Solid Oxide Fuel Cells with Magnetron Sputtered Single-Layer SDC and Multilayer SDC/YSZ/SDC Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. SDC and YSZ Layer Deposition
2.2. Preparation of Single Fuel Cells
2.3. Investigation of Single Fuel Cells
3. Results and Discussion
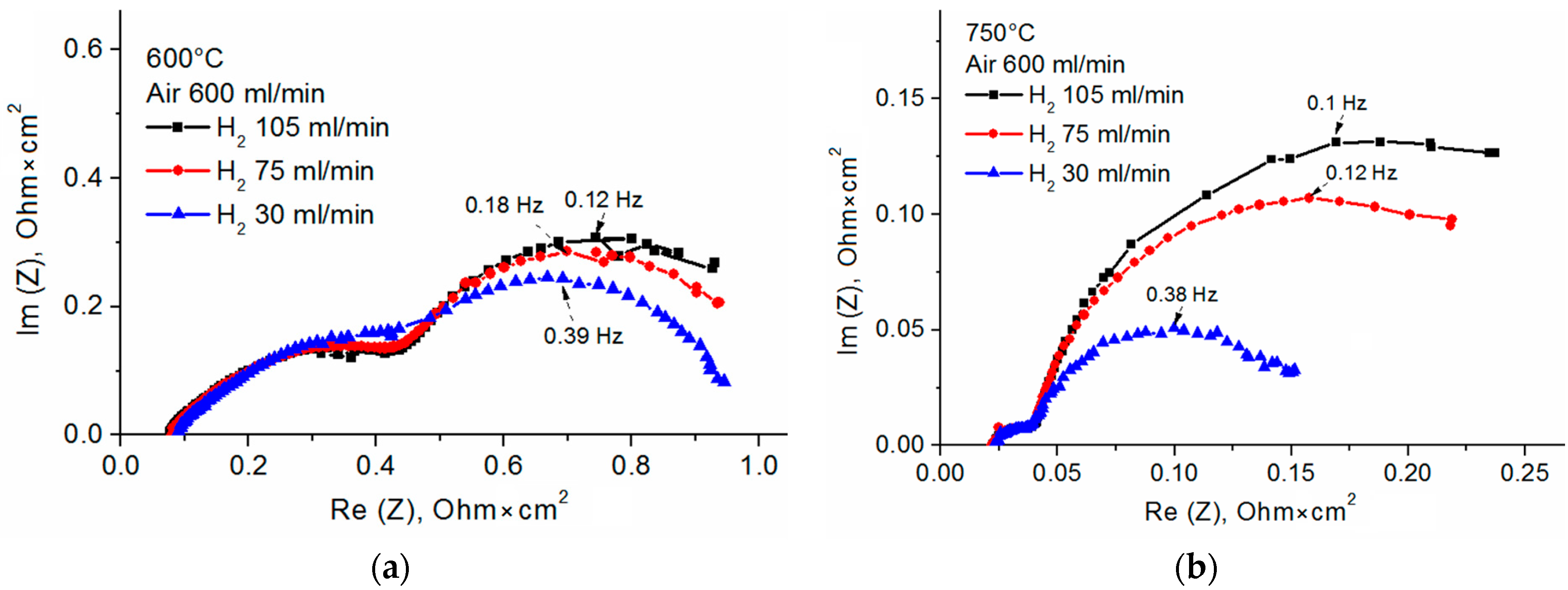
3.1. Fuel Cell with the Single-Layer SDC Electrolyte
3.2. Fuel Cell with Multilayer SDC/YSZ/SDC Electrolyte
3.3. Single Fuel Cell Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tu, H.Y.; Takeda, Y.; Imanishi, N.; Yamamoto, O. Ln0.4Sr0.6Co0.8Fe0.2O3−δ (Ln = La, Pr, Nd, Sm, Gd) for the electrode in solid oxide fuel cells. Solid. State Ion. 1999, 117, 277–281. [Google Scholar] [CrossRef]
- Wright, J.; Virkar, A.V. Conductivity of porous Sm2O3-doped CeO2 as a function of temperature and oxygen partial pressure. J. Power Sources 2011, 196, 6118–6124. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Januschewsky, J.; Ahrens, M.; Opitz, A.; Kubel, F.; Fleig, J. Optimized La0.6Sr0.4CoO3−δ thin-film electrodes with extremely fast oxygen-reduction kinetics. Adv. Funct. Mater. 2009, 19, 3151–3156. [Google Scholar] [CrossRef]
- Leng, Y.; Chan, S.H.; Liu, Q. Development of LSCF–GDC composite cathodes for low-temperature solid oxide fuel cells with thin film GDC electrolyte. Int. J. Hydrog. Energy 2008, 33, 3808–3817. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, L.; Cai, H.; Xu, J.; Wang, L.; Long, W. Improved thermal expansion and electrochemical performance of La0.4Sr0.6Co0.9Sb0.1O3−δ-Ce0.8Sm0.2O1.9 composite cathode for IT-SOFCs. Solid. State Sci. 2019, 91, 126–132. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Ermakova, L.; Kolchugin, A.; Khrustov, A.; Farlenkov, A. Methods to increase electrochemical activity of lanthanum nickelate-ferrite electrodes for intermediate and low temperature SOFCs. Int. J. Hydrog. Energy 2021, 46, 35923–35937. [Google Scholar] [CrossRef]
- Hong, S.; Lee, D.; Lim, Y.; Bae, J.; Kim, Y.B. Yttria-stabilized zirconia thin films with restrained columnar grains for oxygen ion conducting electrolytes. Ceram. Int. 2016, 42, 16703–16709. [Google Scholar] [CrossRef]
- Wang, C.; Worrell, W.L.; Park, S.; Vohs, J.M.; Gorte, R.J. Fabrication and performance of thin-film YSZ solid oxide fuel cells. J. Electrochem. Soc. 2001, 148, A864–A868. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Lebedynskiy, A.M.; Shipilova, A.V.; Ionov, I.V.; Smolyanskiy, E.A.; Lauk, A.L.; Maslov, A.S. Scale-up of solid oxide fuel cells with magnetron sputtered electrolyte. Fuel Cells 2017, 17, 378–382. [Google Scholar] [CrossRef]
- Andersson, D.A.; Simak, S.I.; Skorodumova, N.V.; Abrikosov, I.A.; Johansson, B. Optimisation of ionic conductivity in doped ceria. Proc. Natl. Acad. Sci. USA 2006, 103, 3518–3521. [Google Scholar] [CrossRef] [Green Version]
- Steele, B.C.H. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 °C. Solid. State Ion. 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Solid oxide fuel cells based on ceramic membranes with mixed conductivity: Improving efficiency. Russ. Chem. Rev. 2021, 90, 703–749. [Google Scholar] [CrossRef]
- Venkataramana, K.; Madhuri, C.; Reddy, C.V. Triple-doped ceria–carbonate (Ce0.82La0.06Sm0.06Gd0.06O2−δ-(Li–Na)2CO3) nanocomposite solid electrolyte materials for LT–SOFC applications. Ceram. Int. 2020, 46, 27584–27594. [Google Scholar] [CrossRef]
- Madhuri, C.; Venkataramana, K.; Shanker, J.; Reddy, C.V. Effect of La3+, Pr3+, and Sm3+ triple-doping on structural, electrical, and thermal properties of ceria solid electrolytes for intermediate temperature solid oxide fuel cells. J. Alloy. Compd. 2020, 849, 1566361. [Google Scholar] [CrossRef]
- Xiaomin, L.I.N.; Qiuyue, L.; Lili, Z.H.U.; Xiaomei, L.I.U. Synthesis and characterization of Ce0.8Sm0.2−xPrxO2−δ (x = 0.02–0.08) solid electrolyte materials. J. Rare Earths 2015, 33, 411–416. [Google Scholar]
- Wu, Y.-C.; Chien, C.-H.; Xu, G. Conductivity and microstructure analysis of ceria materials doped with multiple elements. Ceram. Int. 2017, 43, s747–s757. [Google Scholar] [CrossRef]
- Tanwar, K.; Jaiswal, N.; Kumar, D.; Parkash, O. Synthesis & characterization of Dy and Ca Co-doped ceria based solid electrolytes for IT-SOFCs. J. Alloy. Compd. 2018, 684, 683–690. [Google Scholar]
- Babu, A.S.; Bauri, R.; Reddy, G.S. Processing and conduction behavior of nano-crystalline Gd-doped and rare earth co-doped ceria electrolytes. Electrochim. Acta 2016, 209, 541–550. [Google Scholar] [CrossRef]
- Mishima, Y.; Mitsuyasu, H.; Ohtaki, M.; Eguchi, K. Solid oxide fuel cell with composite electrolyte consisting of samaria doped ceria and yttria-stabilized zirconia. J. Electrochem. Soc. 1998, 145, 1004–1007. [Google Scholar] [CrossRef]
- Raghvendra, P.; Singh, P. Electrical conductivity of YSZ-SDC composite solid electrolyte synthesized via glycine-nitrate method. Ceram. Int. 2017, 43, 11692–11698. [Google Scholar] [CrossRef]
- Mukai, T.; Tsukui, S.; Yoshida, K.; Yamaguchi, S.; Hatayama, R.; Adachi, M.; Ishibashi, H.; Kakehi, Y.; Satoh, K.; Kusaka, T.; et al. Fabrication of Y2O3-doped zirconia/gadolinia-doped ceria bilayer electrolyte thin film SOFC cells of SOFCs by single-pulsed laser deposition processing. J. Fuel. Cell. Sci. Technol. 2013, 10, 0610061–0610066. [Google Scholar] [CrossRef] [Green Version]
- Park, T.; Lee, Y.H.; Cho, G.Y.; Ji, S.; Park, J.; Chang, I.; Cha, S.W. Effect of the thickness of sputtered gadolinia-doped ceria as a cathodic interlayer in solid oxide fuel cells. Thin. Solid. Film. 2015, 584, 120–124. [Google Scholar] [CrossRef]
- Fan, Z.; An, J.; Iancu, A.; Prinz, F.B. Thickness effects of yttria-doped ceria interlayers on solid oxide fuel cells. J. Power Sources 2012, 218, 187–191. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Rabotkin, S.V.; Kuterbekov, K.A.; Koketay, T.A.; Nurkenov, S.A.; Opakhai, S.; Shipilova, A.V.; Ionov, I.V.; Eliseeva, G.M. Comparison of sputter-deposited single and multilayer electrolytes based on gadolinia-doped ceria and yttria-stabilized zirconia for solid oxide fuel cells. Int. J. Electrochem. Sci. 2020, 15, 231–240. [Google Scholar] [CrossRef]
- Eguchi, K.; Setoguchi, T.; Inoue, T.; Arai, H. Electrical properties of ceria-based oxides and their application to solid oxide fuel cells. Solid. State Ion. 1992, 52, 165–172. [Google Scholar] [CrossRef]
- Inaba, H.; Tagawa, H. Ceria-based solid electrolytes. Solid. State Ion. 1996, 83, 1–16. [Google Scholar] [CrossRef]
- Suzuki, T.; Hasan, Z.; Funahashi, Y.; Yamaguchi, T.; Fujishiro, Y.; Awano, M. Fabrication and characterization of microtubular SOFCs with multilayered electrolyte. Electrochem. Solid. State Lett. 2008, 11, B87. [Google Scholar] [CrossRef]
- Noh, H.S.; Hong, J.; Kim, H.; Yoon, K.J.; Lee, J.H.; Kim, B.K.; Son, J.W. Sandwiched ultra-thin yttria-stabilized zirconia layer to effectively and reliably block reduction of thin-film gadolinia-doped ceria electrolyte. J. Ceram. Soc. Jpn. 2015, 123, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Yang, J.R.; Zhao, Y.; Zhou, Y.; Yan, Z.; He, J.C.; Yuan, Q.; Wu, J.; Liu, P.; Zhong, Z.; et al. A review of thin film electrolytes fabricated by physical vapor deposition for solid oxide fuel cells. Int. J. Hydrog. Energy 2022, 47, 36926–36952. [Google Scholar] [CrossRef]
- Coppola, N.; Polverino, P.; Carapella, G.; Sacco, C.; Galdi, A.; Ubaldini, A.; Vaiano, V.; Montinaro, D.; Maritato, L.; Pianese, C. Structural and electrical characterization of sputter-deposited Gd0.1Ce0.9O2−δ thin buffer layers at the Y-stabilized zirconia electrolyte interface for IT-solid oxide cells. Catalysts 2018, 8, 571. [Google Scholar] [CrossRef] [Green Version]
- Jordan, N.; Assenmacher, W.; Uhlenbruck, S.; Haanappel, V.A.C.; Buchkremer, H.P.; Stöver, D.; Mader, W. Ce0.8Gd0.2O2−δ protecting layers manufactured by physical vapor deposition for IT-SOFC. Solid. State Ion. 2008, 179, 919–923. [Google Scholar] [CrossRef]
- Yoo, Y. Fabrication and characterization of thin film electrolytes deposited by RF magnetron sputtering for low temperature solid oxide fuel cells. J. Power Sources 2006, 160, 202–206. [Google Scholar] [CrossRef]
- Lin, H.; Ding, C.; Sato, K.; Tsutai, Y.; Hashida, T. Low-temperature anode-supported SOFC with ultra-thin ceria-based electrolytes prepared by modified sol-gel route. J. Solid. Mech. Mater. Eng. 2010, 4, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.; Wang, Y.; Deng, X.; Singh, D.; Legoux, J.-G. Metal supported tubular solid oxide fuel cells fabricated by suspension plasma spray and suspension high velocity oxy-fuel spray. J. Power Sources 2012, 215, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.F.; Shen, J.Y.; Gao, X.J.; Wang, J. Enhanced electrochemical properties of Sm0.2Ce0.8O1.9 film for SOFC electrolyte fabricated by pulsed laser deposition. Rare Met. 2021, 40, 1294–1299. [Google Scholar] [CrossRef]
- Huang, W.; Shuk, P.; Greenblatt, M. Properties of sol-gel prepared Ce1−xSmxO2−x/2 solid electrolytes. Solid. State Ion. 1997, 100, 23–27. [Google Scholar] [CrossRef]
- Matsuda, M.; Hosomia, T.; Murata, K.; Fukui, T.; Miyake, M. Fabrication of bilayered YSZ/SDC electrolyte film by electrophoretic deposition for reduced-temperature operating anode-supported SOFC. J. Power Sources 2007, 165, 102–107. [Google Scholar] [CrossRef]
- Ding, C.S.; Lin, H.F.; Sato, K.; Tsutai, Y.; Ohtaki, H. Preparation of doped ceria electrolyte films for SOFCs by spray coating method. J. Dispers. Sci. Technol. 2009, 30, 240–245. [Google Scholar] [CrossRef]
- Zhao, L.J.; Huang, X.Q.; Zhu, R.B.; Lu, Z.; Sun, W.W. Optimization on technical parameters for fabrication of SDC film by screen-printing used as electrolyte in IT-SOFC. J. Phys. Chem. Solids 2008, 69, 2019–2024. [Google Scholar] [CrossRef]
- Sochugov, N.S.; Soloviev, A.A.; Shipilova, A.V.; Rabotkin, S.V.; Rotshtein, V.P.; Sigfusson, I.T. The effect of pulsed electron beam pretreatment of magnetron sputtered ZrO2:Y2O3 films on the performance of IT-SOFC. Solid. State Ion. 2013, 231, 11–17. [Google Scholar] [CrossRef]
- Bouleau, L.; Coton, N.; Coquoz, P.; Ihringer, R.; Billard, A.; Briois, P. GDC buffer layer synthesized by reactive magnetron sputtering: Effect of total pressure and thickness on SOFC performances. Crystals 2020, 10, 759. [Google Scholar] [CrossRef]
- Zhang, X.; Robertson, M.; Deĉes-Petit, C.; Qu, W.; Kesler, O.; Maric, R.; Ghosh, D. Internal shorting and fuel loss of a low temperature solid oxide fuel cell with SDC electrolyte. J. Power Sources 2007, 164, 668–677. [Google Scholar] [CrossRef]
- Xia, C.; Rauch, W.L.; Chen, F.; Liu, M. Sm0.5Sr0.5CoO3 cathodes for low-temperature SOFCs. Solid. State Ion. 2002, 149, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- DiGiuseppe, G.; Sun, L. On the identification of impedance spectroscopy processes of an SOFC under different hydrogen concentrations. J. Fuel Cell. Sci. Technol. 2012, 9, 051004. [Google Scholar] [CrossRef]
- Nakajima, H.; Kitahara, T.; Konomi, T. Electrochemical impedance spectroscopy analysis of an anode-supported microtubular solid oxide fuel cell. J. Electrochem. Soc. 2010, 157, B1686–B1692. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, N.; Li, C.; Bao, C.; Croiset, E.; Qian, J.; Hu, Q.; Wang, S. Simulation of electrochemical impedance spectra of solid oxide fuel cells using transient physical models. J. Electrochem. Soc. 2008, 155, B270–B280. [Google Scholar] [CrossRef]
- Leonide, A.; Apel, Y.; Ivers-Tiffée, E. SOFC Modeling and parameter identification by means of impedance spectroscopy. ECS Trans. 2009, 19, 81–109. [Google Scholar] [CrossRef]


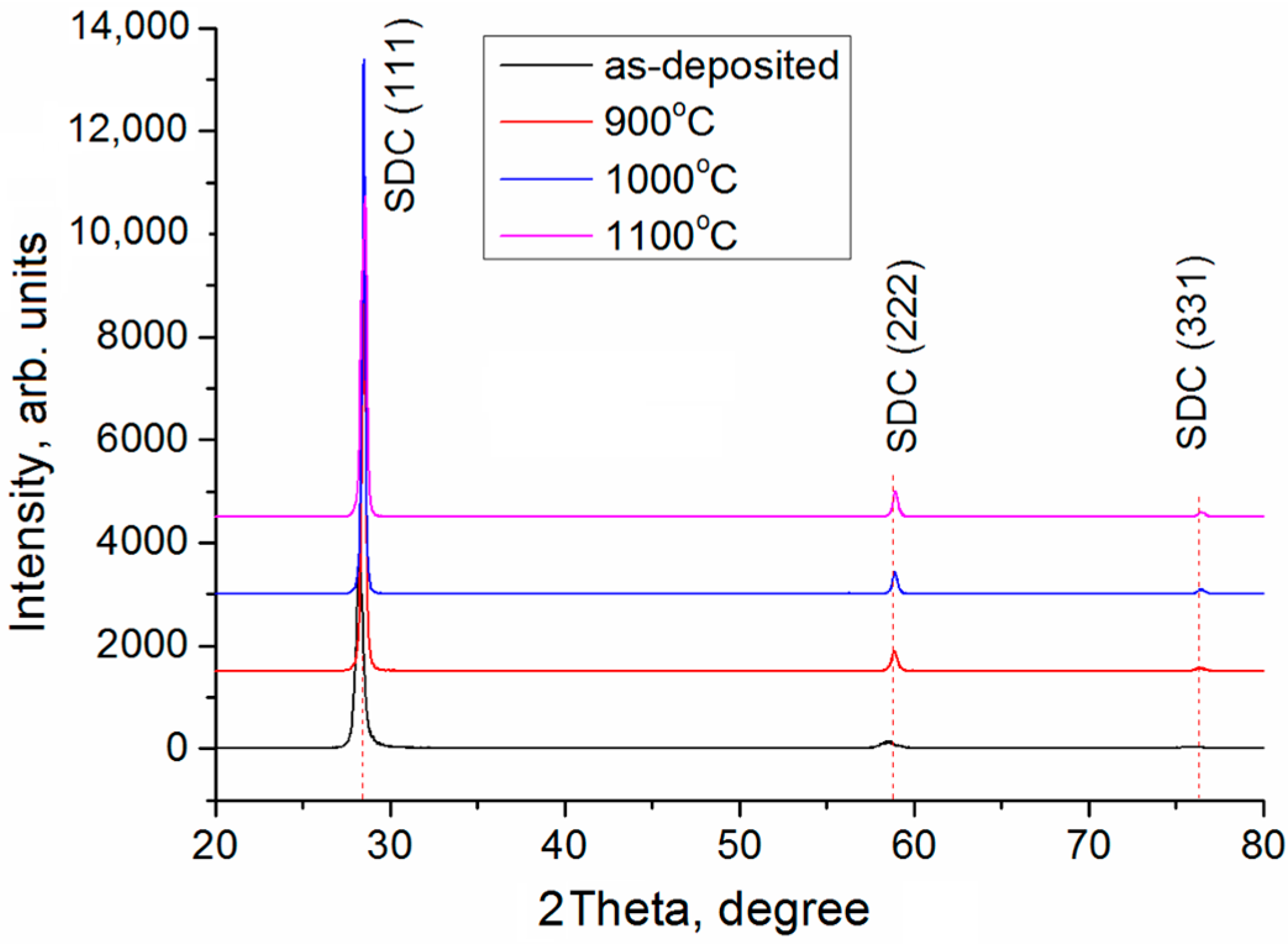
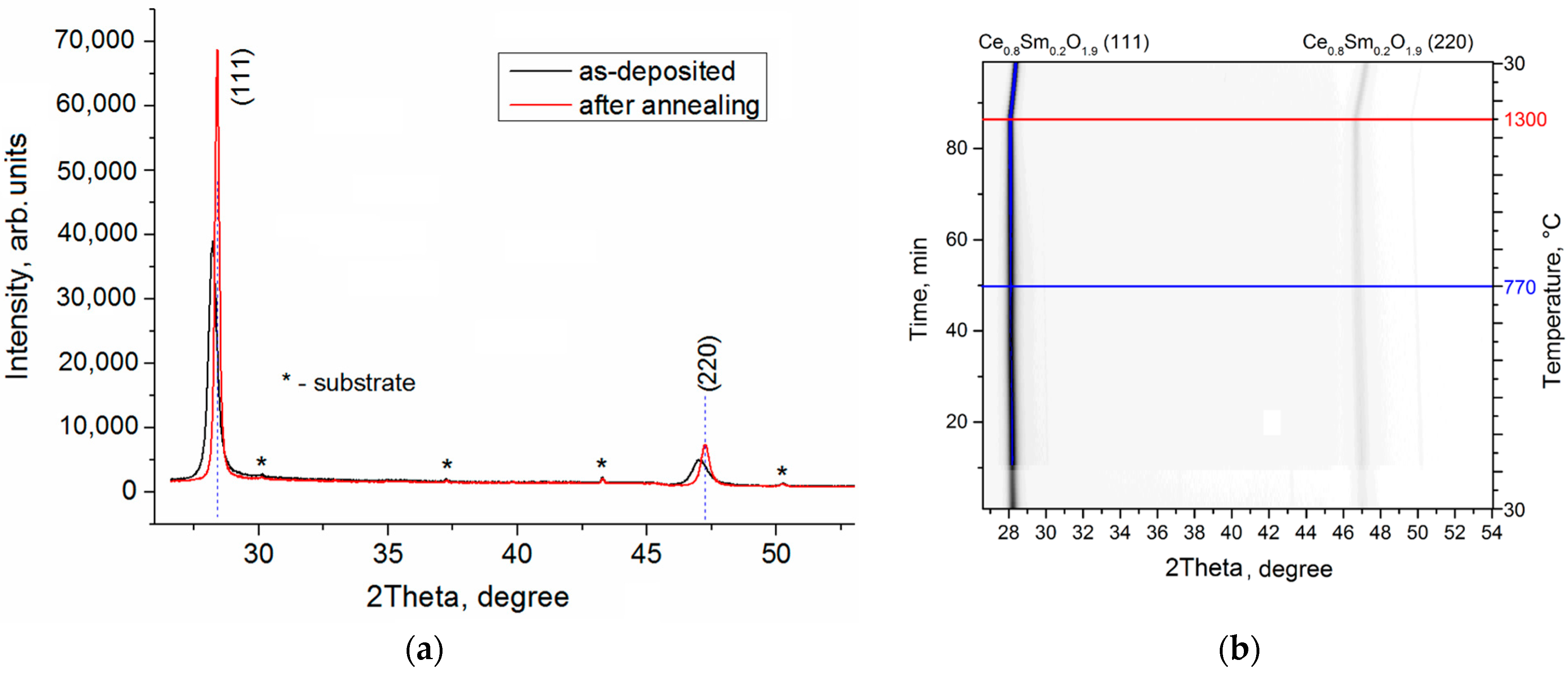
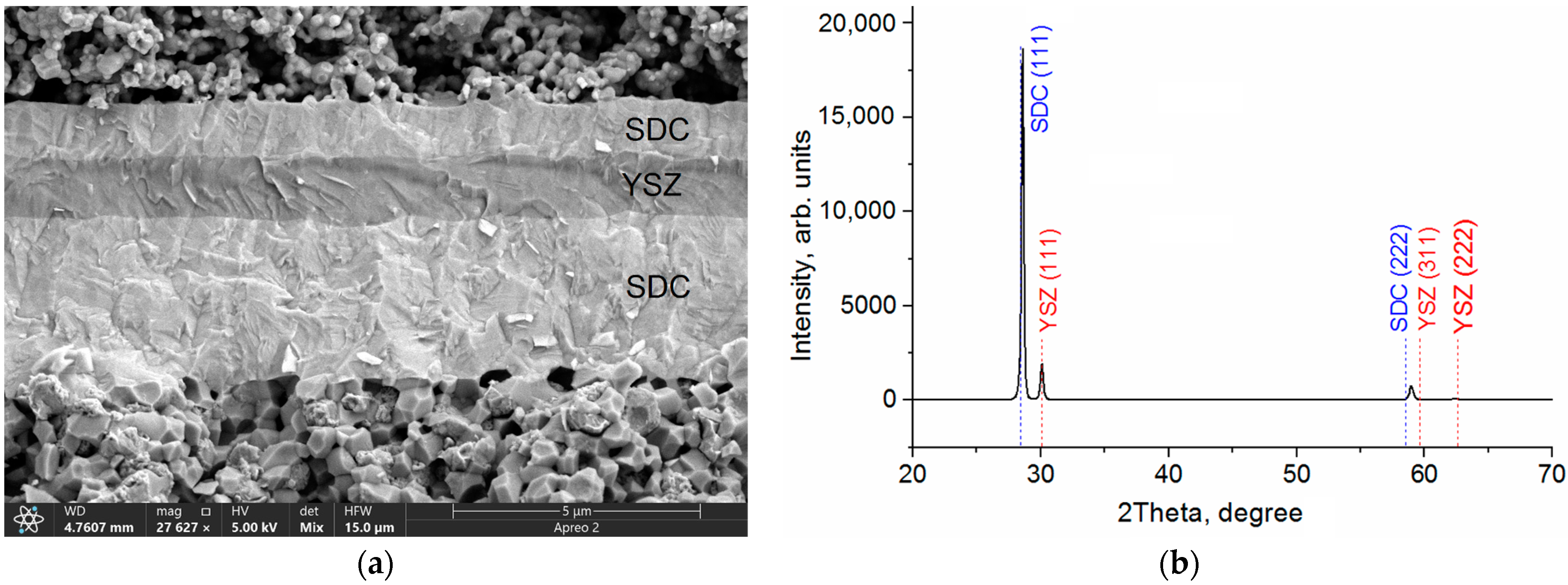


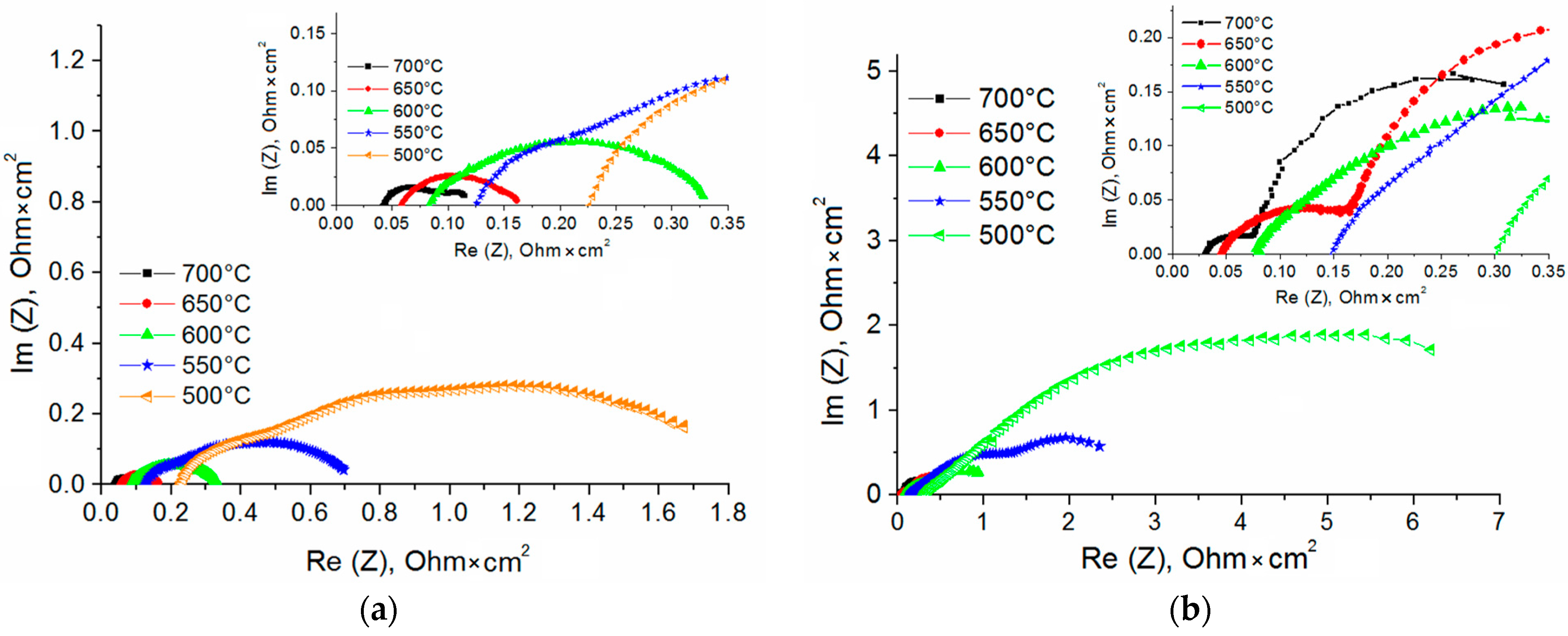
| SDC Films | Phases | Phase Content, vol.% | Lattice Parameters, Ǻ | CSR 1 Size, nm | ∆d/d × 10–3 |
|---|---|---|---|---|---|
| As-deposited | (Ce0.8Sm0.2)O1.9 | 100 | a = 5.4495 | 39 | 1.1 |
| Annealed at 900 °C | (Ce0.8Sm0.2)O1.9 | 100 | a = 5.4375 | 129 | 0.3 |
| Annealed at 1000 °C | (Ce0.8Sm0.2)O1.9 | 100 | a = 5.4309 | 189 | 0.2 |
| Annealed at 1100 °C | (Ce0.8Sm0.2)O1.9 | 100 | a = 5.4348 | 92 | 0.3 |
| Electrolyte Structures | Power Density (mW/cm–2) | ||||||
|---|---|---|---|---|---|---|---|
| 800 °C | 750 °C | 700 °C | 650 °C | 600 °C | 550 °C | 500 °C | |
| SDC (5.5 μm) | - | - | 558 | 651 | 604 | 405 | 208 |
| SDC/YSZ (0.5 μm)/SDC | 1650 | 1523 | 1357 | 1080 | 782 | 469 | 213 |
| SDC/YSZ (1 μm)/SDC | 2263 | 1818 | 1438 | 1132 | 794 | 445 | 201 |
| SDC/YSZ (1.5 μm)/SDC | 1912 | 1450 | 1190 | 935 | 611 | 340 | 143 |
| Electrolyte Structure | Ohmic Resistance (Ω·cm2) | ||||||
|---|---|---|---|---|---|---|---|
| 800 °C | 750 °C | 700 °C | 650 °C | 600 °C | 550 °C | 500 °C | |
| SDC (5.5 μm) | - | - | 0.04 | 0.05 | 0.08 | 0.12 | 0.22 |
| SDC/YSZ (0.5 μm)/SDC | 0.016 | 0.017 | 0.03 | 0.04 | 0.07 | 0.12 | 0.23 |
| SDC/YSZ (1 μm)/SDC | 0.017 | 0.02 | 0.03 | 0.04 | 0.07 | 0.14 | 0.29 |
| SDC/YSZ (1.5 μm)/SDC | 0.018 | 0.02 | 0.03 | 0.05 | 0.1 | 0.19 | 0.39 |
| Electrolyte Structure | Polarization Resistance (Ω·cm2) | ||||||
|---|---|---|---|---|---|---|---|
| 800 °C | 750 °C | 700 °C | 650 °C | 600 °C | 550 °C | 500 °C | |
| SDC (5.5 μm) | - | - | 0.08 | 0.1 | 0.23 | 0.61 | 1.6 |
| SDC/YSZ (1 μm)/SDC | 0.26 | 0.33 | 0.41 | 0.58 | 1.01 | 2.8 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovyev, A.; Shipilova, A.; Smolyanskiy, E. Solid Oxide Fuel Cells with Magnetron Sputtered Single-Layer SDC and Multilayer SDC/YSZ/SDC Electrolytes. Membranes 2023, 13, 585. https://doi.org/10.3390/membranes13060585
Solovyev A, Shipilova A, Smolyanskiy E. Solid Oxide Fuel Cells with Magnetron Sputtered Single-Layer SDC and Multilayer SDC/YSZ/SDC Electrolytes. Membranes. 2023; 13(6):585. https://doi.org/10.3390/membranes13060585
Chicago/Turabian StyleSolovyev, Andrey, Anna Shipilova, and Egor Smolyanskiy. 2023. "Solid Oxide Fuel Cells with Magnetron Sputtered Single-Layer SDC and Multilayer SDC/YSZ/SDC Electrolytes" Membranes 13, no. 6: 585. https://doi.org/10.3390/membranes13060585
APA StyleSolovyev, A., Shipilova, A., & Smolyanskiy, E. (2023). Solid Oxide Fuel Cells with Magnetron Sputtered Single-Layer SDC and Multilayer SDC/YSZ/SDC Electrolytes. Membranes, 13(6), 585. https://doi.org/10.3390/membranes13060585







