PEM Electrochemical Hydrogen Compression with Sputtered Pt Catalysts
Abstract
:1. Introduction
2. Experimental
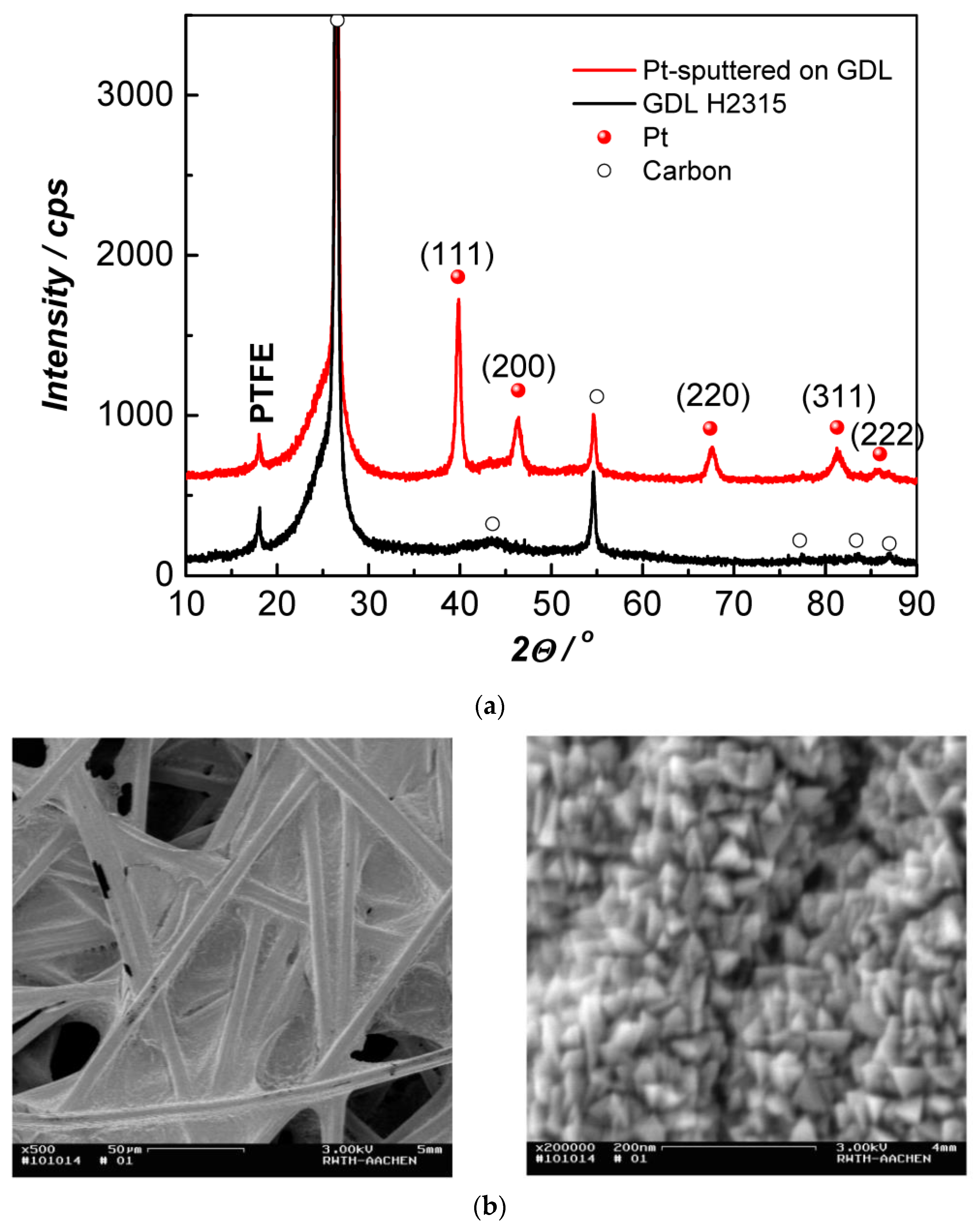
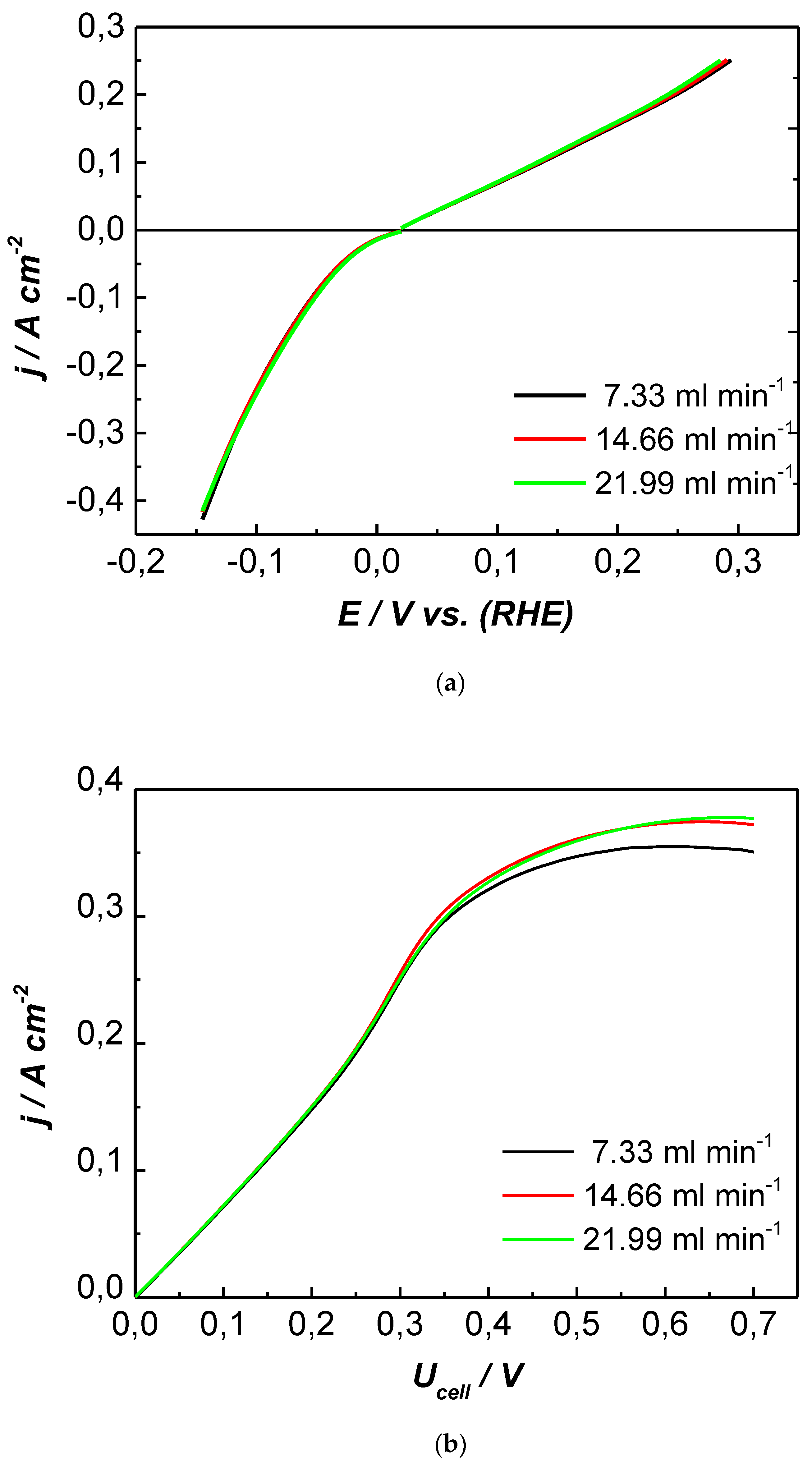
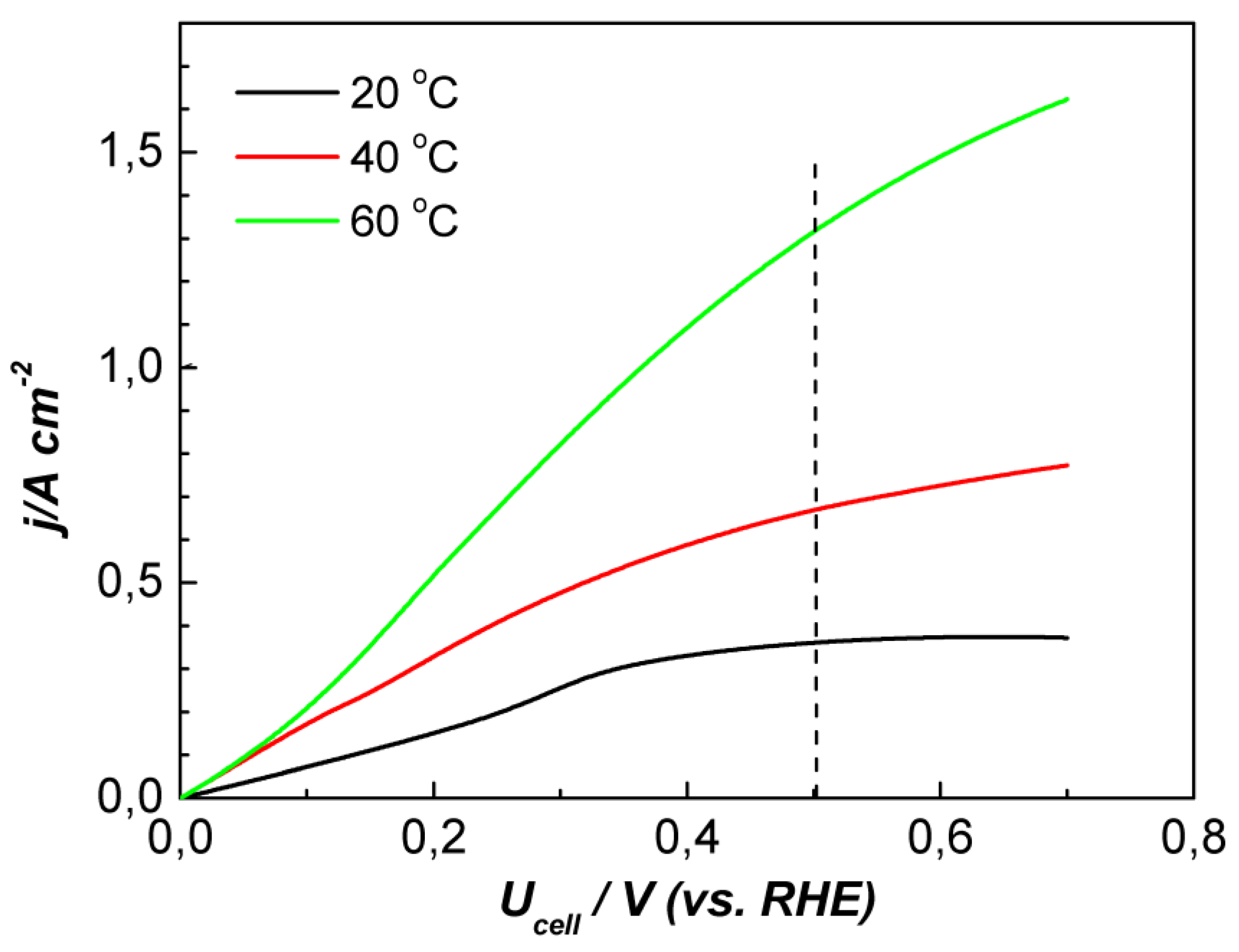
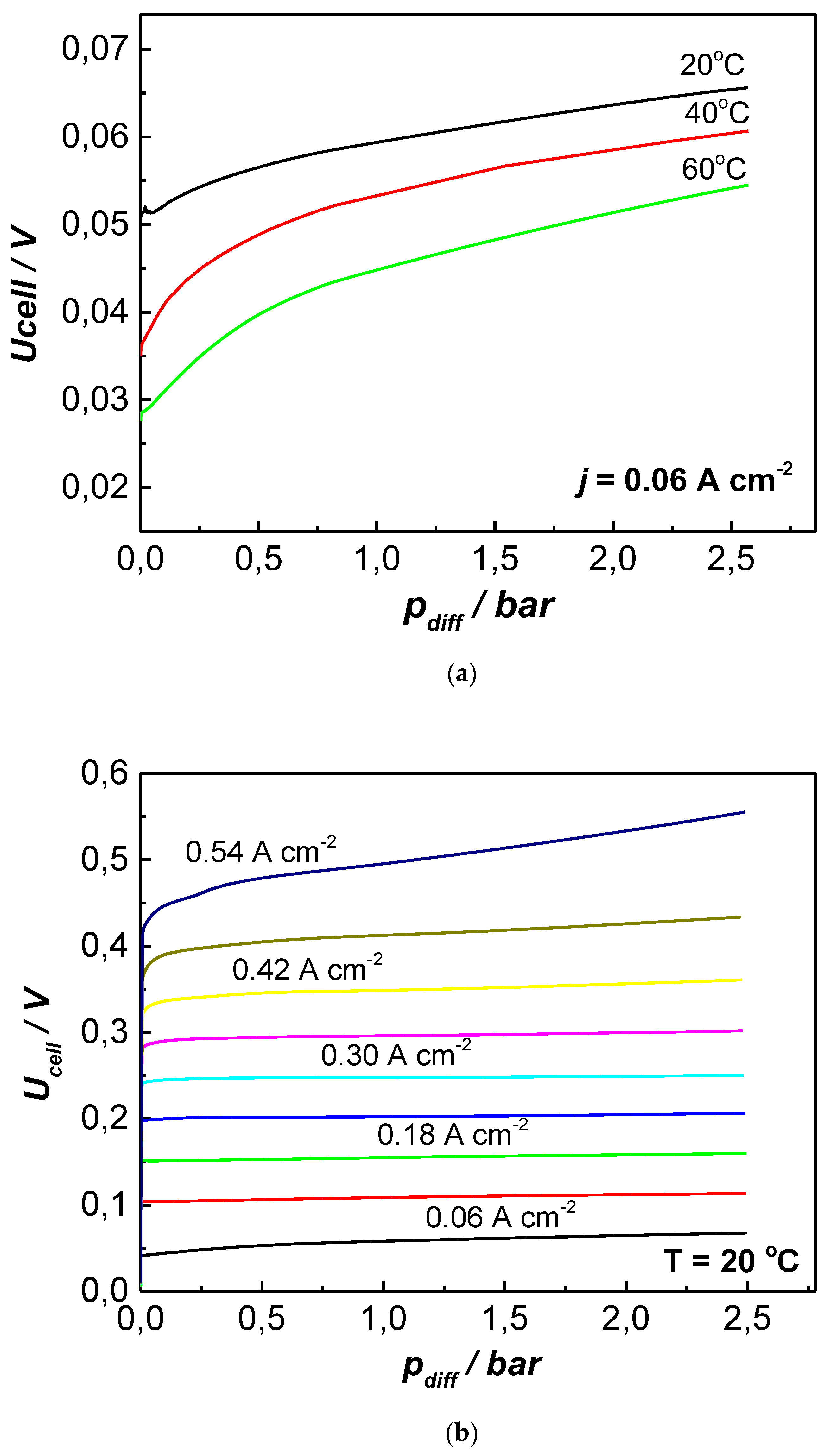
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carmo, M.; Fritz, D.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Smart energy solutions with hydrogen options. Int. J. Hydrogen Energy 2018, 43, 8579–8599. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K. Hydrogen storage and delivery: Review of the state-of-the-art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Rhandi, M.; Trégaro, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part I. Pros and cons. Chin. J. Catal. 2020, 41, 756–769. [Google Scholar] [CrossRef]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Review of the current technologies and performances of hydrogen compression for stationary and automotive applications. Renew. Sustain. Energy Rev. 2019, 102, 150–170. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hamajima, S.; Iwahara, H. Electrochemical hydrogen pump using a high-temperature-type proton conductor: Improvement of pumping capacity. Solid State Ion. 2001, 145, 25–29. [Google Scholar] [CrossRef]
- Sheffield, J.; Martin, K.; Folkson, R. Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance, Towards Zero Carbon Transportation; Elsevier: Amsterdam, The Netherlands, 2014; pp. 117–137. [Google Scholar]
- Grigoriev, S.; Shtatniy, I.; Millet, P.; Porembsky, V.; Fateev, V. Description and characterization of an electrochemical hydrogen compressor/concentrator based on solid polymer electrolyte technology. Int. J. Hydrogen Energy 2011, 36, 4148–4155. [Google Scholar] [CrossRef]
- Nordio, M.; Barain, M.; Raymakers, L.; Annaland, M.; Mulder, M.; Gallucci, G. Effect of CO2 on the performance of an electrochemical hydrogen compressor. Chem. Eng. J. 2020, 392, 123647. [Google Scholar] [CrossRef]
- Langer, S.; Haldeman, R. Electrolytic Hydrogen Purification and Recovery of Same. U.S. Patent 3,475,302, 28 October 1969. [Google Scholar]
- Maget, H. Process for Gas Purification. U.S. Patent 3,489,670, 13 January 1970. [Google Scholar]
- Perry, K.; Eisman, G.; Benicewicz, B. Electrochemical hydrogen pumping using a high-temperature polybenzimidazole (PBI) membrane. J. Power Sources 2008, 177, 478–484. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Yamauchi, T.; Ogaki, K. High temperature type protonic conductor based on SrCeO3 and its application to the extraction of hydrogen gas. Solid State Ion. 1986, 18–19, 1003–1007. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Yamasaki, I. High-temperature steam electrolysis using SrCeO3-based proton conductive solid electrolyteInt. Int. J. Hydrogen Energy 1987, 12, 73–77. [Google Scholar] [CrossRef]
- Hamakawa, S.; Hibino, T.; Iwahara, H. Electrochemical methane coupling using protonic conductors. J. Electrochem. Soc. 1993, 140, 459–462. [Google Scholar] [CrossRef]
- Katahiraa, K.; Matsumotoa, H.; Iwaharaa, H.; Koidea, K.; Iwamoto, T. A solid electrolyte hydrogen sensor with an electrochemically-supplied hydrogen standard. Sens. Actuators B Chem. 2001, 73, 130–134. [Google Scholar] [CrossRef]
- Casati, C.; Longhi, P.; Zanderighi, L.; Bianchi, F. Some fundamental aspects in electrochemical hydrogen purification/compression. J. Power Sources 2008, 180, 103–113. [Google Scholar] [CrossRef]
- Wang l Bliznakov, S.; Isseroff, R.; Zho, Y.; Zuo, X.; Raut, A.; Wang, W.; Cuiffo, M.; Kim, T.; Rafailovich, M. Enhancing proton exchange membrane fuel cell performance via graphene oxide surface synergy. Appl. Energy 2020, 261, 114277. [Google Scholar] [CrossRef]
- Kim, S.; Lee, B.; Ahn, S.; Han, J.; Park, H.; Kim, H.; Yoo, S.; Kim, H.; Cho, E.; Nam, S.; et al. Characterizations of polybenzimidazole based electrochemical hydrogen pumps with various Pt loadings for H2/CO2 gas separation. Int. J. Hydrogen Energy 2013, 38, 14816–14823. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, B.; Jiang, J.; Xu, W. H2 purification process with double layer bcc-PdCu alloy membrane at ambient temperature. Int. J. Hydrogen Energy 2020, 45, 17540–17547. [Google Scholar] [CrossRef]
- Arenas, L.; Hadjigeorgiou, G.; Jones, S.; Dijk, N.; Hodgson, D.; Cruden, A.; León, C. Effect of airbrush type on sprayed platinum and platinum-cobalt catalyst inks: Benchmarking as PEMFC and performance in an electrochemical hydrogen pump. Int. J. Hydrogen Energy 2020, 45, 27392–27403. [Google Scholar] [CrossRef]
- Slavcheva, E.; Radev, I.; Bliznakov, S.; Topalov, G.; Andreev, P.; Budevski, E. Sputtered iridium oxide films as electrocatalysts for water splitting via PEM electrolysis. Electrochim. Acta 2007, 52, 3889–3894. [Google Scholar] [CrossRef]
- Labou, D.; Slavcheva, E.; Schnakenberg, U.; Neophytides, S. Performance of laboratory polymer electrolyte membrane hydrogen generator with sputtered iridium oxide anode. J. Power Sources 2008, 185, 1073–1078. [Google Scholar] [CrossRef]
- Slavcheva, E.; Radev, I.; Topalov, G.; Budevski, E. Sputtered electrocatalysts for PEM electrochemical energy converters. Electrochim. Acta 2007, 53, 362–368. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, W.; Guan, D.; Sunarso, J.; Zhu, Y.; Hu, X.; Zhang, W.; Shao, Z. Two orders of magnitude enhancement in oxygen evolution reactivity on amorphous Ba0.5Sr0.5Co0.8Fe0.2O3−d. Sci. Adv. Chem. 2017, 3, e1603206. [Google Scholar]
- Slavcheva, E.; Ganske, G.; Topalov, G.; Mokwa, W.; Schnakenberg, U. Effect of sputtering parameters on surface morphology and catalytic efficiency of thin platinum films. Appl. Surf. Sci. 2009, 255, 6479–6486. [Google Scholar] [CrossRef]
- Slavcheva, E.; Topalov, G.; Ganske, G.; Radev, I.; Lefterova, E.; Schnakenberg, U. Influence of sputtering pressure on surface structure and oxygen reduction reaction catalytic activity of thin platinum film. Electrochim. Acta 2010, 55, 8992–8997. [Google Scholar] [CrossRef]
- Radev, I.; Slavcheva, E.; Budevski, E. Investigation of nanostructured platinum-based membrane electrode assemblies in EasyTest” cell. Int. J. Hydrogen Energy 2017, 32, 872–877. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisov, G.; Borisov, N.; Heiss, J.; Schnakenberg, U.; Slavcheva, E. PEM Electrochemical Hydrogen Compression with Sputtered Pt Catalysts. Membranes 2023, 13, 594. https://doi.org/10.3390/membranes13060594
Borisov G, Borisov N, Heiss J, Schnakenberg U, Slavcheva E. PEM Electrochemical Hydrogen Compression with Sputtered Pt Catalysts. Membranes. 2023; 13(6):594. https://doi.org/10.3390/membranes13060594
Chicago/Turabian StyleBorisov, Galin, Nevelin Borisov, Jochen Heiss, Uwe Schnakenberg, and Evelina Slavcheva. 2023. "PEM Electrochemical Hydrogen Compression with Sputtered Pt Catalysts" Membranes 13, no. 6: 594. https://doi.org/10.3390/membranes13060594
APA StyleBorisov, G., Borisov, N., Heiss, J., Schnakenberg, U., & Slavcheva, E. (2023). PEM Electrochemical Hydrogen Compression with Sputtered Pt Catalysts. Membranes, 13(6), 594. https://doi.org/10.3390/membranes13060594





