Conjugation of Triterpenic Acids of Ursane and Oleanane Types with Mitochondria-Targeting Cation F16 Synergistically Enhanced Their Cytotoxicity against Tumor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Synthesis of F16-Triterpenoid Conjugates
2.2.1. General Procedure for the Preparation of Alkyl Bromides 5 and 6
2.2.2. General Procedure for the Preparation of the Conjugates 1 and 2
2.3. Cell Culture
2.4. Cytotoxic Assay of Conjugates
2.5. Oxidative Stress Assay
2.6. Isolation of Mitochondria from Rat Liver
2.7. Measurements of Mitochondrial Bioenergetics
2.8. Statistical Analysis
3. Results
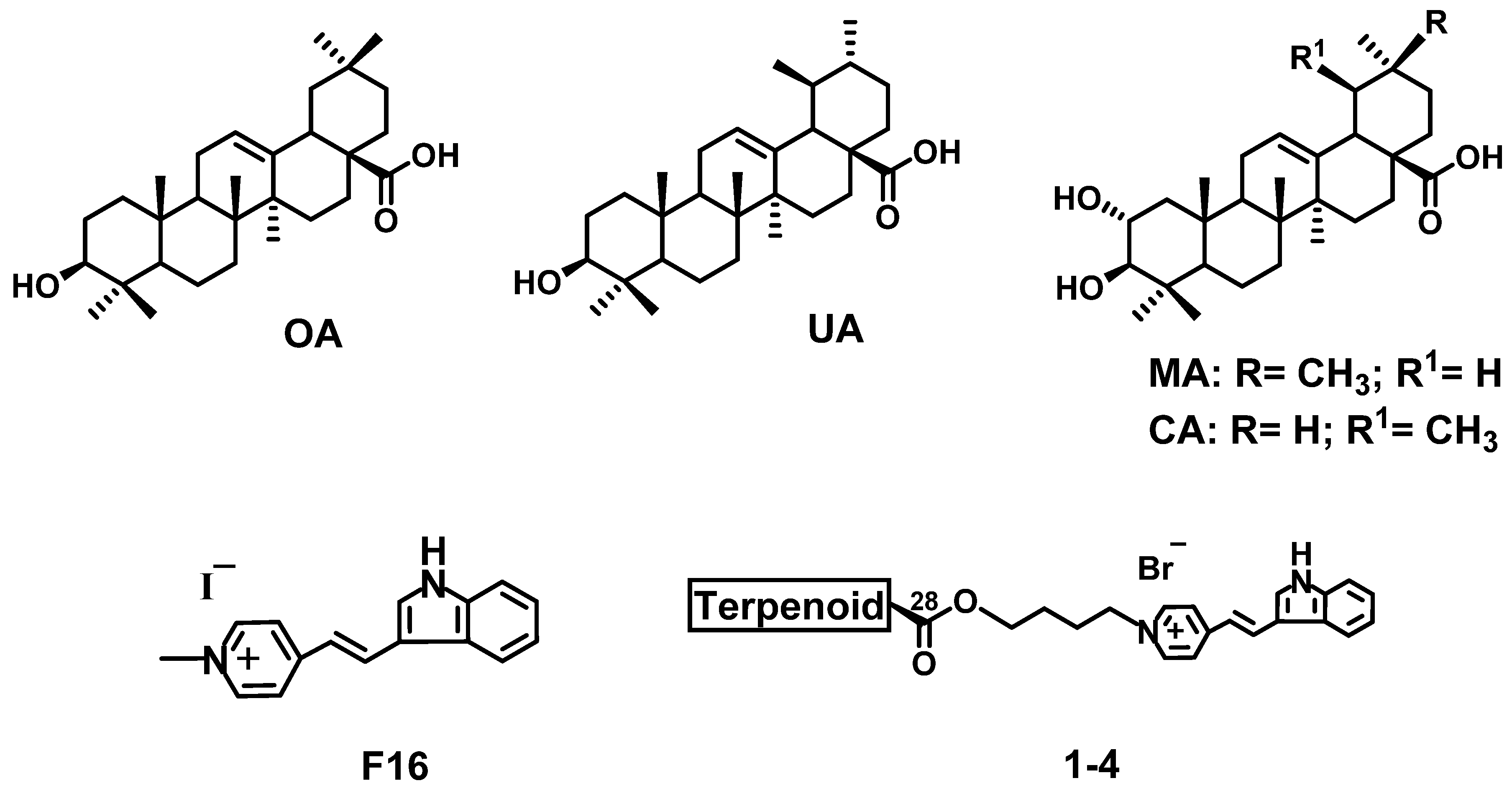
3.1. Chemistry
3.2. Survival of Different Tumor Cells Treated with Conjugates 1 and 2
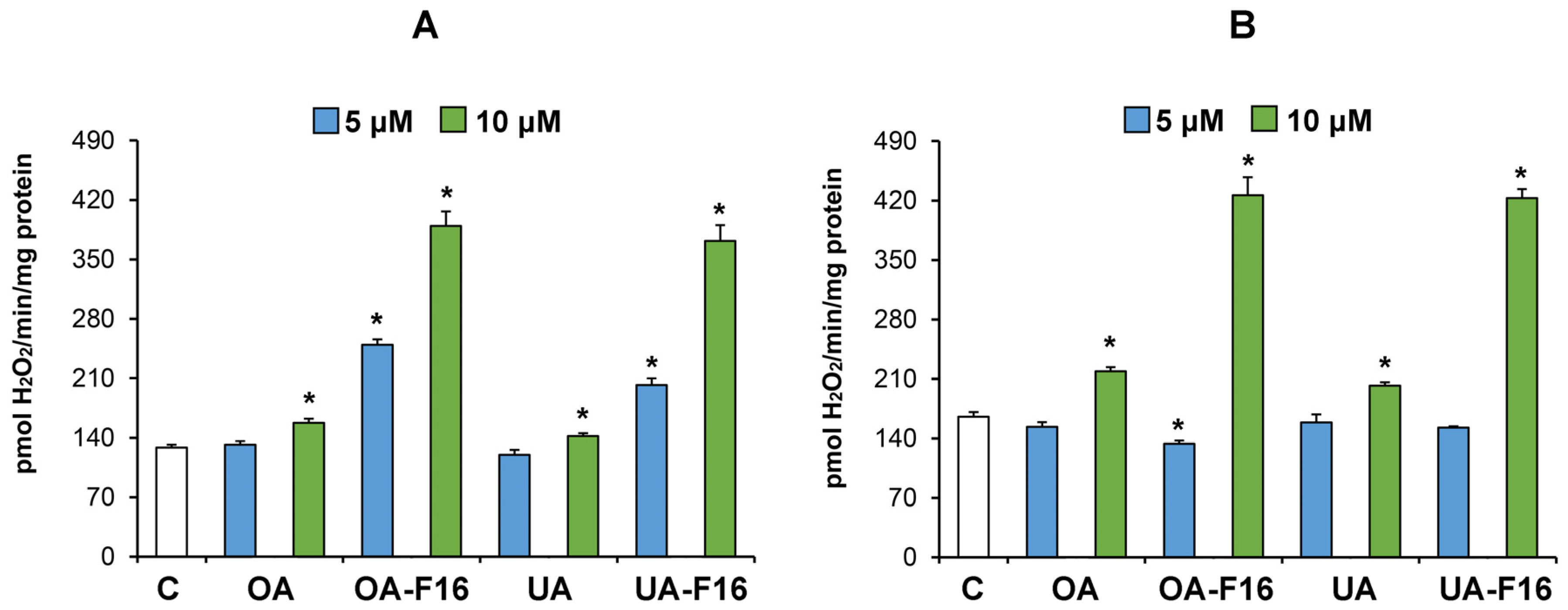
3.3. Effect of Conjugates on the Functioning of Isolated Rat Liver Mitochondria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2020, 37, 962–998. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kinghorn, A.D. Natural Product Triterpenoids and Their Semi-synthetic Derivatives with Potential Anticancer Activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-H.; Chen, W.; Zhao, Y.; Ju, X.-L. Anti-Tumor Activity of Oleanolic, Ursolic and Glycyrrhetinic Acid. Open Nat. Prod. J. 2009, 2, 48–52. [Google Scholar] [CrossRef]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef]
- Lucio, K.A.; Rocha, G.G.; Moncao-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef]
- Yu, L.; Xie, X.; Cao, X.; Chen, J.; Chen, G.; Chen, Y.; Li, G.; Qin, J.; Peng, F.; Peng, C. The Anticancer Potential of Maslinic Acid and Its Derivatives: A Review. Drug Des. Devel. Ther. 2021, 15, 3863–3879. [Google Scholar] [CrossRef]
- Parra, A.; Rivas, F.; Martin-Fonseca, S.; Garcia-Granados, A.; Martinez, A. Maslinic acid derivatives induce significant apoptosis in b16f10 murine melanoma cells. Eur. J. Med. Chem. 2011, 46, 5991–6001. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, R.; Du, J.; Xin, H.; Yi, T.; Sheng, J.; Wang, Y.; Ling, C. Corosolic acid induces apoptosis through mitochondrial pathway and caspases activation in human cervix adenocarcinoma HeLa cells. Cancer Lett. 2009, 284, 229–237. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, H.; Wang, Y.; Zhao, A.; Zhu, Z.; Bao, X.; Sun, Y.; Li, L.; Zhang, Q. Corosolic acid, a natural triterpenoid, induces ER stress-dependent apoptosis in human castration resistant prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and TRIB3. J. Exp. Clin. Cancer Res. 2018, 37, 210. [Google Scholar] [CrossRef]
- Xu, M.F.; Xiong, Y.Y.; Liu, J.K.; Qian, J.J.; Zhu, L.; Gao, J. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol. Sin. 2012, 33, 578–587. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Khalitova, R.R.; Gubaidullin, R.R.; Odinokov, V.N.; Bel’skii, Y.P.; Bel’skaya, N.V.; Khazanov, V.A. Triphenylphosphonium cations of betulinic acid derivatives: Synthesis and antitumor activity. Med. Chem. Res. 2017, 26, 518–531. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Abdullin, T.I.; Grigor’eva, L.R.; Kuznetsova, E.V.; Akhmadishina, R.A.; Ziganshina, L.E.; Cong, H.H.; Mironov, V.F. Design, Synthesis, and Cancer Cell Growth Inhibitory Activity of Triphenylphosphonium Derivatives of the Triterpenoid Betulin. J. Nat. Prod. 2017, 80, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Nedopekina, D.A.; Gubaidullin, R.R.; Odinokov, V.N.; Maximchik, P.V.; Zhivotovsky, B.; Bel’skii, Y.P.; Khazanov, V.A.; Manuylova, A.V.; Gogvadze, V.; Spivak, A.Y. Mitochondria-targeted betulinic and ursolic acid derivatives: Synthesis and anticancer activity. Med. Chem. Med. Chem. Commun. 2017, 8, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, T.; Yuan, H.; Li, D.; Lou, H.; Fan, P. Mitochondria-Targeted Lupane Triterpenoid Derivatives and Their Selective Apoptosis-Inducing Anticancer Mechanisms. J. Med. Chem. 2017, 60, 6353–6363. [Google Scholar] [CrossRef] [PubMed]
- Jina, L.; Dai, L.; Ji, M.; Wang, H. Mitochondria-targeted triphenylphosphonium conjugated glycyrrhetinic acid derivatives as potent anticancer drugs. Bioorg. Chem. 2019, 85, 179–190. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kerzig, C.; Kramell, A.E.; Csuk, R. Rhodamine B conjugates of triterpenoic acids are cytotoxic mitocans even at nanomolar concentrations. Eur. J. Med. Chem. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Wolfram, R.K.; Fischer, L.; Kluge, R.; Strohl, D.; Al-Harrasi, A.; Csuk, R. Homopiperazine-rhodamine B adducts of triterpenoic acids are strong mitocans. Eur. J. Med. Chem. 2018, 155, 869–879. [Google Scholar] [CrossRef]
- Hoenke, S.; Serbian, I.; Deigner, H.P.; Csuk, R. Mitocanic di- and triterpenoid rhodamine B conjugates. Molecules 2020, 25, 5443. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Gubaidullin, R.R.; Davletshin, E.V.; Tukhbatullin, A.A.; D’yakonov, V.A.; Yunusbaeva, M.M.; Dzhemileva, L.U.; Dzhemilev, U.M. Pentacyclic triterpene acid conjugated with mitochondria-targeting cation F16: Synthesis and evaluation of cytotoxic activities. Med. Chem. Res. 2021, 30, 940–951. [Google Scholar] [CrossRef]
- Fantin, V.R.; Berardi, M.J.; Scorrano, L.; Korsmeyer, S.J.; Leder, P. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell 2002, 2, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Belosludtsev, K.N.; Ilzorkina, A.I.; Belosludtseva, N.V.; Sharapov, V.A.; Penkov, N.V.; Serov, D.A.; Karagyaur, M.N.; Nedopekina, D.A.; Davletshin, E.V.; Solovieva, M.E.; et al. Comparative Study of Cytotoxic and Membranotropic Properties of Betulinic Acid-F16 Conjugate on Breast Adenocarcinoma Cells (MCF-7) and Primary Human Fibroblasts. Biomedicines 2022, 10, 2903. [Google Scholar] [CrossRef] [PubMed]
- Siewert, B.; Pianowski, E.; Obernauer, A.; Csuk, R. Towards cytotoxic and selective derivatives of maslinic acid. Bioorg. Med. Chem. 2014, 22, 594–615. [Google Scholar] [CrossRef]
- Huang, L.R.; Hao, X.J.; Li, Q.J.; Wang, D.P.; Zhang, J.X.; Luo, H.; Yang, X.S. 18β-Glycyrrhetinic Acid Derivatives Possessing a Trihydroxylated A Ring Are Potent Gram-Positive Antibacterial Agents. J. Nat. Prod. 2016, 79, 721–731. [Google Scholar] [CrossRef]
- Kraft, O.; Hartmann, A.K.; Brandt, S.; Hoenke, S.; Heise, N.V.; Csuk, R.; Mueller, T. Asiatic acid as a leading structure for derivatives combining sub-nanomolar cytotoxicity, high selectivity, and the ability to overcome drug resistance in human preclinical tumor models. Eur. J. Med. Chem. 2023, 15, 115189. [Google Scholar] [CrossRef]
- Nelson, A.T.; Camelio, A.M.; Claussen, K.R.; Cho, J.; Tremmel, L.; DiGiovanni, J.; Siegel, D. Synthesis of oxygenated oleanolic and ursolic acid derivatives with anti-inflammatory properties. Bioorg. Med. Chem. Lett. 2015, 25, 4342–4346. [Google Scholar] [CrossRef]
- Liu, X.; Zang, X.; Yin, X.; Yang, W.; Huang, J.; Huang, J.; Yu, C.; Ke, C.; Hong, Y. Semi-synthesis of C28-modified triterpene acid derivatives from maslinic acid or corosolic acid as potential α-glucosidase inhibitors. Bioorg. Chem. 2020, 97, 103694. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Samartsev, V.N.; Stepanova, A.E.; Khoroshavina, E.I.; Penkov, N.V.; Yashin, V.A.; Starinets, V.S.; Mikheeva, I.B.; Gudkov, S.V.; Belosludtsev, K.N. Membranotropic effects of ω-hydroxypalmitic acid and Ca2+ on rat liver mitochondria and lecithin liposomes. Aggregation and membrane permeabilization. J. Bioenerg. Biomembr. 2018, 50, 391–401. [Google Scholar] [CrossRef]
- Belosludtseva, N.V.; Starinets, V.S.; Semenova, A.A.; Igoshkina, A.D.; Dubinin, M.V.; Belosludtsev, K.N. S-15176 Difumarate Salt Can Impair Mitochondrial Function through Inhibition of the Respiratory Complex III and Permeabilization of the Inner Mitochondrial Membrane. Biology 2022, 11, 380. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Semenova, A.A.; Nedopekina, D.A.; Davletshin, E.V.; Spivak, A.Y.; Belosludtsev, K.N. Mitochondrial dysfunction induced by F16-betulin conjugate and its role in cell death initiation. Membranes 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Penkov, N.V.; Nedopekina, D.A.; Sharapov, V.A.; Khoroshavina, E.I.; Davletshin, E.V.; Belosludtseva, N.V.; Spivak, A.Y.; et al. Mitochondria-targeted prooxidant effects of betulinic acid conjugated with delocalized lipophilic cation F16. Free Radic. Biol. Med. 2021, 168, 55–69. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Mikheeva, I.B.; Yashin, V.A.; Penkov, N.V.; Vydrina, V.A.; Ishmuratov, G.Y.; Sharapov, V.A.; Khoroshavina, E.I.; et al. Effect of betulin and betulonic acid on isolated rat liver mitochondria and liposomes. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183383. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.C.; Motzer, R.J.; Tong, Y.; Bosl, G.J. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: A rational approach to clinical protocol design. J. Natl. Cancer Inst. 1994, 86, 1517–1524. [Google Scholar] [CrossRef]






| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| H1299 | A549 | MCF7 | BT474 | HPDF | |
| OA-F16 | 0.93 ± 0.07 | 0.74 ± 0.09 | 1.52 ± 0.16 | 4.34 ± 0.28 | 2.05 ± 0.58 |
| MA-F16 | 10.50 ± 2.44 | 13.52 ± 2.02 | 7.44 ± 0.78 | 11.05 ± 2.51 | 9.30 ± 2.56 |
| UA-F16 | 2.80 ± 0.25 | 2.40 ± 0.30 | 1.36 ± 0.74 | 5.18 ± 0.15 | 4.98 ± 0.9 |
| CA-F16 | 4.01 ± 1.69 | 1.87 ± 0.14 | 1.89 ± 0.27 | 6.70 ± 0.35 | 1.15 ± 0.38 |
| OA | 139.33 ± 6.85 | 233.55 ± 22.05 | 113.5 ± 12.58 | 149.9 ± 25.12 | 156.3 ± 10.43 |
| UA | 97.60 ± 5.63 | 68.93 ± 19.63 | 40.6 ± 12.50 | 109.6 ± 19.08 | 59.07 ± 7.73 |
| Additions, μM | State 2 | State 3 | State 4 | State 3UDNP | RC | ADP/O |
|---|---|---|---|---|---|---|
| nmol O2/min/mg Protein | rel. un. | |||||
| - | 7.03 ± 0.11 | 26.01 ± 0.46 | 10.35 ± 0.71 | 34.64 ± 0.25 | 2.55 ± 0.17 | 2.85 ± 0.08 |
| Oleanolic acid | ||||||
| 20 | 8.39 ± 0.09 * | 27.31 ± 0.31 | 13.23 ± 0.18 * | 35.74 ± 0.66 | 2.11 ± 0.12 | 2.53 ± 0.12 |
| OA-F16 | ||||||
| 10 | 6.47 ± 0.27 | 23.82 ± 0.53 * | 10.65 ± 0.24 | 31.34 ± 0.49 * | 2.24 ± 0.11 | 2.80 ± 0.13 |
| 20 | 15.38 ± 0.30 *# | 17.02 ± 0.97 *# | 16.55 ± 0.54 *# | 23.24 ± 0.61 *#γ | 1.03 ± 0.04*# | 0.64 ± 0.05 *# |
| Ursolic acid | ||||||
| 20 | 8.01 ± 0.15 * | 26.92 ± 0.64 | 12.65 ± 0.32 * | 36.66 ± 1.47 | 2.13 ± 0.09 | 2.82 ± 0.03 |
| UA-F16 | ||||||
| 10 | 7.70 ± 0.33 | 25.89 ± 0.86 | 11.78 ± 0.22 | 35.23 ± 0.89 | 2.20 ± 0.04 | 2.65 ± 0.05 |
| 20 | 14.75 ± 0.21 *# | 19.35 ± 0.90 *# | 19.06 ± 1.11 *# | 28.29 ± 1.18 *# | 1.02 ± 0.02 *# | 0.74 ± 0.14 *# |
| Additions, μM | State 2 | State 3 | State 4 | State 3UDNP | RC | ADP/O |
|---|---|---|---|---|---|---|
| nmol O2/min/mg Protein | rel. un. | |||||
| - | 12.62 ± 0.53 | 47.96 ± 0.59 | 14.00 ± 0.57 | 73.28 ± 2.44 | 3.44 ± 0.13 | 1.51 ± 0.06 |
| Oleanolic acid | ||||||
| 20 | 15.06 ± 0.15 * | 48.00 ± 0.60 | 17.18 ± 0.78 * | 69.92 ± 0.68 | 2.80 ± 0.09 * | 1.45 ± 0.05 |
| OA-F16 | ||||||
| 10 | 14.39 ± 0.65 | 48.46 ± 0.82 | 17.22 ± 0.93 * | 69.57 ± 1.73 | 2.83 ± 0.20 * | 1.41 ± 0.09 |
| 20 | 14.05 ± 0.18 * | 41.57 ± 1.36 *# | 33.23 ± 0.75 *# | 56.46 ± 4.21 *# | 1.25 ± 0.10 *# | 1.15 ± 0.05 *# |
| Ursolic acid | ||||||
| 20 | 15.19 ± 0.27 * | 49.15 ± 0.30 | 15.54 ± 0.62 | 75.91 ± 1.74 | 3.32 ± 0.12 | 1.43 ± 0.04 |
| UA-F16 | ||||||
| 10 | 14.42 ± 0.24 * | 46.39 ± 0.30 | 17.51 ± 0.23 * | 67.34 ± 2.12 | 2.65 ± 0.07 * | 1.28 ± 0.04 * |
| 20 | 15.86 ± 0.40 * | 43.98 ± 1.31 *# | 32.20 ± 1.68 *# | 64.18 ± 3.81 # | 1.37 ± 0.11 *# | 0.86 ± 0.05 *#γ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubinin, M.V.; Nedopekina, D.A.; Ilzorkina, A.I.; Semenova, A.A.; Sharapov, V.A.; Davletshin, E.V.; Mikina, N.V.; Belsky, Y.P.; Spivak, A.Y.; Akatov, V.S.; et al. Conjugation of Triterpenic Acids of Ursane and Oleanane Types with Mitochondria-Targeting Cation F16 Synergistically Enhanced Their Cytotoxicity against Tumor Cells. Membranes 2023, 13, 563. https://doi.org/10.3390/membranes13060563
Dubinin MV, Nedopekina DA, Ilzorkina AI, Semenova AA, Sharapov VA, Davletshin EV, Mikina NV, Belsky YP, Spivak AY, Akatov VS, et al. Conjugation of Triterpenic Acids of Ursane and Oleanane Types with Mitochondria-Targeting Cation F16 Synergistically Enhanced Their Cytotoxicity against Tumor Cells. Membranes. 2023; 13(6):563. https://doi.org/10.3390/membranes13060563
Chicago/Turabian StyleDubinin, Mikhail V., Darya A. Nedopekina, Anna I. Ilzorkina, Alena A. Semenova, Vyacheslav A. Sharapov, Eldar V. Davletshin, Natalia V. Mikina, Yuri P. Belsky, Anna Yu. Spivak, Vladimir S. Akatov, and et al. 2023. "Conjugation of Triterpenic Acids of Ursane and Oleanane Types with Mitochondria-Targeting Cation F16 Synergistically Enhanced Their Cytotoxicity against Tumor Cells" Membranes 13, no. 6: 563. https://doi.org/10.3390/membranes13060563
APA StyleDubinin, M. V., Nedopekina, D. A., Ilzorkina, A. I., Semenova, A. A., Sharapov, V. A., Davletshin, E. V., Mikina, N. V., Belsky, Y. P., Spivak, A. Y., Akatov, V. S., Belosludtseva, N. V., Liu, J., & Belosludtsev, K. N. (2023). Conjugation of Triterpenic Acids of Ursane and Oleanane Types with Mitochondria-Targeting Cation F16 Synergistically Enhanced Their Cytotoxicity against Tumor Cells. Membranes, 13(6), 563. https://doi.org/10.3390/membranes13060563











