Stability of Porous Polymeric Membranes in Amine Solvents for Membrane Contactor Applications
Abstract
:1. Introduction
- (a)
- (b)
- (c)
- Membrane deoxygenation of amine solvents: This is a relatively new field of the application of gas–liquid or liquid–liquid membrane contactors. The promise of this approach was first demonstrated by TNO using the Dissolved Oxygen Removal Apparatus (DORA) at TRL6 [11]. The relevance of this problem is due to the fact that oxygen is often present in the mixture being purified, which leads to the oxidative degradation of amines [12,13,14]. In addition to the harm caused by the direct intensification of corrosion in amine solvents, degradation, and the oxidation of amines [15,16,17,18,19,20,21,22,23], it is important to note the effect of oxidation products on the overall performance of the system. Thus, under the influence of oxidation products (carboxylic acids, amides, aldehydes, and amino acids), the physicochemical degradation of the amine occurs by the foaming, erosion, and precipitation of non-regenerated heat-stable salts (HSSs) that accumulate in the system and lead to general pollution. In addition, these compounds are direct corrosion catalysts [24,25], suggesting the autocatalytic degradation of the absorbent.
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Membranes
2.1.2. Amine Solutions
2.1.3. Heat-Stable Salt Anions
2.2. Membranes Characterization
2.2.1. Long-Term Treatment of Porous Polymer Membranes with Amine Solvents
2.2.2. FTIR Spectroscopy
2.2.3. Atomic Force Microscopy
2.2.4. Pore Size Measurements
2.2.5. Gas Permeance Measurements
3. Results and Discussion
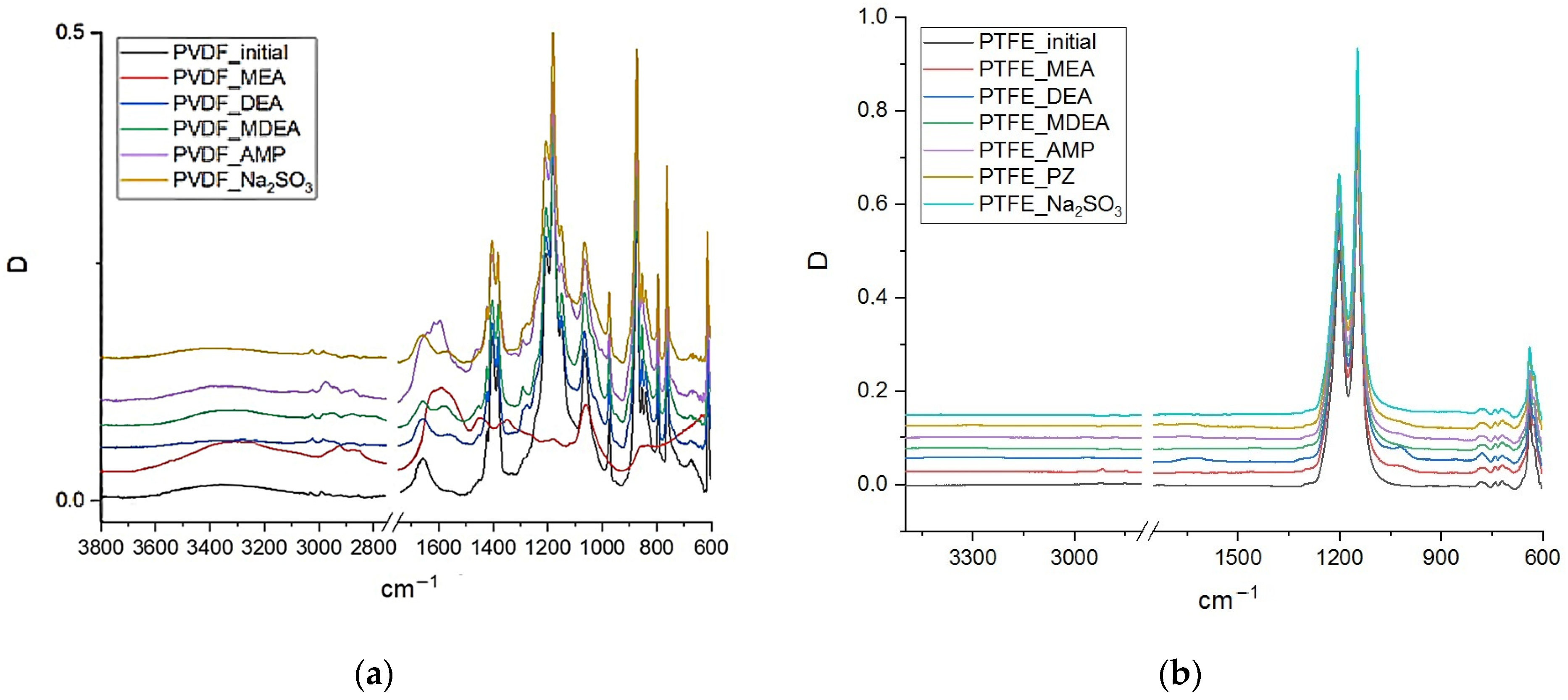
3.1. Changes in the Chemical Structure of Polymeric Membranes
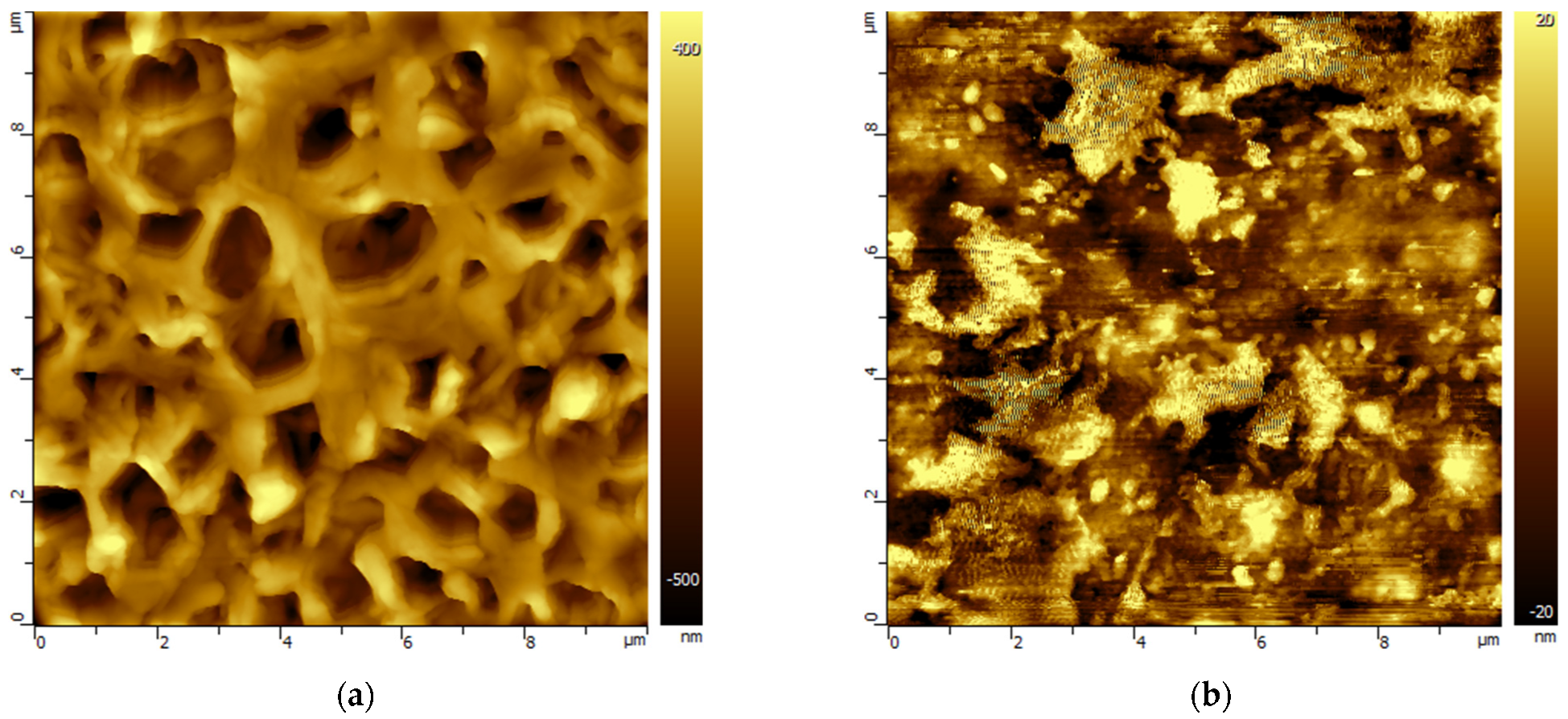
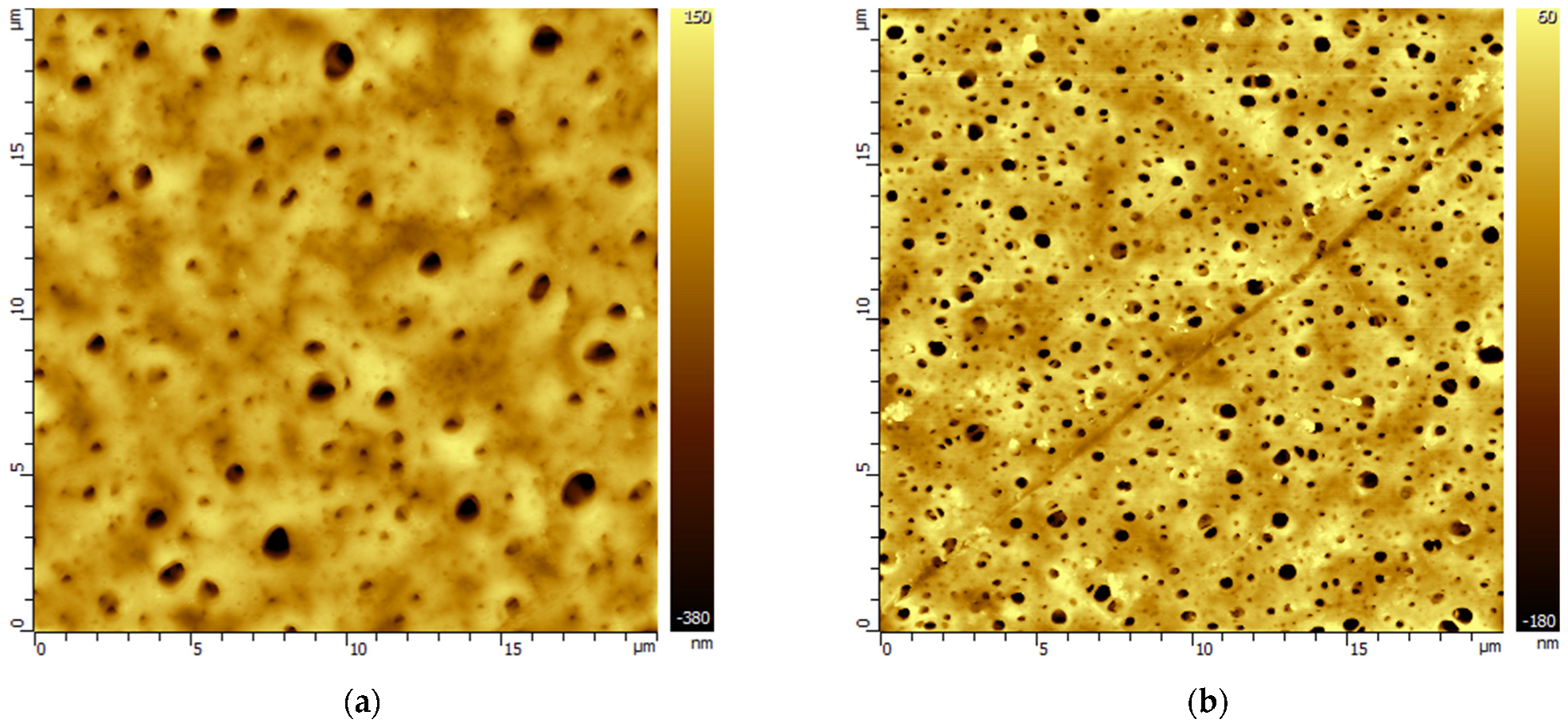
3.2. Changes in the Morphology of Polymeric Membranes
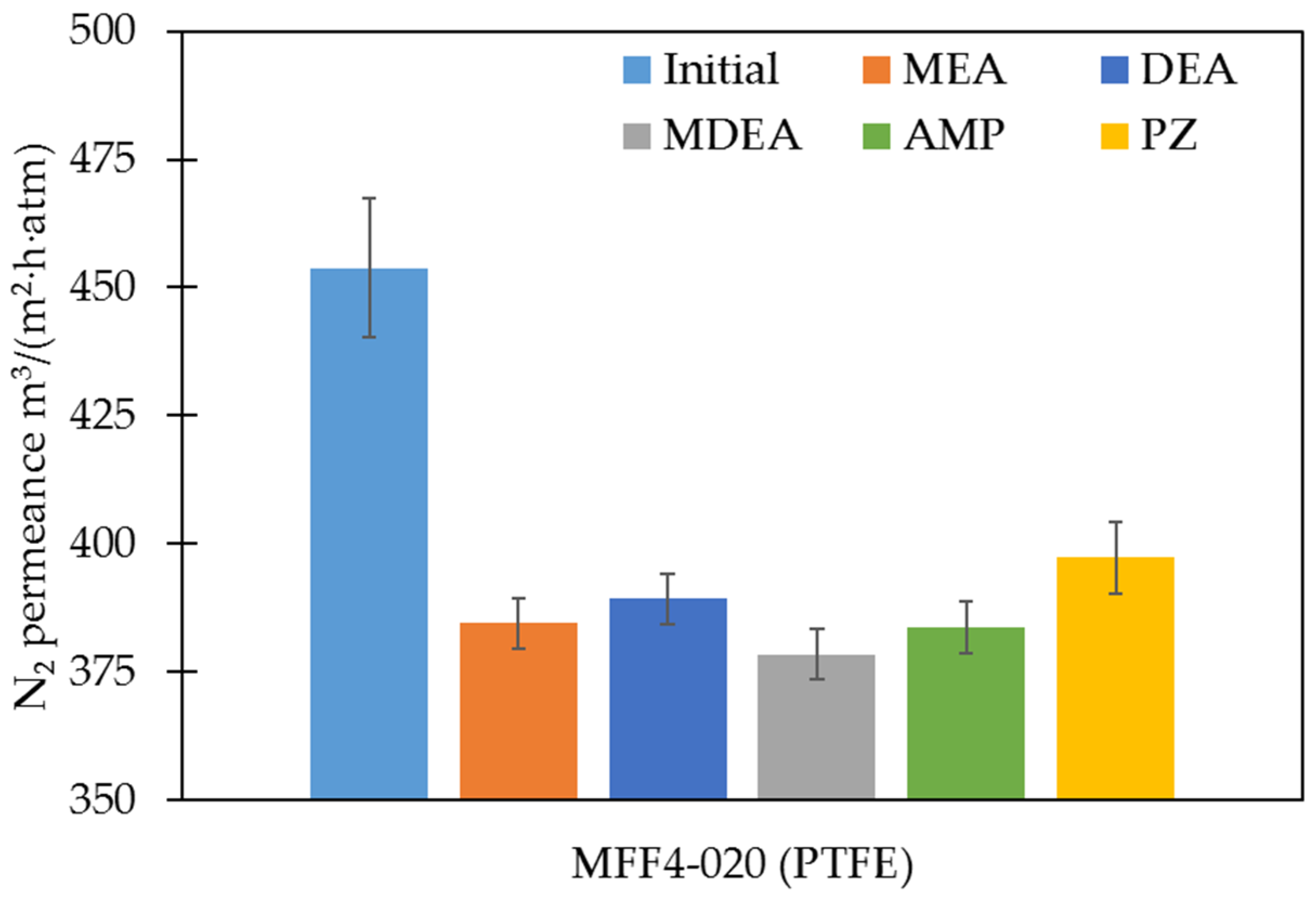
3.3. Changes in Pore Size, Transport, and Separating Properties of Polymeric Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horowitz, C.A. Paris Agreement. Int. Leg. Mater. 2016, 55, 740–755. [Google Scholar] [CrossRef]
- Adam, S.; Delestre, I.; Levell, P.; Miller, H. Tax Policies to Reduce Carbon Emissions. Fisc. Stud. 2022, 43, 235–263. [Google Scholar] [CrossRef]
- Ghazouani, A.; Xia, W.; Ben Jebli, M.; Shahzad, U. Exploring the Role of Carbon Taxation Policies on CO2 Emissions: Contextual Evidence from Tax Implementation and Non-Implementation European Countries. Sustainability 2020, 12, 8680. [Google Scholar] [CrossRef]
- Shi, J.Q.; Durucan, S. CO2 Storage in Deep Unminable Coal Seams; Stockage Du CO2 Dans Des Veines de Charbon Non Exploitables. Oil Gas Sci. Technol. 2005, 60, 547–558. [Google Scholar] [CrossRef]
- Gozalpour, F.; Ren, S.R.; Tohidi, B. CO2 EOR and storage in oil reservoir. Oil Gas Sci. Technol. 2005, 60, 537–546. [Google Scholar] [CrossRef]
- Bazhenov, S.; Chuboksarov, V.; Maximov, A.; Zhdaneev, O. Technical and Economic Prospects of CCUS Projects in Russia. Sustain. Mater. Technol. 2022, 33, e00452. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.Y.; Bae, T.H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2019, 59, 6773–6794. [Google Scholar] [CrossRef]
- Kim, S.; Scholes, C.A.; Heath, D.E.; Kentish, S.E. Gas-liquid membrane contactors for carbon dioxide separation: A review. Chem. Eng. J. 2021, 411, 128468. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Gomez-Coma, L.; Garea, A.; Irabien, A. Hollow fiber membrane contactors in CO2 desorption: A review. Energy Fuels 2020, 35, 111–136. [Google Scholar] [CrossRef]
- Li, T.; Keener, T.C. A review: Desorption of CO2 from rich solutions in chemical absorption processes. Int. J. Greenh. Gas Control 2016, 51, 290–304. [Google Scholar] [CrossRef]
- Figueiredo, R.V.; Srivastava, T.; Skaar, T.; Warning, N.; Gravesteijn, P.; van Os, P.; Ansaloni, L.; Deng, L.; Knuutila, H.; Monteiro, J.; et al. Impact of dissolved oxygen removal on solvent degradation for post-combustion CO2 capture. Int. J. Greenh. Gas Control 2021, 112, 103493. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Novitskii, E.G.; Vasilevskii, V.P.; Grushevenko, E.A.; Bienko, A.A.; Volkov, A.V. Heat-Stable Salts and Methods for Their Removal from Alkanolamine Carbon Dioxide Absorbents. Russ. J. Appl. Chem. 2019, 92, 1045–1063. [Google Scholar] [CrossRef]
- Buvik, V.; Høisæter, K.K.; Vevelstad, S.J.; Knuutila, H.K. A Review of Degradation and Emissions in Post-Combustion CO2 Capture Pilot Plants. Int. J. Greenh. Gas Control 2021, 106, 103246. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Gas Purification, 5th ed.; Gulf Publishing Company: Houston, TX, USA, 1997. [Google Scholar]
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion Problems during Oil and Gas Production and Its Mitigation. Int. J. Ind. Chem. 2013, 4, 35. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Duan, D.; Nešić, S.; Vitse, F.; Bedell, S.A.; Worley, C. Effect of Oxygen and Heat Stable Salts on the Corrosion of Carbon Steel in MDEA-Based CO2 Capture Process. Corrosion 2010, 66, 125004–125010. [Google Scholar] [CrossRef]
- Soosaiprakasam, I.R.; Veawab, A. Corrosion and Polarization Behavior of Carbon Steel in MEA-Based CO2 Capture Process. Int. J. Greenh. Gas Control 2008, 2, 553–562. [Google Scholar] [CrossRef]
- Kladkaew, N.; Idem, R.; Tontiwachwuthikul, P.; Saiwan, C. Studies on Corrosion and Corrosion Inhibitors for Amine Based Solvents for CO2 Absorption from Power Plant Flue Gases Containing CO2, O2 and SO2. Energy Procedia 2011, 4, 1761–1768. [Google Scholar] [CrossRef]
- Zheng, L.; Landon, J.; Matin, N.S.; Thomas, G.A.; Liu, K. Corrosion Mitigation via a PH Stabilization Method in Monoethanolamine-Based Solutions for Post-Combustion CO2 Capture. Corros. Sci. 2016, 106, 281–292. [Google Scholar] [CrossRef]
- Xiang, Y.; Xie, W.; Ni, S.; He, X. Comparative Study of A106 Steel Corrosion in Fresh and Dirty MEA Solutions during the CO2 Capture Process: Effect of NO3−. Corros. Sci. 2020, 167, 108521. [Google Scholar] [CrossRef]
- Moser, P.; Wiechers, G.; Schmidt, S.; Monteiro, J.G.M.-S.; Charalambous, C.; Garcia, S.; Fernandez, E.S. Results of the 18-Month Test with MEA at the Post-Combustion Capture Pilot Plant at Niederaussem–New Impetus to Solvent Management, Emissions and Dynamic Behaviour. Int. J. Greenh. Gas Control 2020, 95, 102945. [Google Scholar] [CrossRef]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.-L. Amine Degradation in CO2 Capture. I. A Review. Int. J. Greenh. Gas Control 2012, 10, 244–270. [Google Scholar] [CrossRef]
- Bedell, S.A. Oxidative Degradation Mechanisms for Amines in Flue Gas Capture. Energy Procedia 2009, 1, 771–778. [Google Scholar] [CrossRef]
- Wang, T.; Hovland, J.; Jens, K.J. Amine Reclaiming Technologies in Post-Combustion Carbon Dioxide Capture. J. Environ. Sci. 2015, 27, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.J.; Rochelle, G.T. Catalysts and Inhibitors for Oxidative Degradation of Monoethanolamine. Int. J. Greenh. Gas Control 2009, 3, 704–711. [Google Scholar] [CrossRef]
- Kalmykov, D.; Balynin, A.; Yushkin, A.; Grushevenko, E.; Sokolov, S.; Malakhov, A.; Volkov, A.; Bazhenov, S. Membranes Based on PTMSP/PVTMS Blends for Membrane Contactor Applications. Membranes 2022, 12, 1160. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W.; deMontigny, D. Comparison of thin film composite and microporous membrane contactors for CO2 absorption into monoethanolamine. Int. J. Greenh. Gas Control 2015, 42, 66–74. [Google Scholar] [CrossRef]
- Franco, J.A.; deMontigny, D.; Kentish, S.E.; Perera, J.M.; Stevens, G.W. Effect of amine degradation products on the membrane gas absorption process. Chem. Eng. Sci. 2009, 64, 4016–4023. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Komkova, M.A.; Eliseev, A.A.; Poyarkov, A.A.; Eliseev, A.A. Nanoporous Polypropylene Membrane Contactors for CO2 and H2S Capture Using Alkali Absorbents. Chem. Eng. Res. Des. 2022, 177, 448–460. [Google Scholar] [CrossRef]
- Kamo, J.; Hirai, T.; Kamada, K. Solvent-Induced Morphological Change of Microporous Hollow Fiber Membranes. J. Membr. Sci. 1992, 70, 217–224. [Google Scholar] [CrossRef]
- Barbe, A. Surface Morphology Changes during Initial Usage of Hydrophobic, Microporous Polypropylene Membranes. J. Membr. Sci. 2000, 172, 149–156. [Google Scholar] [CrossRef]
- Faiz, R.; Fallanza, M.; Boributh, S.; Jiraratananon, R.; Ortiz, I.; Li, K. Long Term Stability of PTFE and PVDF Membrane Contactors in the Application of Propylene/Propane Separation Using AgNO3 Solution. Chem. Eng. Sci. 2013, 94, 108–119. [Google Scholar] [CrossRef]
- Wang, R.; Li, D.F.; Zhou, C.; Liu, M.; Liang, D.T. Impact of DEA Solutions with and without CO2 Loading on Porous Polypropylene Membranes Intended for Use as Contactors. J. Membr. Sci. 2004, 229, 147–157. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, M.; Ma, Q.; Yu, H.; Wei, C.-C.; Luo, Z. Investigation of Membrane Wetting in Different Absorbents at Elevated Temperature for Carbon Dioxide Capture. J. Membr. Sci. 2014, 455, 219–228. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; El-Naas, M.H.; Zhang, Z.; Van der Bruggen, B. CO2 capture using hollow fiber membranes: A review of membrane wetting. Energy Fuels 2018, 32, 963–978. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zhao, B.; Zhang, H.; Lu, X.; Yang, Q. Effect of Long-Term Operation on the Performance of Polypropylene and Polyvinylidene Fluoride Membrane Contactors for CO2 Absorption. Sep. Purif. Technol. 2013, 116, 300–306. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Wang, L.; Zhao, B.; Li, J.; Zhang, H. Wetting Mechanism of a PVDF Hollow Fiber Membrane in Immersed Membrane Contactors for CO2 Capture in the Presence of Monoethanolamine. RSC Adv. 2017, 7, 13451–13457. [Google Scholar] [CrossRef]
- Rezaiyan, Z.; Keshavarz, P.; Khorram, M. Experimental Investigation of the Effects of Different Chemical Absorbents on Wetting and Morphology of Poly(Vinylidene Fluoride) Membrane. J. Appl. Polym. Sci. 2017, 134, 45543. [Google Scholar] [CrossRef]
- Sedghi, S.M.; Brisson, J.; Rodrigue, D.; Iliuta, M.C. Chemical Alteration of LDPE Hollow Fibers Exposed to Monoethanolamine Solutions Used as Absorbent for CO2 Capture Process. Sep. Purif. Technol. 2011, 80, 338–344. [Google Scholar] [CrossRef]
- Mosadegh-Sedghi, S.; Brisson, J.; Rodrigue, D.; Iliuta, M.C. Morphological, Chemical and Thermal Stability of Microporous LDPE Hollow Fiber Membranes in Contact with Single and Mixed Amine Based CO2 Absorbents. Sep. Purif. Technol. 2012, 96, 117–123. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Precombustion CO2 capture in polymeric hollow fiber membrane contactors using ionic liquids: Porous membrane versus nonporous composite membrane. Ind. Eng. Chem. Res. 2016, 55, 5983–5992. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, Y.; Wang, F.; Zhang, H.; Guo, Y. Long-Term Stability of Polytetrafluoroethylene (PTFE) Hollow Fiber Membranes for CO2 Capture. Energy Fuels 2016, 30, 492–503. [Google Scholar] [CrossRef]
- Naim, R.; Khulbe, K.C.; Ismail, A.F.; Matsuura, T. Characterization of PVDF Hollow Fiber Membrane for CO2 Stripping by Atomic Force Microscopy Analysis. Sep. Purif. Technol. 2013, 109, 98–106. [Google Scholar] [CrossRef]
- Rosli, A.; Ahmad, A.L.; Low, S.C. Functionalization of Silica Nanoparticles to Reduce Membrane Swelling in CO2 Absorption Process. J. Chem. Technol. Biotechnol. 2020, 95, 1073–1084. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Leo, C.P.; Ahmad, A.L.; Nur Izwanne, M. Swelling Reduction of Polyvinylidenefluoride Hollow Fiber Membrane Incorporated with Silicoaluminophosphate-34 Zeotype Filler for Membrane Gas Absorption. Sep. Purif. Technol. 2019, 212, 941–951. [Google Scholar] [CrossRef]
- Emori, W.; Jiang, S.L.; Duan, D.L.; Ekerenam, O.O.; Zheng, Y.G.; Okafor, P.C.; Qiao, Y.X. Corrosion Behavior of Carbon Steel in Amine-Based CO2 Capture System: Effect of Sodium Sulfate and Sodium Sulfite Contaminants. Mater. Corros. 2017, 68, 674–682. [Google Scholar] [CrossRef]
- Morken, A.K.; Pedersen, S.; Nesse, S.O.; Flø, N.E.; Johnsen, K.; Feste, J.K.; de Cazenove, T.; Faramarzi, L.; Vernstad, K. CO2 Capture with Monoethanolamine: Solvent Management and Environmental Impacts during Long Term Operation at the Technology Centre Mongstad (TCM). Int. J. Greenh. Gas Control 2019, 82, 175–183. [Google Scholar] [CrossRef]
- Dibrov, G.A.; Volkov, V.V.; Vasilevsky, V.P.; Shutova, A.A.; Bazhenov, S.D.; Khotimsky, V.S.; van de Runstraat, A.; Goetheer, E.L.V.; Volkov, A.V. Robust high-permeance PTMSP composite membranes for CO2 membrane gas desorption at elevated temperatures and pressures. J. Membr. Sci. 2014, 470, 439–450. [Google Scholar] [CrossRef]
- Volkov, A.V.; Tsarkov, S.E.; Goetheer, E.L.V.; Volkov, V.V. Amine-based solvents regeneration in gas-liquid membrane contactor based on asymmetric PVTMS. Pet. Chem. 2015, 55, 716–723. [Google Scholar] [CrossRef]
- Volkov, A.; Vasilevsky, V.; Bazhenov, S.; Volkov, V.; Rieder, A.; Unterberger, S.; Schallert, B. Reclaiming of Monoethanolamine (MEA) Used in Post-Combustion CO2-Capture with Electrodialysis. Energy Procedia 2014, 51, 148–153. [Google Scholar] [CrossRef]
- Rieder, A.; Unterberger, S. EnBW’s Post-Combustion Capture Pilot Plant at Heilbronn–Results of the First Year’s Testing Programme. Energy Procedia 2013, 37, 6464–6472. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Malakhov, A.O.; Karpacheva, G.P.; Bondarenko, G.; Marbelia, L.; Vankelecom, I.F.J.; Volkov, A.V. Creation of Highly Stable Porous Polyacrylonitrile Membranes Using Infrared Heating. React. Funct. Polym. 2021, 158, 104793. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Maksimov, A.L.; Volkov, A.V. Composite Membranes Based on the Poly(1-Trimethylsylyl-1-Propine): Influence of the Porous Aromatic Frameworks Produced from the Friedel–Crafts Reaction and Introduced into the Polymer Matrix. Russ. J. Appl. Chem. 2020, 93, 252–257. [Google Scholar] [CrossRef]
- Khaisri, S.; deMontigny, D.; Tontiwachwuthikul, P.; Jiraratananon, R. Comparing membrane resistance and absorption performance of three different membranes in a gas absorption membrane contactor. Sep. Purif. Technol. 2009, 65, 290–297. [Google Scholar] [CrossRef]
- Bottino, A.; Comite, A.; Costa, C.; Di Felice, R.; Varosio, E. Wetting of polypropylene membranes by aqueous solutions in CO2 absorbing devices. Sep. Sci. Technol. 2015, 50, 1860–1869. [Google Scholar] [CrossRef]
- Rongwong, W.; Jiraratananon, R.; Atchariyawut, S. Experimental study on membrane wetting in gas–liquid membrane contacting process for CO2 absorption by single and mixed absorbents. Sep. Purif. Technol. 2009, 69, 118–125. [Google Scholar] [CrossRef]

| Material | Designation | Thickness, μm | Trading Name, Company | |
|---|---|---|---|---|
| Fluoropolymers | ||||
| Polyvinylidenefluoride | 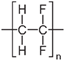 | PVDF | 123 ± 2 | PVDF-022, Technofilter RME, Vladimir, Russia |
| Polytetrafluoroethylene | 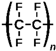 | PTFE | 45 ± 2 | MFF4-020, Technofilter RME, Vladimir, Russia |
| Polyolefin | ||||
| Polypropylene | 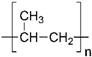 | PP | 95 ± 2 | PolySep™, GE Osmonics Labstore, Minnetonka, MN, USA |
| Polyethersulfone | ||||
| Polyethersulfone | 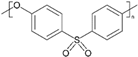 | PES | 79 ± 1 | PES-020, Technofilter RME, Vladimir, Russia |
| Polyamide | ||||
| Polyamide (nylon) |  | PA | 111 ± 4 | MCM-010, Technofilter RME, Vladimir, Russia |
| Amine | Abbreviation | Amine Structure | Concentration, wt.%. | Comments |
|---|---|---|---|---|
| Monoethanolamine | MEA | 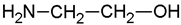 | 30 | Solution in distilled water |
| N-methyldiethanolamine | MDEA | 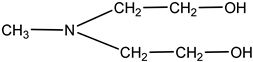 | 40 | Solution in distilled water |
| 2-Amino-2-methylpropan-1-ol | AMP | 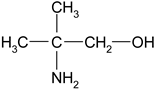 | 30 | Solution in distilled water |
| Piperazine | PZ | 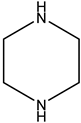 | 40 | Solution in distilled water |
| Diethanolamine | DEA | 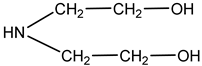 | ~29 | A sample of a real industrial degraded solvent. The content of degradation products (heat-stable salts) is ~8000 ppm |
| HSS Anion | Concentration | |
|---|---|---|
| mg/L | mmol-equiv/L | |
| Formate | 1200 | 26.65 |
| Oxalate | 500 | 11.36 |
| Acetate | 50 | 0.85 |
| Nitrate | 200 | 3.23 |
| Sulfate | 400 | 8.33 |
| Chloride | 10 | 0.28 |
| Total content of HSS anions | 2360 | 50.7 |
| Solution | Statistical Quantities | Porosity | Roughness | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum, nm | Maximum, nm | Δ, nm | Total Area, µm2 | Pore Area, µm2 | Porosity, % | Square Roughness Rq, nm | Average Roughness Ra, nm | |
| PVDF-022 (PVDF) | ||||||||
| Initial | −400 | 300 | 700 | 403.0 ± 5.0 | 42.7 ± 3.0 | 11 ± 1 | 149.7 ± 5.0 | 119.6 ± 5.0 |
| MEA | −190 | 190 | 380 | 402.7 ± 5.0 | 46.5 ± 3.0 | 12 ± 1 | 66.3 ± 3.5 | 52.1 ± 3.5 |
| DEA | −500 | 400 | 900 | 401.0 ± 5.0 | 40.6 ± 3.0 | 10 ± 1 | 182.1 ± 5.0 | 148.3 ± 5.0 |
| MDEA | −400 | 400 | 800 | 400.0 ± 5.0 | 32.4 ± 3.0 | 8 ± 1 | 167.2 ± 5.0 | 136.4 ± 5.0 |
| AMP | −500 | 500 | 1000 | 401.0 ± 5.0 | 92.0 ± 3.0 | 23 ± 1 | 244.8 ± 5.0 | 203.6 ± 5.0 |
| PZ * | − | - | - | - | - | - | - | - |
| Na2SO3 | −1700 | 1400 | 3100 | 404.0 ± 5.0 | 53.3 ± 3.0 | 13 ± 1 | 708.2 ± 7.0 | 568.2 ± 7.0 |
| MFF4-020 (PTFE) | ||||||||
| Initial | −360 | 200 | 560 | 400.0 ± 5.0 | 47.2 ± 3.0 | 12 ± 1 | 116.3 ± 5.0 | 92.8 ± 5.0 |
| MEA | −400 | 300 | 700 | 398.9 ± 5.0 | 39.2 ± 3.0 | 10 ± 1 | 143.7 ± 5.0 | 115.1 ± 5.0 |
| DEA | −500 | 200 | 700 | 400.0 ± 5.0 | 18.5 ± 3.0 | 5 ± 1 | 118.4 ± 5.0 | 89.6 ± 5.0 |
| MDEA | −600 | 300 | 900 | 401.1 ± 5.0 | 29.0 ± 3.0 | 7 ± 1 | 140.6 ± 5.0 | 106.9 ± 5.0 |
| AMP | −600 | 300 | 900 | 400.0 ± 5.0 | 25.4 ± 3.0 | 6 ± 1 | 155.3 ± 5.0 | 122.1 ± 5.0 |
| PZ | −500 | 300 | 800 | 398.9 ± 5.0 | 36.0 ± 3.0 | 9 ± 1 | 151.2 ± 5.0 | 117.7 ± 5.0 |
| Na2SO3 | −600 | 300 | 900 | 400.0 ± 5.0 | 11.5 ± 2.0 | 3 ± 1 | 154.7 ± 5.0 | 118.7 ± 5.0 |
| PolySep (PP) | ||||||||
| Initial | −300 | 200 | 500 | 436.9 ± 5.0 | 74.3 ± 3.0 | 17 ± 1 | 107.7 ± 5.0 | 83.2 ± 5.0 |
| MEA | −400 | 200 | 600 | 437.0 ± 5.0 | 41.2 ± 3.0 | 9 ± 1 | 112.4 ± 5.0 | 84.7 ± 5.0 |
| DEA | −500 | 300 | 800 | 441.1 ± 5.0 | 49.0 ± 3.0 | 11 ± 1 | 159.8 ± 5.0 | 125.6 ± 5.0 |
| MDEA | −800 | 800 | 1600 | 439.0 ± 5.0 | 40.3 ± 3.0 | 9 ± 1 | 421.1 ± 5.0 | 335.0 ± 5.0 |
| AMP | −390 | 300 | 690 | 442.3 ± 5.0 | 86.8 ± 3.0 | 20 ± 1 | 143.8 ± 5.0 | 112.1 ± 5.0 |
| PZ | −500 | 500 | 1000 | 452.2 ± 5.0 | 43.4 ± 3.0 | 10 ± 1 | 186.2 ± 5.0 | 143.0 ± 5.0 |
| Na2SO3 | −180 | 100 | 280 | 443.6 ± 5.0 | 61.5 ± 3.0 | 14 ± 1 | 64.9 ± 3.5 | 51.4 ± 3.5 |
| PES-020 (PES) | ||||||||
| Initial | −380 | 150 | 530 | 399.0 ± 5.0 | 14.3 ± 2.0 | 4 ± 1 | 62.8 ± 3.5 | 39.6 ± 3.5 |
| MEA | −180 | 60 | 240 | 399.0 ± 5.0 | 31.8 ± 3.0 | 8 ± 1 | 45.1 ± 3.5 | 26.3 ± 3.5 |
| DEA | −400 | 180 | 580 | 399.0 ± 5.0 | 31.5 ± 3.0 | 8 ± 1 | 101.6 ± 5.0 | 61.4 ± 5.0 |
| MDEA | −300 | 150 | 450 | 400.0 ± 5.0 | 22.8 ± 3.0 | 6 ± 1 | 76.1 ± 5.0 | 46,0 ± 3.5 |
| AMP | −170 | 50 | 220 | 399.0 ± 5.0 | 31.1 ± 3.0 | 8 ± 1 | 45.9 ± 3.5 | 27.9 ± 3.5 |
| PZ * | − | - | - | - | - | - | - | - |
| Na2SO3 | −600 | 200 | 800 | 400.0 ± 5.0 | 16.8 ± 2.0 | 4 ± 1 | 123.3 ± 5.0 | 73.9 ± 5.0 |
| MCM-010 (Nylon PA) | ||||||||
| Initial | −400 | 400 | 800 | 399.0 ± 5.0 | 31.6 ± 3.0 | 8 ± 1 | 158.3 ± 5.0 | 128.9 ± 5.0 |
| MEA * | − | - | - | - | - | - | - | - |
| DEA | −500 | 300 | 800 | 399.0 ± 5.0 | 30.9 ± 3.0 | 8 ± 1 | 152.4 ± 5.0 | 121.5 ± 5.0 |
| MDEA | −600 | 700 | 1300 | 399.0 ± 5.0 | 47.6 ± 3.0 | 12 ± 1 | 214.8 ± 5.0 | 171.8 ± 5.0 |
| AMP | −600 | 400 | 1000 | 399.0 ± 5.0 | 49.8 ± 3.0 | 12 ± 1 | 196.0 ± 5.0 | 159.7 ± 5.0 |
| PZ | −390 | 200 | 590 | 400.0 ± 5.0 | 62.2 ± 3.0 | 16 ± 1 | 151.7 ± 5.0 | 122.7 ± 5.0 |
| Na2SO3 | −400 | 300 | 700 | 400.0 ± 5.0 | 67.8 ± 3.0 | 17 ± 1 | 165.5 ± 5.0 | 134.8 ± 5.0 |
| Membrane | Solvent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEA | DEA | MDEA | PZ | AMP | Na2SO3 | |||||||||||||||||||
| I | II | III | Σ | I | II | III | Σ | I | II | III | Σ | I | II | III | Σ | I | II | III | Σ | I | II | III | Σ | |
| PVDF-022 (PVDF) | −/+ | + | −/+ | +/− | +/− | + | +/− | −/+ | +/− | +/− | +/− | +/− | − | − | − | − | −/+ | − | −/+ | −/+ | +/− | +/− | − | −/+ |
| MFF4-020 (PTFE) | +/− | +/− | +/− | +/− | +/− | −/+ | + | +/− | + | +/− | +/− | +/− | + | +/− | +/− | +/− | + | +/− | +/− | +/− | + | −/+ | +/− | +/− |
| PolySep (PP) | +/− | +/− | + | +/− | +/− | +/− | +/− | +/− | +/− | +/− | – | −/+ | +/− | −/+ | −/+ | −/+ | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− |
| PES-020 (PES) | +/− | − | +/− | −/+ | +/− | − | −/+ | −/+ | +/− | +/− | +/− | +/− | − | − | − | − | +/− | − | +/− | −/+ | +/− | −/+ | −/+ | −/+ |
| MCM-010 (Nylon PA) | − | − | − | − | −/+ | + | + | +/− | −/+ | +/− | +/− | −/+ | +/− | − | + | −/+ | +/− | +/− | +/− | +/− | −/+ | − | + | −/+ |
| Membrane | Initial, ° | After Exposure to MEA, ° |
|---|---|---|
| PVDF-022 (PVDF) | 98.9 ± 2.0 | 86.9 ± 2.0 |
| MFF4-020 (PTFE) | 111.9 ± 2.0 | 110.9 ± 2.0 |
| PolySep (PP) | 100.3 ± 2.0 | 102.2 ± 2.0 |
| PES-020 (PES) | 76.1 ± 2.0 | 68.1 ± 2.0 |
| MCM-010 (Nylon PA) | 45.2 ± 2.0 | 33.8 ± 2.0 |
| Solution | dmin, μm | dMFP, μm | dmax, μm |
|---|---|---|---|
| Initial | 0.27 ± 0.01 | 0.43 ± 0.01 | 0.56 ± 0.01 |
| MEA | 0.22 ± 0.01 | 0.43 ± 0.01 | 0.95 ± 0.01 |
| DEA | 0.32 ± 0.01 | 0.43 ± 0.01 | 0.53 ± 0.01 |
| MDEA | 0.32 ± 0.01 | 0.43 ± 0.01 | 0.53 ± 0.01 |
| AMP | 0.36 ± 0.01 | 0.45 ± 0.01 | 0.53 ± 0.01 |
| PZ | 0.34 ± 0.01 | 0.44 ± 0.01 | 0.54 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalmykov, D.; Shirokikh, S.; Grushevenko, E.A.; Legkov, S.A.; Bondarenko, G.N.; Anokhina, T.S.; Molchanov, S.; Bazhenov, S.D. Stability of Porous Polymeric Membranes in Amine Solvents for Membrane Contactor Applications. Membranes 2023, 13, 544. https://doi.org/10.3390/membranes13060544
Kalmykov D, Shirokikh S, Grushevenko EA, Legkov SA, Bondarenko GN, Anokhina TS, Molchanov S, Bazhenov SD. Stability of Porous Polymeric Membranes in Amine Solvents for Membrane Contactor Applications. Membranes. 2023; 13(6):544. https://doi.org/10.3390/membranes13060544
Chicago/Turabian StyleKalmykov, Denis, Sergey Shirokikh, Evgenia A. Grushevenko, Sergey A. Legkov, Galina N. Bondarenko, Tatyana S. Anokhina, Sergey Molchanov, and Stepan D. Bazhenov. 2023. "Stability of Porous Polymeric Membranes in Amine Solvents for Membrane Contactor Applications" Membranes 13, no. 6: 544. https://doi.org/10.3390/membranes13060544
APA StyleKalmykov, D., Shirokikh, S., Grushevenko, E. A., Legkov, S. A., Bondarenko, G. N., Anokhina, T. S., Molchanov, S., & Bazhenov, S. D. (2023). Stability of Porous Polymeric Membranes in Amine Solvents for Membrane Contactor Applications. Membranes, 13(6), 544. https://doi.org/10.3390/membranes13060544











