Long-Term Stable Complementary Electrochromic Device Based on WO3 Working Electrode and NiO-Pt Counter Electrode
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of WO3 Films
2.3. Preparation for NiO, Pt and NiO-Pt Composite Films
2.4. Preparation of Electrolyte
2.5. Assembly of Electrochromic Devices
2.6. Characterization
3. Results and Discussion
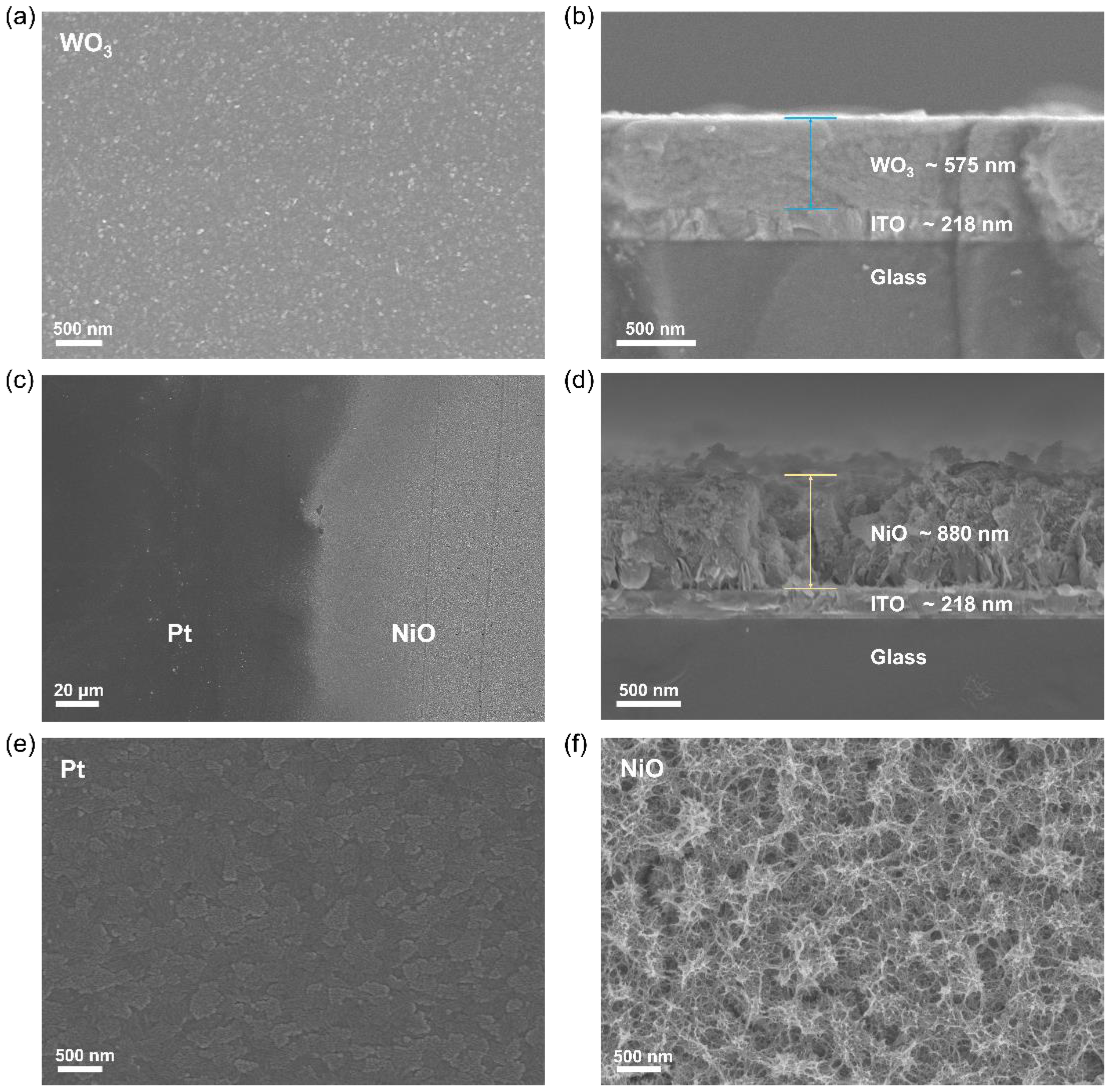
3.1. Structure and Morphology of Thin Films
3.2. The Complementary Effects of WO3 and NiO Electrochromic Processes
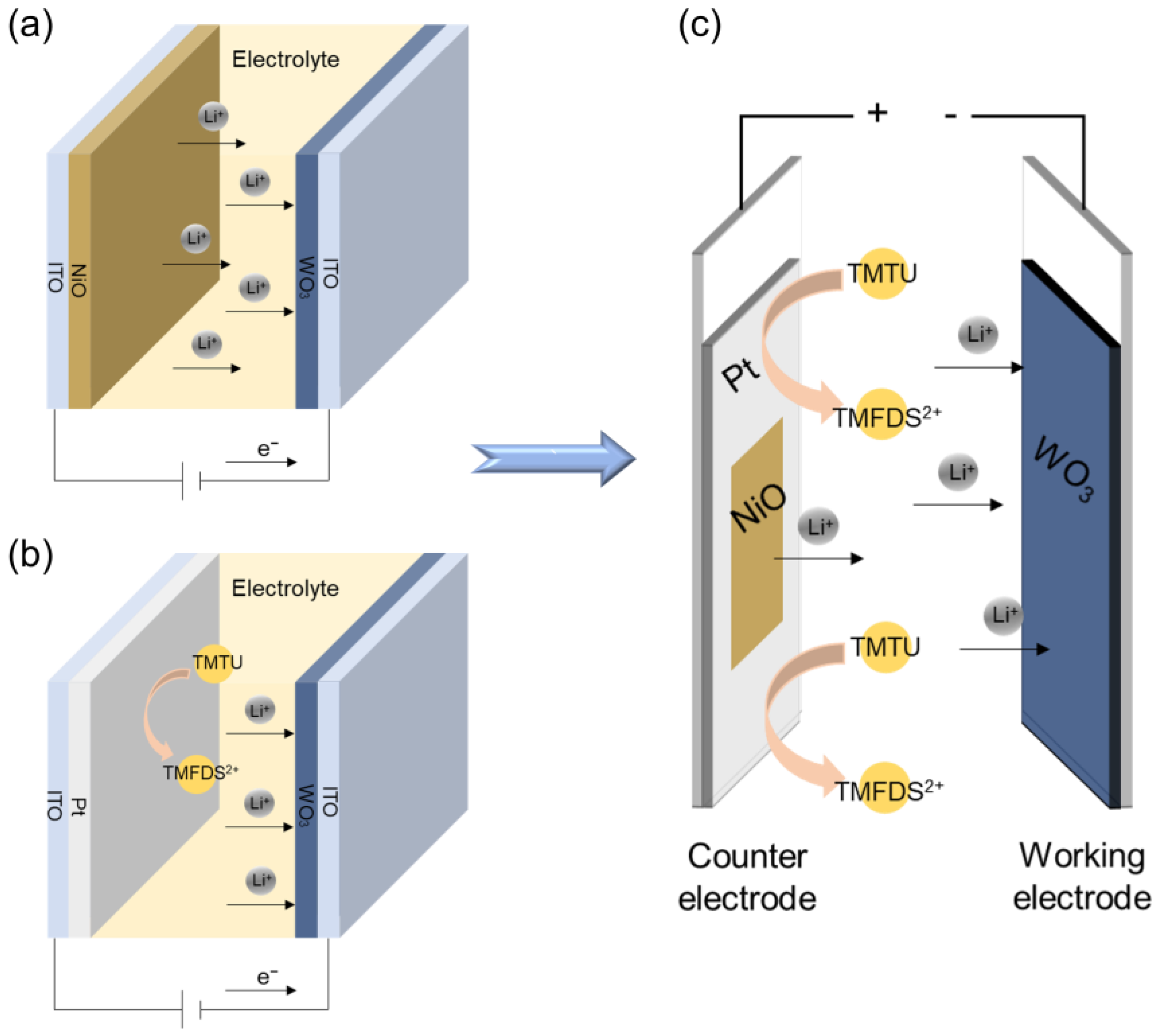
3.3. Structure Configuration and Working Mechanism of the ECDs
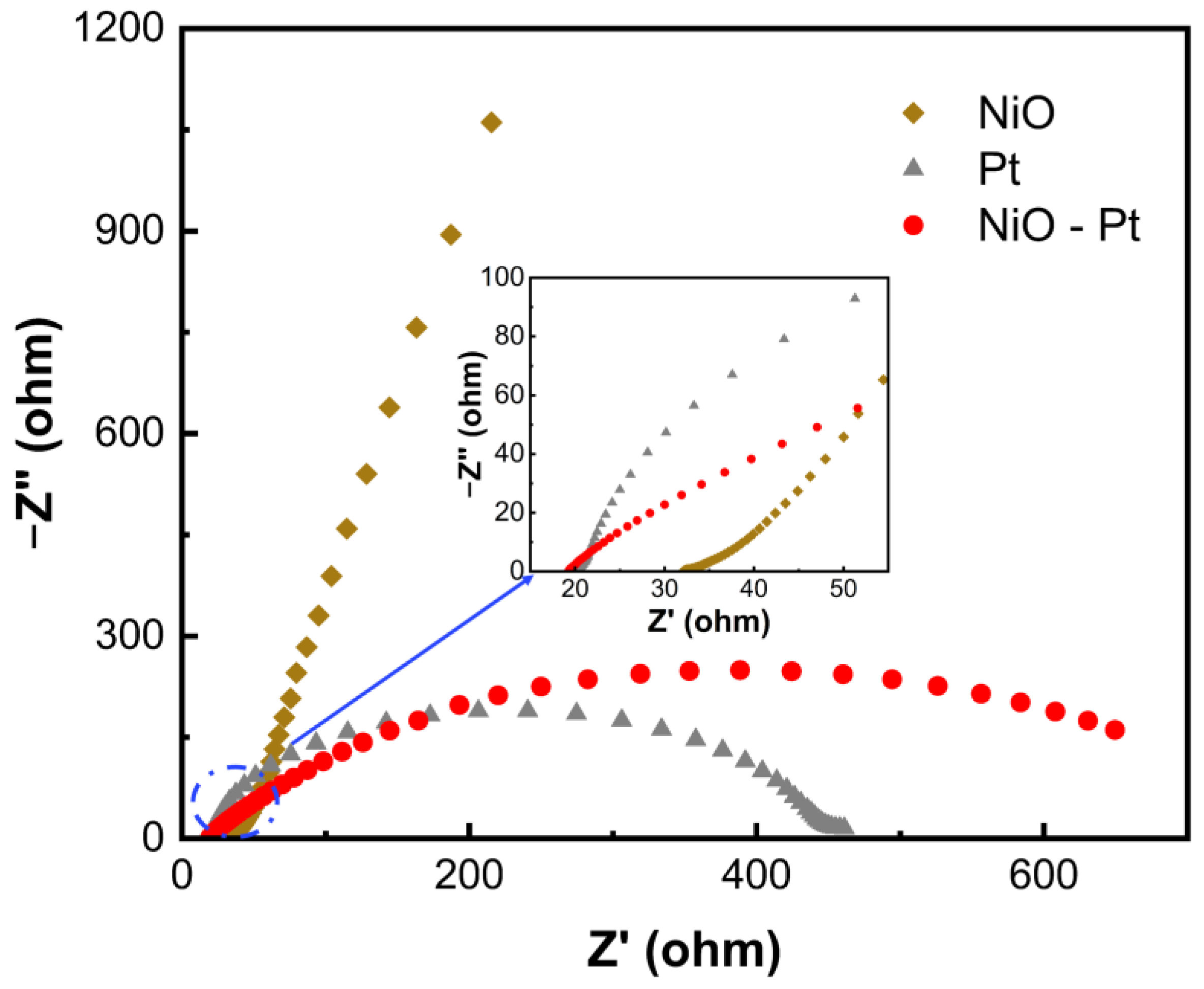
3.4. Electrochemical Properties of Different Counter Electrodes
3.5. Electrochromic Performance of ECDs
3.6. Cycle Stability of the ECDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, V.K.; Ding, G.; Ma, J.; Lee, P.S.; Lu, X. Hybrid materials and polymer electrolytes for electrochromic device applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef]
- Ke, Y.J.; Zhou, C.Z.; Zhou, Y.; Wang, S.C.; Chan, S.H.; Long, Y. Emerging thermal-responsive materials and integrated techniques targeting the energy-efficient smart window application. Adv. Funct. Mater. 2018, 28, 1800113. [Google Scholar] [CrossRef]
- Wang, M.; Xing, X.; Perepichka, I.F.; Shi, Y.H.; Zhou, D.Y.; Wu, P.H.; Meng, H. Electrochromic smart windows can achieve an absolute private state through thermochromically engineered electrolyte. Adv. Energy Mater. 2019, 9, 1900433. [Google Scholar] [CrossRef]
- Yan, C.; Kang, W.; Wang, J.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Stretchable and wearable electrochromic devices. ACS Nano. 2014, 8, 316–322. [Google Scholar] [CrossRef]
- Mortimer, R.J. Electrochromic materials. Annu. Rev. Mater. Res. 2011, 41, 241–268. [Google Scholar] [CrossRef]
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic systems and the prospects for devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Kalagi, S.S.; Mali, S.S.; Dalavi, D.S.; Inamdar, A.I.; Im, H.; Patil, P.S. Limitations of dual and complementary inorganic–organic electrochromic device for smart window application and its colorimetric analysis. Synth. Met. 2011, 161, 1105–1112. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ko, T.F.; Hsu, S.C.; Li, K.D.; Wu, J.Y. Fast response of complementary electrochromic device based on WO3/NiO electrodes. Sci. Rep. 2020, 10, 8430. [Google Scholar] [CrossRef]
- Eren, E.; Karaca, G.Y.; Koc, U.; Oksuz, L.; Oksuz, A.U. Electrochromic characteristics of radio frequency plasma sputtered WO3 thin films onto flexible polyethylene terephthalate substrates. Thin Solid Film. 2017, 634, 40–50. [Google Scholar] [CrossRef]
- Alam, M.W.; BaQais, A.; Mir, T.A.; Nahvi, I.; Zaidi, N.; Yasin, A. Effect of Mo doping in NiO nanoparticles for structural modification and its efficiency for antioxidant, antibacterial applications. Sci. Rep. 2023, 13, 1328. [Google Scholar] [CrossRef]
- Scherer, M.R.; Steiner, U. Efficient electrochromic devices made from 3D nanotubular gyroid networks. Nano Lett. 2013, 13, 3005–3010. [Google Scholar] [CrossRef]
- Karaca, G.Y.; Eren, E.; Alver, C.; Koc, U.; Uygun, E.; Oksuz, L.; Oksuz, A.U. Plasma modified V2O5/PEDOT hybrid based flexible electrochromic devices. Electroanalysis 2017, 29, 1324–1331. [Google Scholar] [CrossRef]
- Choi, D.; Son, M.; Im, T.; Ahn, S.H.; Lee, C.S. Crack-free fabrication of Prussian blue-based blending film for the dramatic enhancement of dual electrochromic device. Ceram. Int. 2020, 46, 21008–21013. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, R.; Wang, Y.; Ye, X.; Wan, Z.; Jia, C.; Weng, X.; Xie, J.; Deng, L. A high-performance electrochromic device assembled with hexagonal WO3 and NiO/PB composite nanosheet electrodes towards energy storage smart window. Sol. Energy Mater. Sol. Cells 2020, 207, 110337. [Google Scholar] [CrossRef]
- Qin, M.S.; Liu, M.C.; Zeng, Z.Q.; Wu, Q.; Wu, Y.K.; Zhang, H.; Lei, S.; Cheng, S.J.; Xie, J. Rejuvenating propylene carbonate-based electrolyte through nonsolvating interactions for wide-temperature Li-ions batteries. Adv. Energy Mater. 2022, 12, 2201801. [Google Scholar] [CrossRef]
- Qiu, D.; Ji, H.; Zhang, X.; Zhang, H.; Cao, H.; Chen, G.; Tian, T.; Chen, Z.; Guo, X.; Liang, L.; et al. Electrochromism of nanocrystal-in-glass tungsten oxide thin films under various conduction cations. Inorg. Chem. 2019, 58, 2089–2098. [Google Scholar] [CrossRef]
- Arvizu, M.A.; Triana, C.A.; Stefanov, B.I.; Granqvist, C.G.; Niklasson, G.A. Electrochromism in sputter-deposited W–Ti oxide films: Durability enhancement due to Ti. Sol. Energy Mater. Sol. Cells 2014, 125, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-M.; Yun, S.-W.; Kim, J.-H.; You, S.-H.; Park, J.; Lee, S.; Chang, S.H.; Chae, S.C.; Joo, S.H.; Jung, Y.; et al. Selective electrocatalysis imparted by metal–insulator transition for durability enhancement of automotive fuel cells. Nat. Catal. 2020, 3, 639–648. [Google Scholar] [CrossRef]
- Yan, S.; Fu, H.; Zhang, L.; Dong, Y.; Li, W.; Ouyang, M.; Zhang, C. Conjugated polymer multilayer by in situ electrochemical polymerization for black-to-transmissive eletrochromism. Chem. Eng. J. 2021, 406, 126819. [Google Scholar] [CrossRef]
- Alam, M.W.; Khalid, N.R.; Naeem, S.; Niaz, N.A.; Ahmad Mir, T.; Nahvi, I.; Souayeh, B.; Zaidi, N. Novel Nd-N/TiO2 nanoparticles for photocatalytic and antioxidant applications using hydrothermal approach. Materials 2022, 15, 6658. [Google Scholar] [CrossRef]
- Georg, A.; Georg, A. Electrochromic device with a redox electrolyte. Sol. Energy Mater. Sol. Cells 2009, 93, 1329–1337. [Google Scholar] [CrossRef]
- Sheng, K.; Xu, F.; Shen, K.; Zheng, J.; Xu, C. Electrocatalytic PProDOT–Me2 counter electrode for a Br−/Br3− redox couple in a WO3-based electrochromic device. Electrochem. Commun. 2020, 111, 106646. [Google Scholar] [CrossRef]
- Bogati, S.; Georg, A.; Jerg, C.; Graf, W. Tetramethylthiourea (TMTU) as an alternative redox mediator for electrochromic devices. Sol. Energy Mater. Sol. Cells 2016, 157, 454–461. [Google Scholar] [CrossRef]
- Bae, J.; Kim, H.; Moon, H.C.; Kim, S.H. Low-voltage, simple WO3-based electrochromic devices by directly incorporating an anodic species into the electrolyte. J. Mater. Chem. C 2016, 4, 10887–10892. [Google Scholar] [CrossRef]
- Hočevar, M.; Opara Krašovec, U. Solid electrolyte containing a colorless redox couple for electrochromic device. Sol. Energy Mater. Sol. Cell 2019, 196, 9–15. [Google Scholar] [CrossRef]
- Wang, M.Y.; He, Y.C.; Da Rocha, M.; Rougier, A.; Diao, X.G. Temperature dependence of the electrochromic properties of complementary NiO//WO3 based devices. Sol. Energy Mater. Sol. Cells 2021, 230, 111239. [Google Scholar] [CrossRef]
- Alam, M.W.; Wang, Z.K.; Naka, S.; Okada, H. Performance enhancement of top-contact pentacene-based organic thin-film transistors with bilayer WO3/Au electrodes. Jpn. J. Appl. Phys. 2013, 52, 03bb08. [Google Scholar] [CrossRef]
- Dong, D.; Wang, W.; Rougier, A.; Dong, G.; Da Rocha, M.; Presmanes, L.; Zrikem, K.; Song, G.; Diao, X.; Barnabe, A. Life-cycling and uncovering cation-trapping evidence of a monolithic inorganic electrochromic device: Glass/ITO/WO3/LiTaO3/NiO/ITO. Nanoscale 2018, 10, 16521–16530. [Google Scholar] [CrossRef] [Green Version]
- Phan, G.T.; Van Pham, D.; Patil, R.A.; Tsai, C.H.; Lai, C.C.; Yeh, W.C.; Liou, Y.; Ma, Y.R. Fast-switching electrochromic smart windows based on NiO-nanorods counter electrode. Sol. Energy Mater. Sol. Cells 2021, 231, 111306. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Wang, Y.; Wang, L.; Guo, W.; Qi, D.; Peng, X.; Hagfeldt, A.; Gratzel, M.; Ma, T. Economical Pt-free catalysts for counter electrodes of dye-sensitized solar cells. J. Am. Chem. Soc. 2012, 134, 3419–3428. [Google Scholar] [CrossRef]
- Du, Z.; Pan, Z.; Fabregat-Santiago, F.; Zhao, K.; Long, D.; Zhang, H.; Zhao, Y.; Zhong, X.; Yu, J.S.; Bisquert, J. Carbon counter-electrode-based quantum-dot-sensitized solar cells with certified efficiency sxceeding 11%. J. Phys. Chem. Lett. 2016, 7, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Bang, J.H. Rising Again: Opportunities and Challenges for Platinum-Free Electrocatalysts. Chem. Mater. 2015, 27, 7218–7235. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, J.; Xu, C. A newly-designed self-powered electrochromic window. Sci. China Chem. 2016, 60, 84–89. [Google Scholar] [CrossRef]
- Vijaya, S.; Landi, G.; Wu, J.J.; Anandan, S. MoS2 nanosheets based counter electrodes: An alternative for Pt-free dye-sensitized solar cells. Electrochim. Acta 2019, 294, 134–141. [Google Scholar] [CrossRef]
- Qin, M.; Zeng, Z.; Wu, Q.; Yan, H.; Liu, M.; Wu, Y.; Zhang, H.; Lei, S.; Cheng, S.; Xie, J. Dipole–dipole interactions for inhibiting solvent co-intercalation into a graphite anode to extend the horizon of electrolyte design. Energy Environ. Sci. 2023, 16, 546–556. [Google Scholar] [CrossRef]
- Sheng, K.; Khalifa, M.A.; Wang, Z.; Zheng, J.; Xu, C. Rapid switching of a Pt-free photovoltachromic device based on WO3 using PProDOT-Me2 catalyst. Sol. Energy 2022, 232, 139–145. [Google Scholar] [CrossRef]
- Khalifa, M.A.; Sheng, K.; Wang, Z.; Zheng, J.; Xu, C. Partly covered PProDOT-Me2 on MoS2 nanosheets counter electrode for high-performance self-powered electrochromic device. Adv. Mater. Interfaces 2022, 9, 2100945. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, K.; Xie, H.; Xue, B.; Zheng, J.; Xu, C. Robust non-complementary electrochromic device based on WO3 film and CoS catalytic counter electrode with TMTU/TMFDS2+ redox couple. Chem. Eng. J. 2021, 426, 131314. [Google Scholar] [CrossRef]
- Cai, G.; Cui, M.; Kumar, V.; Darmawan, P.; Wang, J.; Wang, X.; Lee-Sie Eh, A.; Qian, K.; Lee, P.S. Ultra-large optical modulation of electrochromic porous WO3 film and the local monitoring of redox activity. Chem. Sci. 2016, 7, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Giannuzzi, R.; Scarfiello, R.; Sibillano, T.; Nobile, C.; Grillo, V.; Giannini, C.; Cozzoli, P.D.; Manca, M. From capacitance-controlled to diffusion-controlled electrochromism in one-dimensional shape-tailored tungsten oxide nanocrystals. Nano Energy 2017, 41, 634–645. [Google Scholar] [CrossRef]


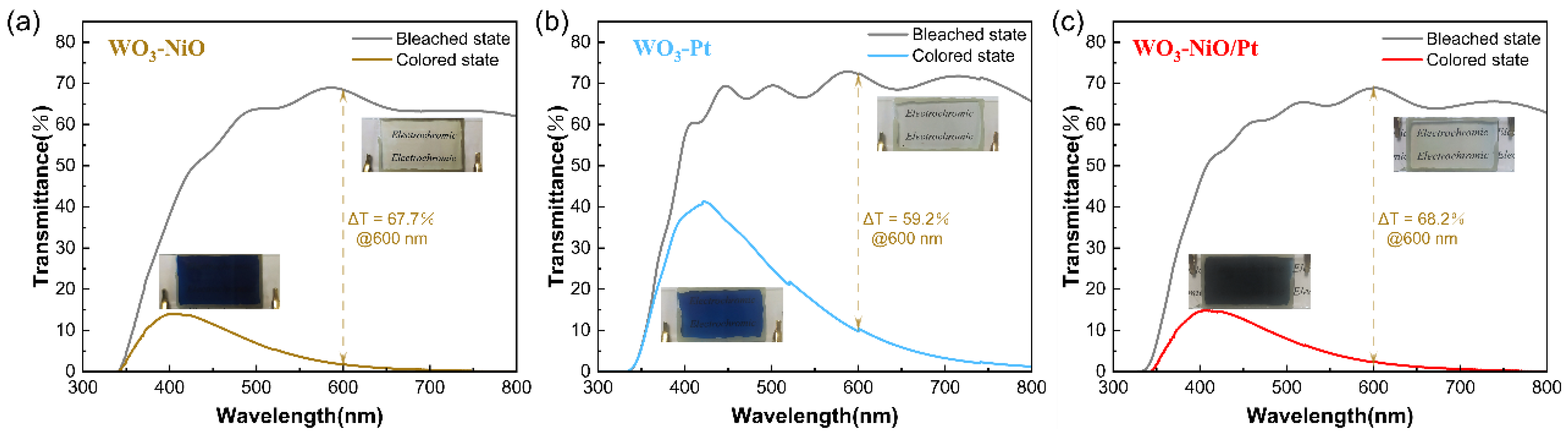
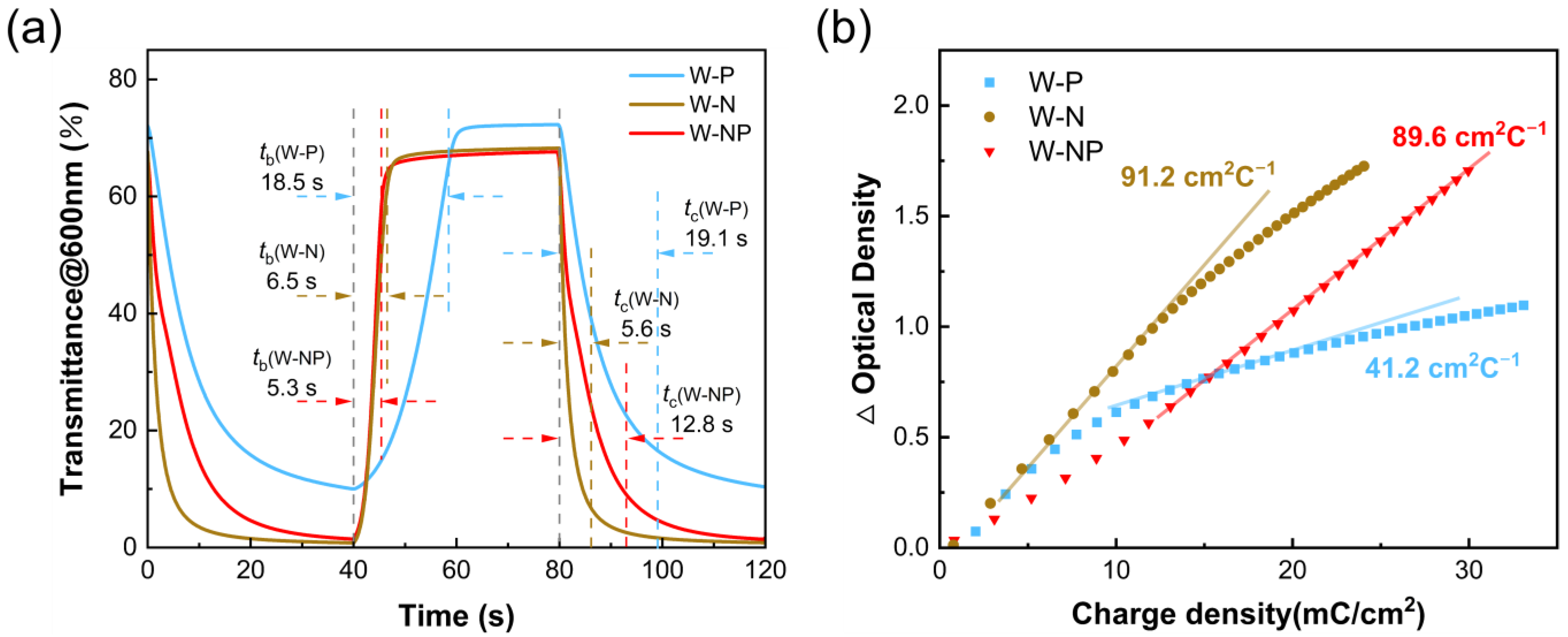

| ECDs | Transmittance Modulation @ 600 nm (%) | tc (s) | tb (s) | Coloration Efficiency (cm2·C−1) |
|---|---|---|---|---|
| W-N | 67.7 | 6.5 | 5.6 | 91.2 |
| W-P | 59.2 | 18.5 | 19.1 | 41.2 |
| W-NP | 68.2 | 5.3 | 12.8 | 89.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Y.; Wang, Z.; Xie, H.; Khalifa, M.A.; Zheng, J.; Xu, C. Long-Term Stable Complementary Electrochromic Device Based on WO3 Working Electrode and NiO-Pt Counter Electrode. Membranes 2023, 13, 601. https://doi.org/10.3390/membranes13060601
Ke Y, Wang Z, Xie H, Khalifa MA, Zheng J, Xu C. Long-Term Stable Complementary Electrochromic Device Based on WO3 Working Electrode and NiO-Pt Counter Electrode. Membranes. 2023; 13(6):601. https://doi.org/10.3390/membranes13060601
Chicago/Turabian StyleKe, Yajie, Zitao Wang, Haiyi Xie, Mahmoud A. Khalifa, Jianming Zheng, and Chunye Xu. 2023. "Long-Term Stable Complementary Electrochromic Device Based on WO3 Working Electrode and NiO-Pt Counter Electrode" Membranes 13, no. 6: 601. https://doi.org/10.3390/membranes13060601
APA StyleKe, Y., Wang, Z., Xie, H., Khalifa, M. A., Zheng, J., & Xu, C. (2023). Long-Term Stable Complementary Electrochromic Device Based on WO3 Working Electrode and NiO-Pt Counter Electrode. Membranes, 13(6), 601. https://doi.org/10.3390/membranes13060601





