Marcialis, M.A.; Loddo, B. Antiviral Activity of Glycyrrhizic Acid. Experientia 1980, 36, 304. [Google Scholar] [CrossRef]
- Hoever, G.; Baltina, L.; Michaelis, M.; Kondratenko, R.; Baltina, L.; Tolstikov, G.A.; Doerr, H.W.; Cinatl, J. Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS-Coronavirus. J. Med. Chem. 2005, 48, 1256–1259. [Google Scholar] [CrossRef]
- Chrzanowski, J.; Chrzanowska, A.; Graboń, W. Glycyrrhizin: An Old Weapon against a Novel Coronavirus. Phyther. Res. 2021, 35, 629–636. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An Alternative Drug for the Treatment of COVID-19 Infection and the Associated Respiratory Syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef]
- Fomenko, V.V.; Rudometova, N.B.; Yarovaya, O.I.; Rogachev, A.D.; Fando, A.A.; Zaykovskaya, A.V.; Komarova, N.I.; Shcherbakov, D.N.; Pyankov, O.V.; Pokrovsky, A.G.; et al. Synthesis and In Vitro Study of Antiviral Activity of Glycyrrhizin Nicotinate Derivatives against HIV-1 Pseudoviruses and SARS-CoV-2 Viruses. Molecules 2022, 27, 295. [Google Scholar] [CrossRef]
- Kang, H.; Lieberman, P.M. Mechanism of Glycyrrhizic Acid Inhibition of Kaposi’s Sarcoma-Associated Herpesvirus: Disruption of CTCF-Cohesin-Mediated RNA Polymerase II Pausing and Sister Chromatid Cohesion. J. Virol. 2011, 85, 11159–11169. [Google Scholar] [CrossRef] [PubMed]
- Sekizawa, T.; Yanagi, K.; Itoyama, Y. Glycyrrhizin Increases Survival of Mice with Herpes Simplex Encephalitis. Acta Virol. 2001, 45, 51–54. [Google Scholar] [PubMed]
- Baba, M.; Shigeta, S. Antiviral Activity of Glycyrrhizin against Varicella-Zoster Virus in Vitro. Antiviral Res. 1987, 7, 99–107. [Google Scholar] [CrossRef]
- Lin, J.C. Mechanism of Action of Glycyrrhizic Acid in Inhibition of Epstein-Barr Virus Replication in Vitro. Antiviral Res. 2003, 59, 41–47. [Google Scholar] [CrossRef]
- Duan, E.; Wang, D.; Fang, L.; Ma, J.; Luo, J.; Chen, H.; Li, K.; Xiao, S. Suppression of Porcine Reproductive and Respiratory Syndrome Virus Proliferation by Glycyrrhizin. Antiviral Res. 2015, 120, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Harada, S. The Broad Anti-Viral Agent Glycyrrhizin Directly Modulates the Fluidity of Plasma Membrane and HIV-1 Envelope. Biochem. J. 2005, 392, 191–199. [Google Scholar] [CrossRef]
- Crance, J.M.; Lévêque, F.; Biziagos, E.; van Cuyck-Gandré, H.; Jouan, A.; Deloince, R. Studies on Mechanism of Action of Glycyrrhizin against Hepatitis A Virus Replication in Vitro. Antiviral Res. 1994, 23, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Yin, J.; Ren, X. Antiviral Effect of Diammonium Glycyrrhizinate and Lithium Chloride on Cell Infection by Pseudorabies Herpesvirus. Antiviral Res. 2010, 85, 346–353. [Google Scholar] [CrossRef]
- Schröfelbauer, B.; Raffetseder, J.; Hauner, M.; Wolkerstorfer, A.; Ernst, W.; Szolar, O.H.J. Glycyrrhizin, the Main Active Compound in Liquorice, Attenuates pro-Inflammatory Responses by Interfering with Membrane-Dependent Receptor Signalling. Biochem. J. 2009, 421, 473–482. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Matsuura, T.; Aoyagi, H.; Matsuda, M.; Hmwe, S.S.; Date, T.; Watanabe, N.; Watashi, K.; Suzuki, R.; Ichinose, S.; et al. Antiviral Activity of Glycyrrhizin against Hepatitis C Virus In Vitro. PLoS ONE 2013, 8, e68992. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Polyakov, N.E.; Korneev, D.V.; Zaitsev, B.N. Influence of Glycyrrhizin on Permeability and Elasticity of Cell Membrane: Perspectives for Drugs Delivery. Drug Deliv. 2016, 23, 848–855. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Polyakov, N.E. Glycyrrhizic Acid as a Multifunctional Drug Carrier—From Physicochemical Properties to Biomedical Applications: A Modern Insight on the Ancient Drug. Int. J. Pharm. 2019, 559, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Glazachev, Y.I.; Schlotgauer, A.A.; Timoshnikov, V.A.; Kononova, P.A.; Selyutina, O.Y.; Shelepova, E.A.; Zelikman, M.V.; Khvostov, M.V.; Polyakov, N.E. Effect of Glycyrrhizic Acid and Arabinogalactan on the Membrane Potential of Rat Thymocytes Studied by Potential-Sensitive Fluorescent Probe. J. Membr. Biol. 2020, 253, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Shelepova, E.A.; Paramonova, E.D.; Kichigina, L.A.; Khalikov, S.S.; Polyakov, N.E. Glycyrrhizin-Induced Changes in Phospholipid Dynamics Studied by 1H NMR and MD Simulation. Arch. Biochem. Biophys. 2020, 686, 108368. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Apanasenko, I.E.; Kim, A.V.; Shelepova, E.A.; Khalikov, S.S.; Polyakov, N.E. Spectroscopic and Molecular Dynamics Characterization of Glycyrrhizin Membrane-Modifying Activity. Colloids Surf. B Biointerfaces 2016, 147, 459–466. [Google Scholar] [CrossRef]
- Chernyshev, A. Pharmaceutical Targeting the Envelope Protein of SARS-CoV-2: The Screening for Inhibitors in Approved Drugs. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Pervushin, K.; Tan, E.; Parthasarathy, K.; Lin, X.; Jiang, F.L.; Yu, D.; Vararattanavech, A.; Tuck, W.S.; Ding, X.L.; Torres, J. Structure and Inhibition of the SARS Coronavirus Envelope Protein Ion Channel. PLOS Pathog. 2009, 5, e1000511. [Google Scholar] [CrossRef]
- Struppe, J.; Whiles, J.A.; Void, R.R. Acidic Phospholipid Bicelles: A Versatile Model Membrane System. Biophys. J. 2000, 78, 281–289. [Google Scholar] [CrossRef]
- Piai, A.; Fu, Q.; Dev, J.; Chou, J.J. Optimal Bicelle q for Solution NMR Studies of Protein Transmembrane Partition. Chemistry 2017, 23, 1361. [Google Scholar] [CrossRef]
- Zeng, C.-X.; Hu, Q. Determination of the Polyacid Dissociation Constants of Glycyrrhizic Acid. Indian J. Chem. 2008, 47, 71–74. [Google Scholar]
- Lazaridis, T.; Mallik, B.; Chen, Y. Implicit Solvent Simulations of DPC Micelle Formation. J. Phys. Chem. B 2005, 109, 15098–15106. [Google Scholar] [CrossRef] [PubMed]
- Leftin, A.; Molugu, T.R.; Job, C.; Beyer, K.; Brown, M.F. Area per Lipid and Cholesterol Interactions in Membranes from Separated Local-Field 13C NMR Spectroscopy. Biophys. J. 2014, 107, 2274. [Google Scholar] [CrossRef] [PubMed]
- Björnerås, J.; Nilsson, M.; Mäler, L. Analysing DHPC/DMPC Bicelles by Diffusion NMR and Multivariate Decomposition. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2910–2917. [Google Scholar] [CrossRef]
- Salditt, T.; Li, C.; Spaar, A. Structure of Antimicrobial Peptides and Lipid Membranes Probed by Interface-Sensitive X-Ray Scattering. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, V.S.P.; Yu, O.; GuoHong, M. NMR Analysis and Hydrolysis Studies of Glycyrrhizic Acid, a Major Constituent of Glycyrrhia Glabra. Eur. Chem. Bull. 2014, 3, 104–107. [Google Scholar]
- Panahi, A.; Brooks, C.L. Membrane Environment Modulates the PKa Values of Transmembrane Helices. J. Phys. Chem. B 2015, 119, 4601–4607. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Kononova, P.A.; Polyakov, N.E. Experimental and Theoretical Study of Emodin Interaction with Phospholipid Bilayer and Linoleic Acid. Appl. Magn. Reson. 2020, 51, 951–960. [Google Scholar] [CrossRef]
- Thurlkill, R.L.; Grimsley, G.R.; Scholtz, J.M.; Pace, C.N. Hydrogen Bonding Markedly Reduces the PK of Buried Carboxyl Groups in Proteins. J. Mol. Biol. 2006, 362, 594–604. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononova, P.A.; Selyutina, O.Y.; Polyakov, N.E. The Interaction of the Transmembrane Domain of SARS-CoV-2 E-Protein with Glycyrrhizic Acid in Lipid Bilayer. Membranes 2023, 13, 505. https://doi.org/10.3390/membranes13050505
Kononova PA, Selyutina OY, Polyakov NE. The Interaction of the Transmembrane Domain of SARS-CoV-2 E-Protein with Glycyrrhizic Acid in Lipid Bilayer. Membranes. 2023; 13(5):505. https://doi.org/10.3390/membranes13050505
Chicago/Turabian StyleKononova, Polina A., Olga Yu. Selyutina, and Nikolay E. Polyakov. 2023. "The Interaction of the Transmembrane Domain of SARS-CoV-2 E-Protein with Glycyrrhizic Acid in Lipid Bilayer" Membranes 13, no. 5: 505. https://doi.org/10.3390/membranes13050505
APA StyleKononova, P. A., Selyutina, O. Y., & Polyakov, N. E. (2023). The Interaction of the Transmembrane Domain of SARS-CoV-2 E-Protein with Glycyrrhizic Acid in Lipid Bilayer. Membranes, 13(5), 505. https://doi.org/10.3390/membranes13050505







