Sintering Aid Strategy for Promoting Oxygen Reduction Reaction on High-Performance Double-Layer LaNi0.6Fe0.4O3–? Composite Electrode for Devices Based on Solid-State Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Dense Electrolyte Substrates
2.2. Synthesis of Powders for Electrodes
2.3. Sample Preparation
2.4. High-Temperature Measurements
3. Results
3.1. Chemical Compatibility
3.2. Electrical Conductivity of Collector Layers
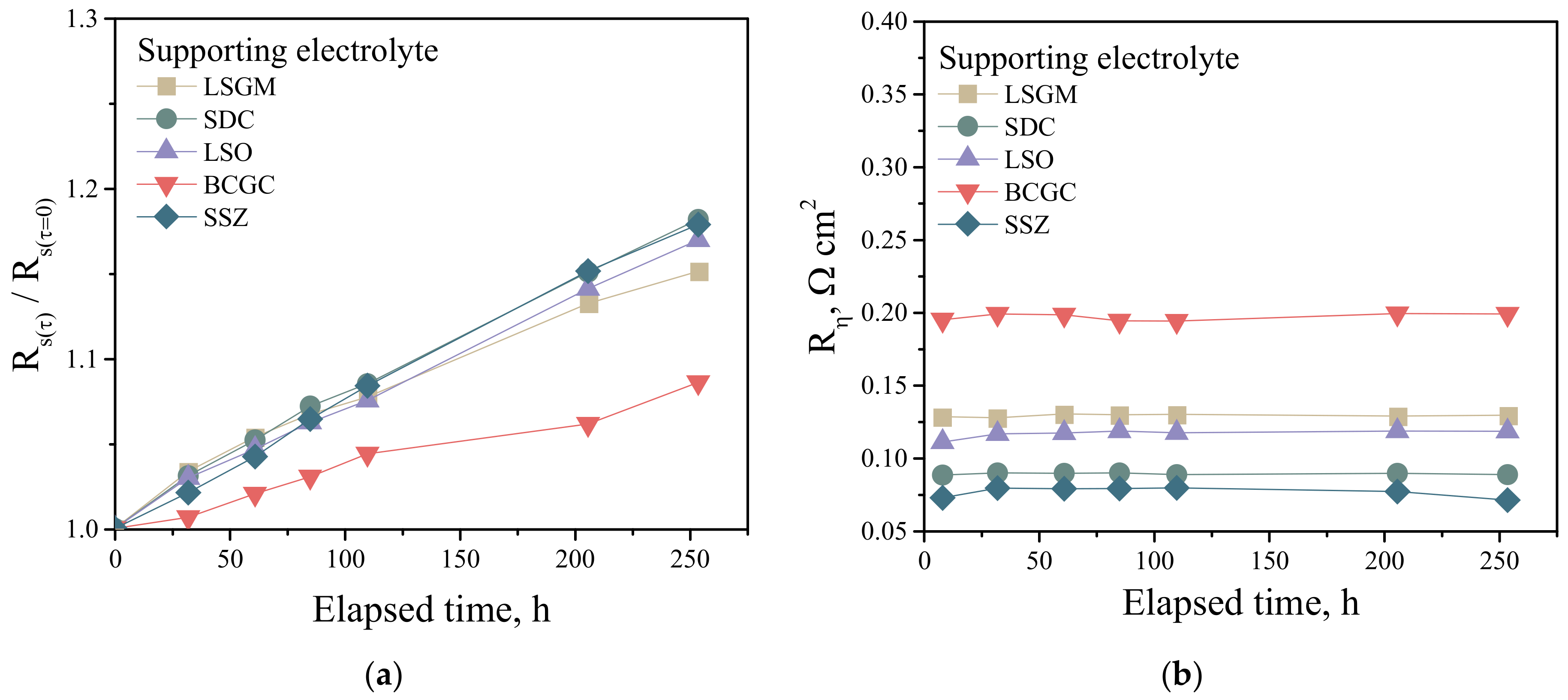
3.3. Electrochemical Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Si, P.; Zheng, Z.; Gu, Y.; Geng, C.; Guo, Z.; Qin, J.; Wen, W. Nanostructured TiO2 arrays for energy storage. Materials 2023, 16, 3864. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, H.; Zhang, T.; Song, B.; Wang, X.; Gu, Z. Recent Advances in ZnO nanomaterial-mediated biological applications and action mechanisms. Nanomaterials 2023, 13, 1500. [Google Scholar] [CrossRef]
- Zhai, H.; Wu, Z.; Fang, Z. Recent progress of Ga2O3-based gas sensors. Ceram. Int. 2022, 48, 24213–24233. [Google Scholar] [CrossRef]
- Lai, H.; II, T.A.A. Life cycle analyses of SOFC/gas turbine hybrid power plants accounting for long-term degradation effects. J. Clean. Product. 2023, 412, 137411. [Google Scholar] [CrossRef]
- Alaedini, A.H.; Tourani, H.K.; Saidi, M. A review of waste-to-hydrogen conversion technologies for solid oxide fuel cell (SOFC) applications: Aspect of gasification process and catalyst development. J. Environ. Manag. 2023, 329, 117077. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Qu, J.; Zhu, Y.; Wu, B.; Wu, Y.; Xiao, Z.; Liu, J. Progress and prospect of the novel integrated SOFC-ICE hybrid power system: System design, mass and heat integration, system optimization and techno-economic analysis. Energy Conver. Manag. 2023, 18, 100350. [Google Scholar] [CrossRef]
- Iliev, I.K.; Filimonova, A.A.; Chichirov, A.A.; Chichirova, N.D.; Pechenkin, A.V.; Vinogradov, A.S. Theoretical and experimental studies of combined heat and power systems with SOFCs. Energies 2023, 16, 1898. [Google Scholar] [CrossRef]
- Vinchhi, P.; Khandla, M.; Chaudhary, L.; Pati, R. Recent advances on electrolyte materials for SOFC: A review. Inorg. Chem. Comm. 2023, 152, 110724. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Horri, B.A. Progress in material development for low-temperature solid oxide fuel cells: A Review. Energies 2021, 14, 1280. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G.; Pikalova, N.S.; Filonova, E.A. High-entropy materials in SOFC technology: Theoretical foundations for their creation, features of synthesis, and recent achievements. Materials 2022, 15, 8783. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Ahmad, S.H.; Chen, R.S.; Ismail, A.F.; Hazan, R.; Baharuddin, N.A. Review on recent advancement in cathode material for lower and intermediate temperature solid oxide fuel cells application. Int. J. Hydrogen Energy 2022, 47, 1103–1120. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. A review on recent advances and trends in symmetrical electrodes for solid oxide cells. J. Power Sources 2022, 520, 230852. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Wang, F.; Yan, K.; Budiman, R.A.; Kishimoto, H.; Ishiyama, T.; Bagarinao, K.D.; Yamaji, K.; Horita, T.; Yokokawa, H. The correlation of sulfur distribution in LSCF and performance degradation under different operation temperatures. ECS Trans. 2017, 78, 927–933. [Google Scholar] [CrossRef]
- Budiman, R.A.; Ishiyama, T.; Kishimoto, H.; Bagarinao, K.D.; Yamaji, K.; Horita, T.; Yokokawa, H. Evaluation of electrochemical properties of La0.6Sr0.4Co0.2Fe0.8O3−δ porous electrode with sulfur poisoning. ECS Trans. 2017, 78, 759–764. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Montini, T.; Tavagnacco, C.; Vicario, G.; Fornasiero, P.; Graziani, M. Influence of synthesis route on morphology and electrical properties of LaNi0.6Fe0.4O3. Solid State Ion. 2006, 177, 2957–2965. [Google Scholar] [CrossRef]
- Chen, J.Y.; Rebello, J.; Vashook, V.; Trots, D.M.; Wang, S.R.; Wen, T.L.; Zosel, J.; Guth, U. Thermal stability, oxygen non-stoichiometry and transport properties of LaNi0.6Fe0.4O3. Solid State Ion. 2011, 192, 424–430. [Google Scholar] [CrossRef]
- Basu, R.N.; Tietz, F.; Wessel, E.; Buchkremer, H.P.; Stover, D. Microstructure and electrical conductivity of LaNi0.6Fe0.4O3 prepared by combustion synthesis routes. Mater. Res. Bull. 2004, 39, 1335–1345. [Google Scholar] [CrossRef]
- Niwa, E.; Hashimoto, T. Dependence of thermal expansion of LaNi0.6Fe0.4O3−δ and La0.6Sr0.4Co0.2Fe0.8O3−δ on oxygen partial pressure. Solid State Ion. 2016, 285, 187–194. [Google Scholar] [CrossRef]
- Huang, B.; Zhu, X.; Ren, R.; Hu, Y.; Ding, X.; Liu, Y.; Liu, Z. Chromium poisoning and degradation at Gd0.2Ce0.8O2-impregnated LaNi0.6Fe0.4O3−δ cathode for solid oxide fuel cell. J. Power Sources 2012, 216, 89–98. [Google Scholar] [CrossRef]
- Stodolny, M.K.; Boukamp, B.A.; Blank, D.H.A.; van Berkel, F.P.F. Impact of Cr-poisoning on the conductivity of LaNi0.6Fe0.4O3. J. Power Sources 2011, 196, 9290–9298. [Google Scholar] [CrossRef] [Green Version]
- Chiba, R.; Tabata, Y.; Komatsu, T.; Orui, H.; Nozawa, K.; Arakawa, M.; Arai, H. Property change of a LaNi0.6Fe0.4O3 cathode in the initial current loading process and the influence of a ceria interlayer. Solid State Ion. 2008, 178, 1701–1709. [Google Scholar] [CrossRef]
- Nishi, M.; Horita, T.; Yamaji, K.; Yokokawa, H.; Shimonosono, T.; Kishimoto, H.; Brito, M.E.; Cho, D.; Wang, F. Oxide ion conductivity of LaNi0.6Fe0.4O3. ECS Trans. 2012, 45, 171–180. [Google Scholar] [CrossRef]
- Huang, B.; Zhu, X.; Nie, H.; Niu, Y.; Li, Y.; Cheng, N. Comparison of the electrochemical properties of impregnated and functionally gradient LaNi0.6Fe0.4O3-Gd0.2Ce0.8O2 composite cathodes for Solid Oxide Fuel Cell. J. Power Sources 2013, 235, 20–28. [Google Scholar] [CrossRef]
- Huang, B.; Zhu, X.; Lv, Y.; Liu, H. High-performance Gd0.2Ce0.8O2-impregnated LaNi0.6Fe0.4O3−δ cathodes for intermediate temperature solid oxide fuel cell. J. Power Sources 2012, 209, 209–219. [Google Scholar] [CrossRef]
- Huang, S.; Feng, S.; Wang, H.; Li, Y.; Wang, C. LaNi0.6Fe0.4O3-Ce0.8Sm0.2O1.9-Ag composite cathode for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 10968–10974. [Google Scholar] [CrossRef]
- Budiman, R.A.; Miyazaki, T.; Hashimoto, S.; Yashiro, K.; Kawada, T. Determination of oxygen surface exchange constant of LaNi0.6Fe0.4O3−δ coated with Ce0.9Gd0.1O1.95 by isotope exchange technique. Solid State Ion. 2016, 286, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Budiman, R.A.; Hashimoto, S.; Fujimaki, Y.; Nakamura, T.; Yashiro, K.; Amezawa, K.; Kawada, T. Evaluation of electrochemical properties of LaNi0.6Fe0.4O3−δ-Ce0.9Gd0.1O1.95 composite as air electrode for SOFC. Solid State Ion. 2019, 332, 70–76. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Wen, T.; Li, J. Optimization of LaNi0.6Fe0.4O3−δ cathode for intermediate temperature solid oxide fuel cells. J. Alloys Comp. 2009, 487, 377–381. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Bogdanovich, N.M.; Kolchugin, A.A.; Osinkin, D.A.; Bronin, D.I. Electrical and electrochemical properties of La2NiO4+δ-based cathodes in contact with Ce0.8Sm0.2O2−δ electrolyte. Proc. Eng. 2014, 98, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Millar, L.; Taherparvar, H.; Filkin, N.; Slater, P.; Yeomans, J. Interaction of (La1−xSrx)1−yMnO3-Zr1−zYzO2−d cathodes and LaNi0.6Fe0.4O3 current collecting layers for solid oxide fuel cell application. Solid State Ion. 2008, 179, 732–739. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Ermakova, L.; Khrustov, A.; Farlenkov, A.; Bronin, D. Methods to increase electrochemical activity of lanthanum nickelate-ferrite electrodes for intermediate and low temperature SOFCs. Int. J. Hydrogen Energy 2021, 46, 35923–35937. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Shubin, K.; Ermakovam, L.; Eremeev, N.; Farlenkov, A.; Khrustov, A.; Filonova, E.; Sadykov, V. Development of composite LaNi0.6Fe0.4O3−δ-based air electrodes for solid oxide fuel cells with a thin-film bilayer electrolyte. Int. J. Hydrogen Energy 2021, 46, 16947–16964. [Google Scholar] [CrossRef]
- Gordeev, E.; Belyakov, S.; Antonova, E.; Osinkin, D. Highly conductive Fe-doped (La,Sr)(Ga,Mg)O3−δ solid-state membranes for electrochemical application. Membranes 2023, 13, 502. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Antonova, E.P.; Porotnikova, N.M.; Bogdanovich, N.M. Features of the electrochemical reaction of hydrogen oxidation on the composite SrFeO3-based anode for a protonic ceramic fuel cell. Int. J. Energy Res. 2022, 46, 12597–12607. [Google Scholar] [CrossRef]
- Kotov, Y.A. Electric explosion of wires as a method for preparation of nanopowders. J. Nanopart. Res. 2003, 5, 539–550. [Google Scholar] [CrossRef]
- Osinkin, D.A. The parallel pathways of hydrogen oxidation reaction on high active decorated Ni–YSZ electrode in electrochemical cell with GDC protective layer. J. Electroanal. Chem. 2022, 927, 116999. [Google Scholar] [CrossRef]
- Gavrilyuk, A.L.; Osinkin, D.A.; Bronin, D.I. The use of Tikhonov regularization method for calculating the distribution function of relaxation times in impedance spectroscopy. Russ. J. Electrochem. 2017, 53, 575–588. [Google Scholar] [CrossRef]
- Niwa, E.; Uematsu, C.; Hashimoto, T. Sintering temperature dependence of conductivity, porosity and specific surfacearea of LaNi0.6Fe0.4O3 ceramics as cathode material for solid oxide fuel cells—Superiority of Pechini method among various solution mixing processes. Mater. Res. Bull. 2013, 48, 1–6. [Google Scholar] [CrossRef]
- Antonova, E.P.; Osinkin, D.A.; Bogdanovich, N.M.; Gorshkov, M.Y.; Bronin, D.I. Electrochemical performance of Ln2NiO4+δ (Ln-La, Nd, Pr) and Sr2Fe1.5Mo0.5O6−δ oxide electrodes in contact with apatite-type La10(SiO6)4O3 electrolyte. Solid State Ion. 2019, 329, 82–89. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Unraveling the influence of the electrolyte on the polarization resistance of nanostructured La0.6Sr0.4Co0.2Fe0.8O3−δ cathodes. Nanomaterials 2022, 12, 3936. [Google Scholar] [CrossRef] [PubMed]
- Pelosato, R.; Donazzi, A.; Dotelli, G.; Cristiani, C.; Sora, I.N.; Mariani, M. Electrical characterization of co-precipitated LaBaCo2O5+δ and YBaCo2O5+δ oxides. J. Eur. Ceram. Soc. 2014, 34, 4257–4272. [Google Scholar] [CrossRef]
- Antonova, E.P.; Khodimchuk, A.V.; Tropin, E.S.; Porotnikova, N.M.; Farlenkov, A.S.; Vlasov, M.I.; Ananyev, M.V. Influence of modifying additives on electrochemical performance of La2NiO4+δ-based oxygen electrodes. Solid State Ion. 2020, 346, 115215. [Google Scholar] [CrossRef]
- Hou, M.; Sun, W.; Li, P.; Feng, J.; Yang, G.; Qiao, J.; Wang, Z.; Rooney, D.; Feng, J.; Sun, K. Investigation into the effect of molybdenum-site substitution on the performance of Sr2Fe1.5Mo0.5O6−δ for intermediate temperature solid oxide fuel cells. J. Power Sources 2014, 272, 759–765. [Google Scholar] [CrossRef]
- Meng, F.; Xia, T.; Wang, J.; Shi, Z.; Lian, J.; Zhao, H.; Bassat, J.-M.; Grenier, J.-C. Evaluation of layered perovskites YBa1−xSrxCo2O5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 4531–4543. [Google Scholar] [CrossRef]
- Liu, X.; Han, D.; Zhou, Y.; Meng, X.; Wu, H.; Li, J.; Zeng, F.; Zhan, Z. Sc-substituted La0.6Sr0.4FeO3−δ mixed conducting oxides as promising electrodes for symmetrical solid oxide fuel cells. J. Power Sources 2014, 246, 457–463. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, K.; Liu, Y.; He, B. A novel layered perovskite as symmetric electrode for direct hydrocarbon solid oxide fuel cells. J. Power Sources 2017, 342, 313–319. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, T.; Yang, Z.; Han, M. Co-synthesis of LSCFN-GDC electrode for symmetric solid oxide fuel cell running on propane. Electrochim. Acta 2018, 265, 259–264. [Google Scholar] [CrossRef]
- Jin, F.; Li, L.; He, T. NdBaCo2/3Fe2/3Cu2/3O5+δ double perovskite as a novel cathode material for CeO2− and LaGaO3-based solid oxide fuel cells. J. Power Sources 2015, 273, 591–599. [Google Scholar] [CrossRef]
- Porras-Vazquez, J.M.; Kemp, T.F.; Hanna, J.V.; Slater, P.R. Synthesis and characterisation of oxyanion-doped manganites for potential application as SOFC cathodes. J. Mater. Chem. 2012, 22, 8287–8293. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Baratov, S.A.; Tarutina, L.R.; Vdovin, G.K.; Medvedev, D.A. Ba-doped Pr2NiO4+δ electrodes for proton-conducting electrochemical cells. Part 2: Transport and electrochemical properties. Int. J. Hydrogen Energy 2023, 48, 57, in press. [Google Scholar] [CrossRef]
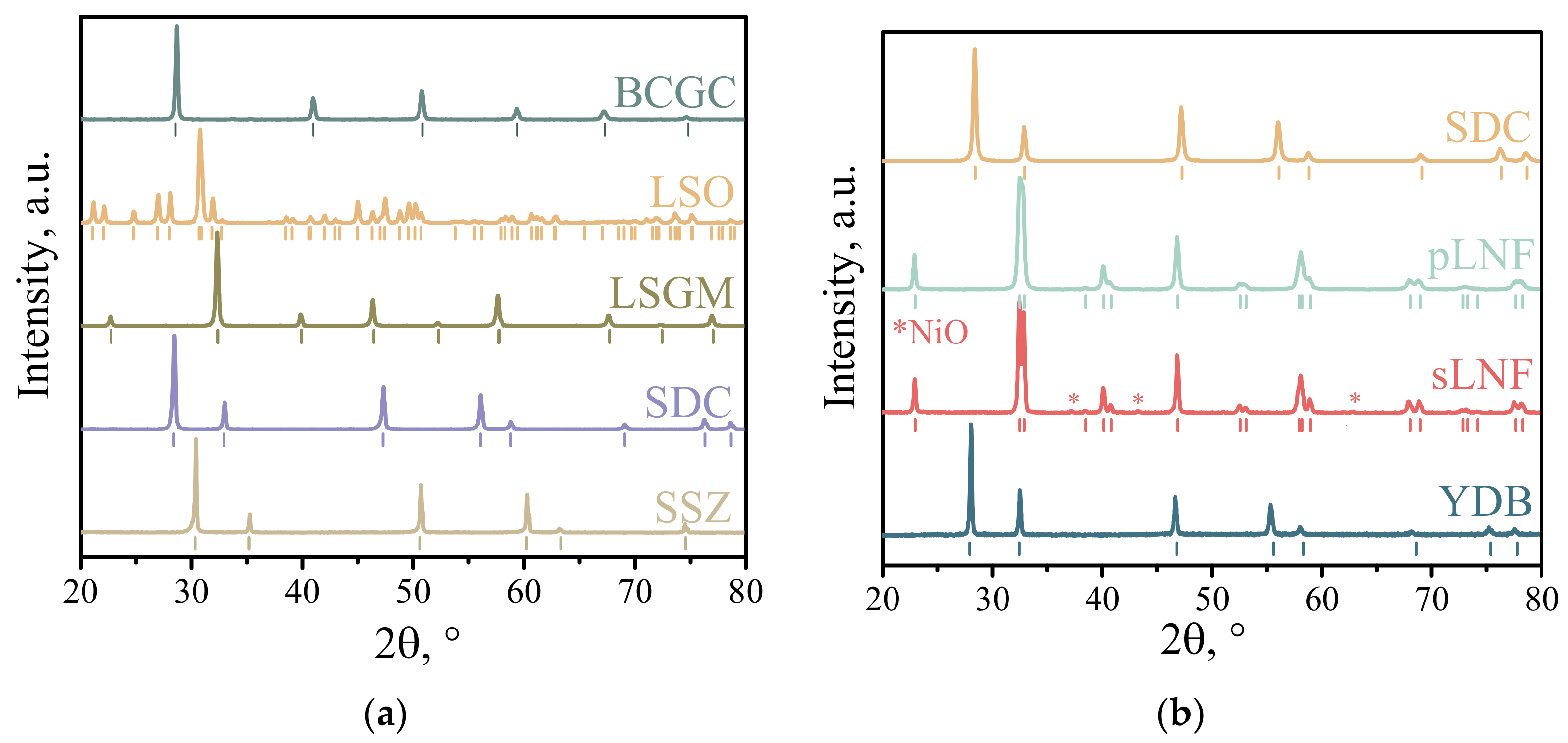
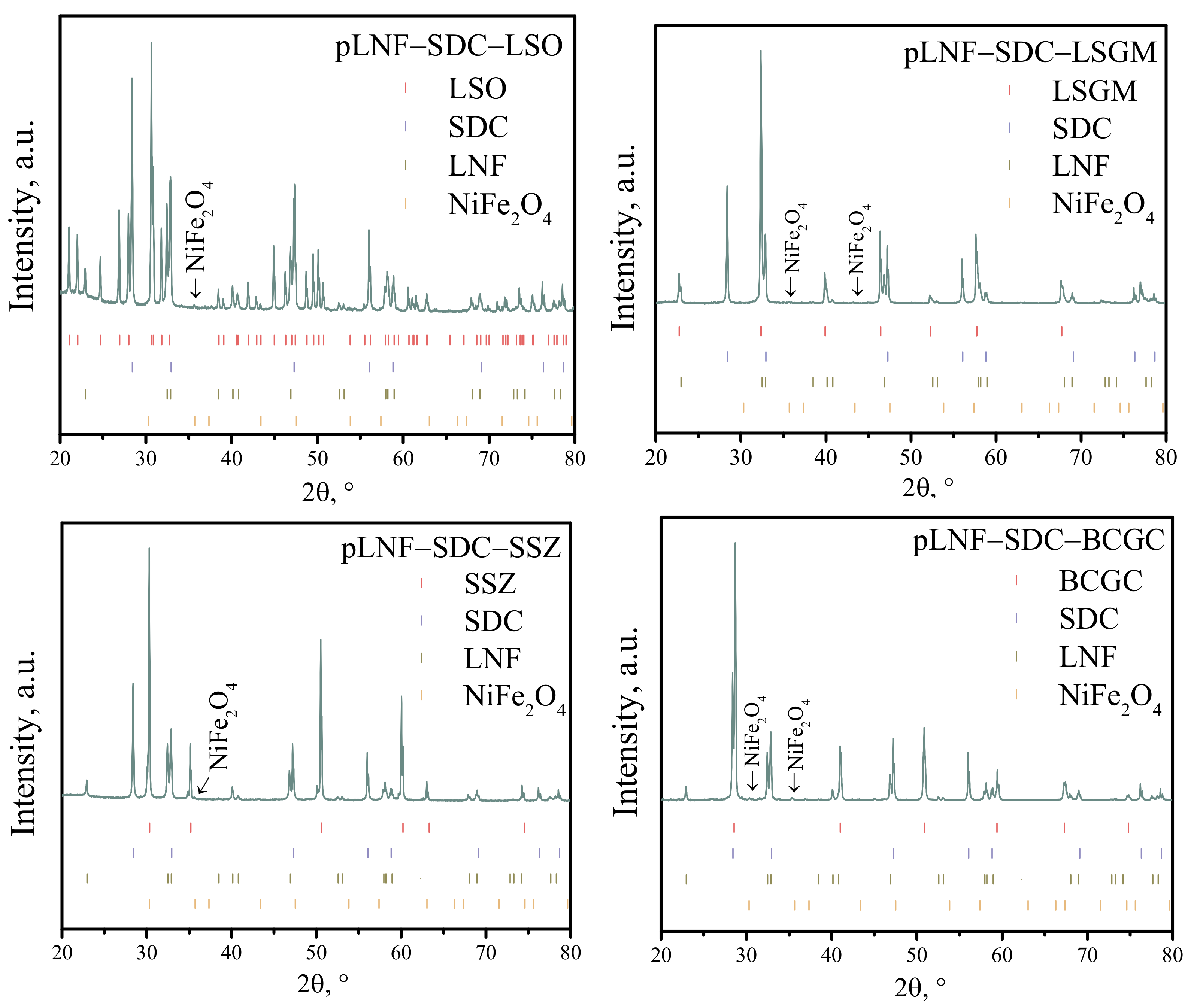
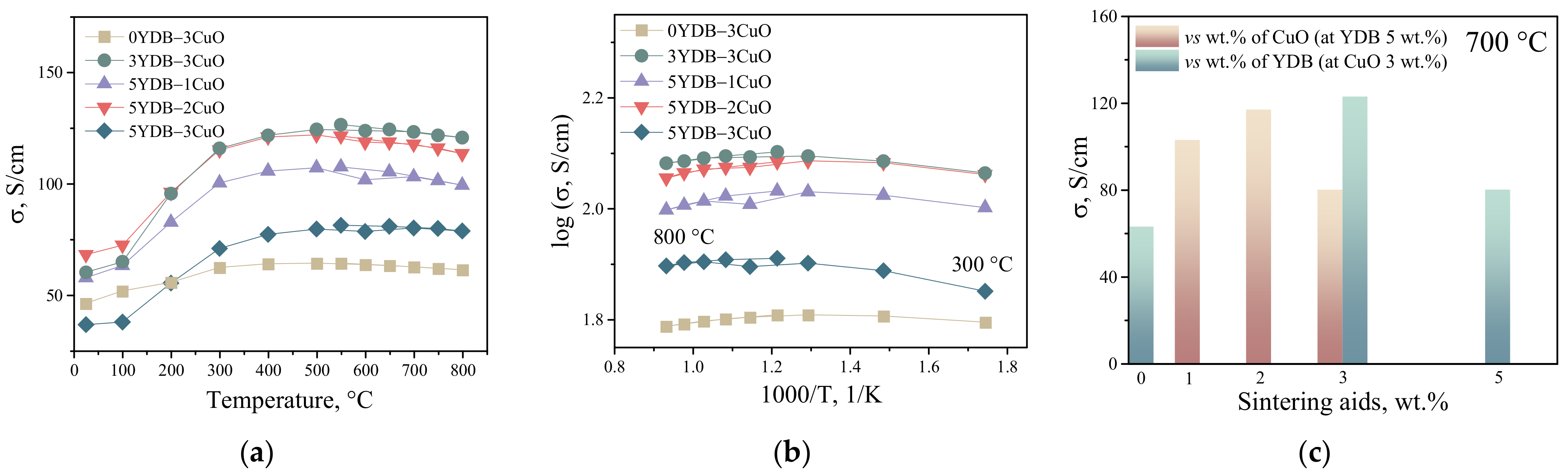

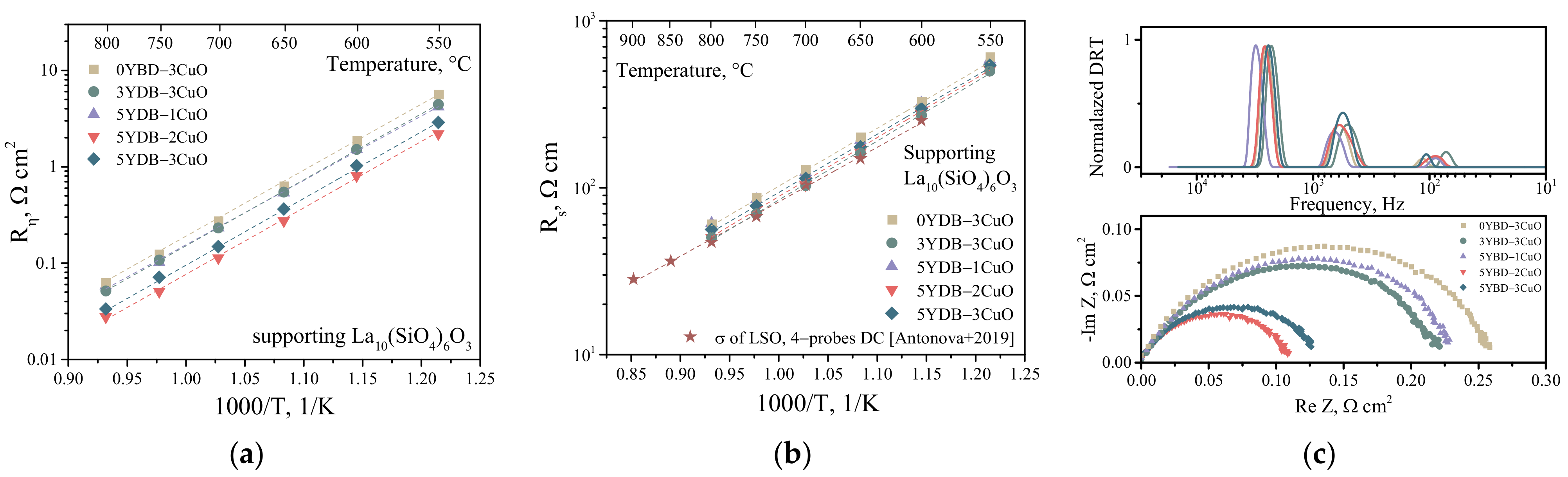

| Composition of the Collector Layer/wt.% | Designations |
|---|---|
| 60% sLNF + 32% pLNF + 5% YDB + 3% CuO | 5YDB–3CuO |
| 60% sLNF + 33% pLNF + 5% YDB + 2% CuO | 5YDB–2CuO |
| 60% sLNF + 34% pLNF + 5% YDB + 1% CuO | 5YDB–1CuO |
| 60% sLNF + 34% pLNF + 3% YDB + 3% CuO | 3YDB–3CuO |
| 60% sLNF + 37% pLNF + 3% CuO | 0YDB–3CuO |
| Sample | Supporting Electrolyte and Polarization Resistance (Ohm cm2) at 800/700/600 °C | ||||
|---|---|---|---|---|---|
| LSO | LSGM | SDC | SSZ | BCGC | |
| 0YDB–3CuO | 0.06/0.28/1.83 | 0.09/0.45/3.75 | 0.05/0.19/1.11 | 0.06/0.25/1.36 | 0.25/1.03/6.15 |
| 5YDB–2CuO | 0.03/0.11/0.79 | 0.02/0.13/1.27 | 0.02/0.08/0.47 | 0.02/0.07/0.39 | 0.04/0.19/0.89 |
| Composition | Polarization Resistance (Ohm cm2) | Reference |
|---|---|---|
| YBaCo2O5–δ | 0.13 (700 °C) | [43] |
| La2NiO4+δ + Pr2NiO4+δ | 0.15 (700 °C) | [44] |
| Sr2Fe1.5Mo0.5O6–δ | 0.14 (800 °C) | [45] |
| YBa0.8Sr0.2Co2O5-δ | 0.20 (700 °C) | [46] |
| La0.6Sr0.4Fe0.9Sc0.1O3-δ | 0.015 (800 °C) | [47] |
| PrBaMn2O5-δ | 0.30 (800 °C) | [48] |
| La0.4Sr0.6Co0.2Fe0.7Nb0.1O3–δ + GDC | 0.034 (800 °C) | [49] |
| NdBaCo2/3Fe2/3Cu2/3O5+δ | 0.05 (800 °C) | [50] |
| CaMn0.95P0.05O3−δ | 0.3 (800 °C) | [51] |
| Pr1.7Ba0.3NiO4+δ | 0.4 (700 °C) | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osinkin, D.; Bogdanovich, N. Sintering Aid Strategy for Promoting Oxygen Reduction Reaction on High-Performance Double-Layer LaNi0.6Fe0.4O3–? Composite Electrode for Devices Based on Solid-State Membranes. Membranes 2023, 13, 603. https://doi.org/10.3390/membranes13060603
Osinkin D, Bogdanovich N. Sintering Aid Strategy for Promoting Oxygen Reduction Reaction on High-Performance Double-Layer LaNi0.6Fe0.4O3–? Composite Electrode for Devices Based on Solid-State Membranes. Membranes. 2023; 13(6):603. https://doi.org/10.3390/membranes13060603
Chicago/Turabian StyleOsinkin, Denis, and Nina Bogdanovich. 2023. "Sintering Aid Strategy for Promoting Oxygen Reduction Reaction on High-Performance Double-Layer LaNi0.6Fe0.4O3–? Composite Electrode for Devices Based on Solid-State Membranes" Membranes 13, no. 6: 603. https://doi.org/10.3390/membranes13060603
APA StyleOsinkin, D., & Bogdanovich, N. (2023). Sintering Aid Strategy for Promoting Oxygen Reduction Reaction on High-Performance Double-Layer LaNi0.6Fe0.4O3–? Composite Electrode for Devices Based on Solid-State Membranes. Membranes, 13(6), 603. https://doi.org/10.3390/membranes13060603







