A Combined Gas and Water Permeances Method for Revealing the Deposition Morphology of GO Grafting on Ceramic Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of a-Alumina Disks
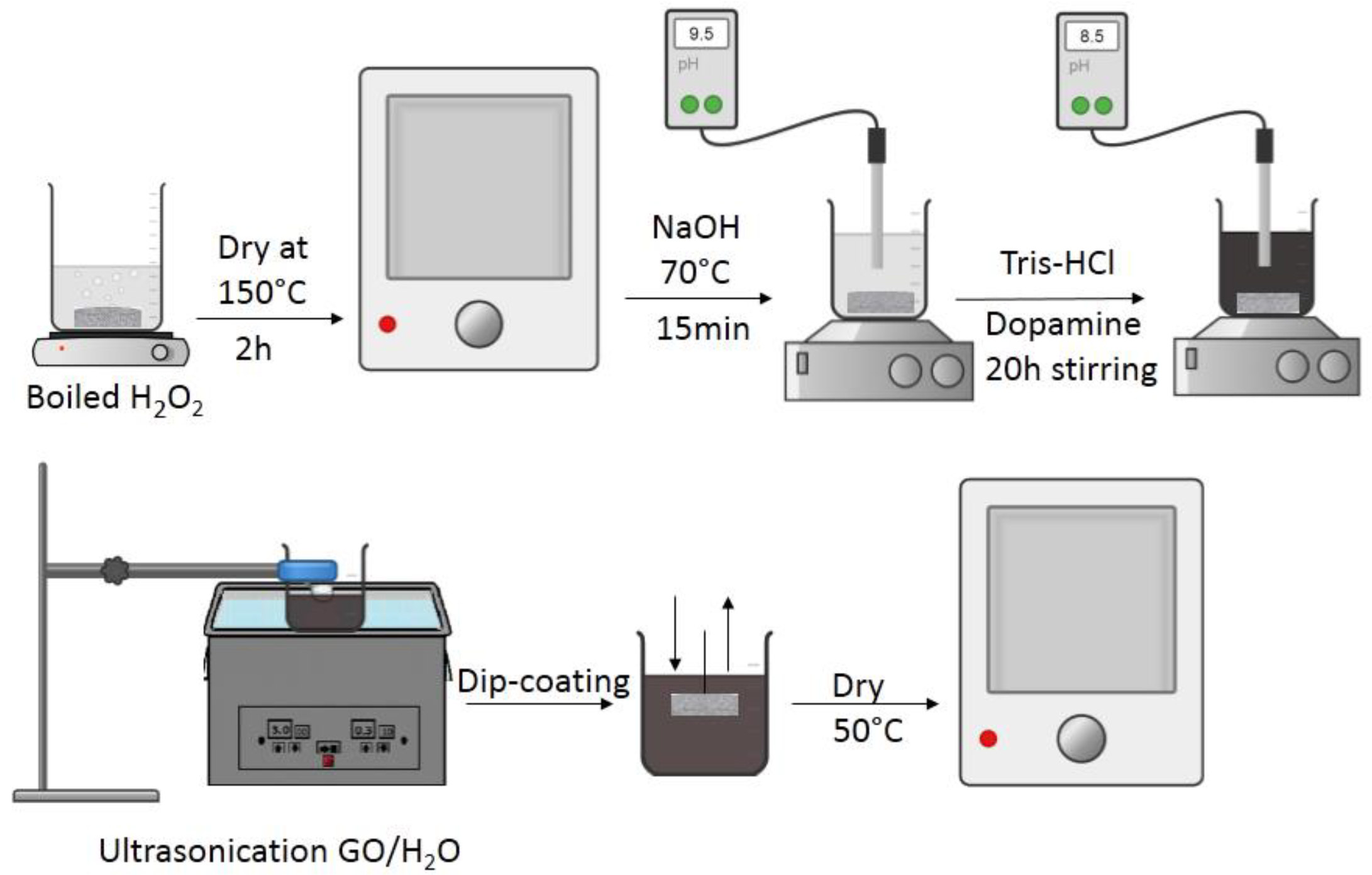
2.1.1. Preparation of Composite Membranes with PDA as Linker
2.1.2. Preparation of Composite Membranes with APTMS as Linker
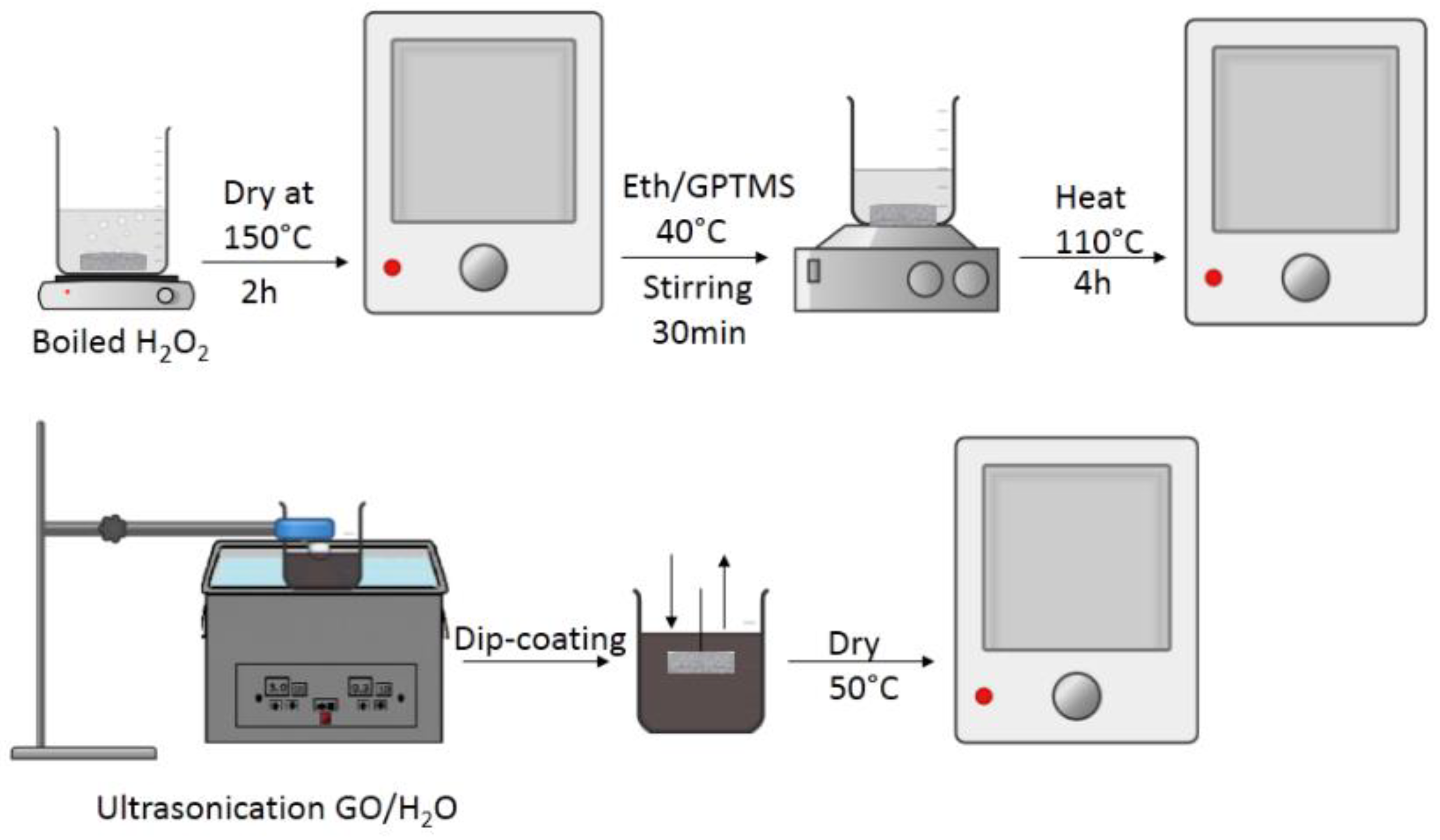
2.1.3. Preparation of Composite Membranes with GPTMS as Linker
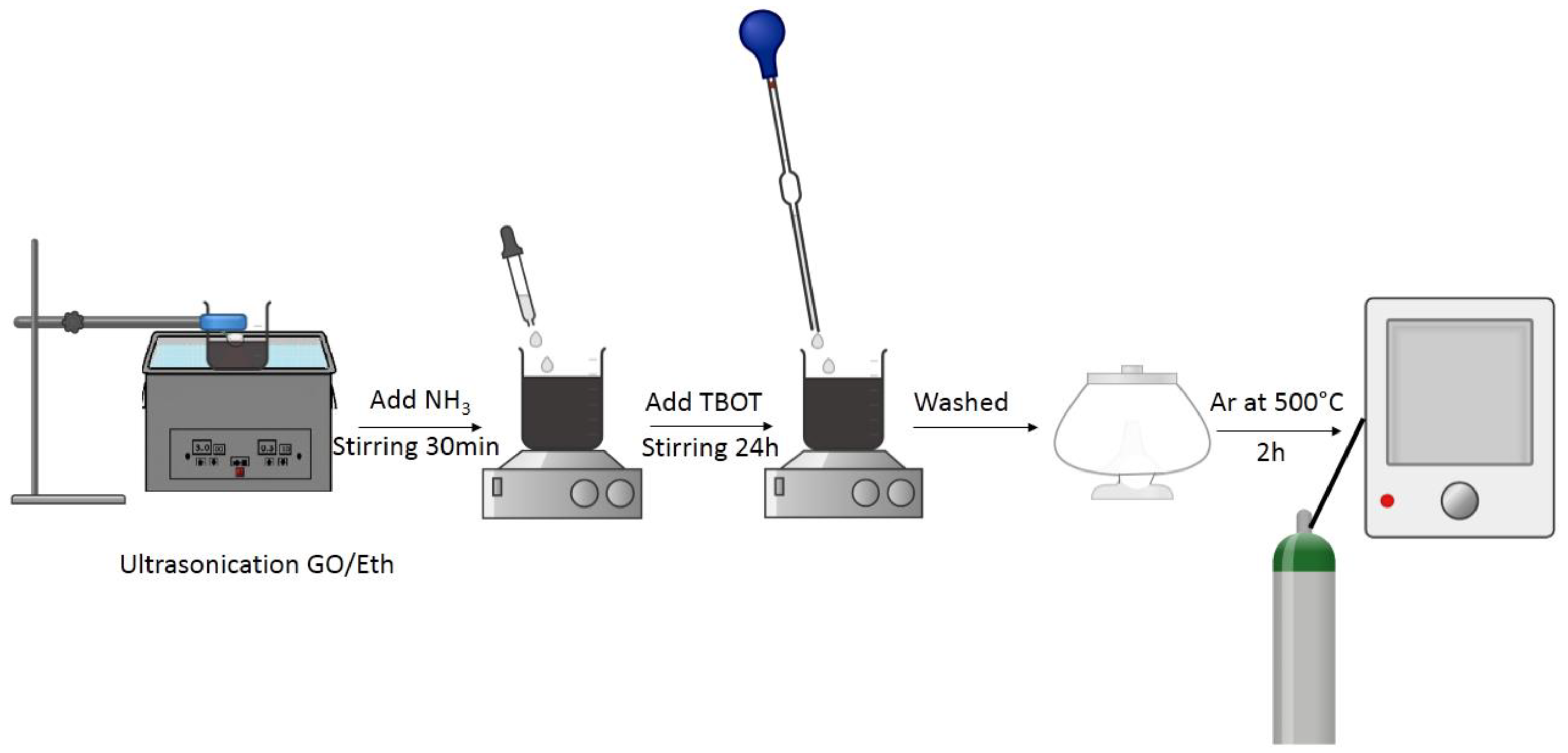
2.1.4. Preparation of Composite Membranes with Vacuum Filtration Method and EDA as Linker between the GO Sheets
2.2. Filtering Device
2.3. Gas Permeance Device
2.4. Water Permeability Device
2.5. Characterization Techniques
3. Results
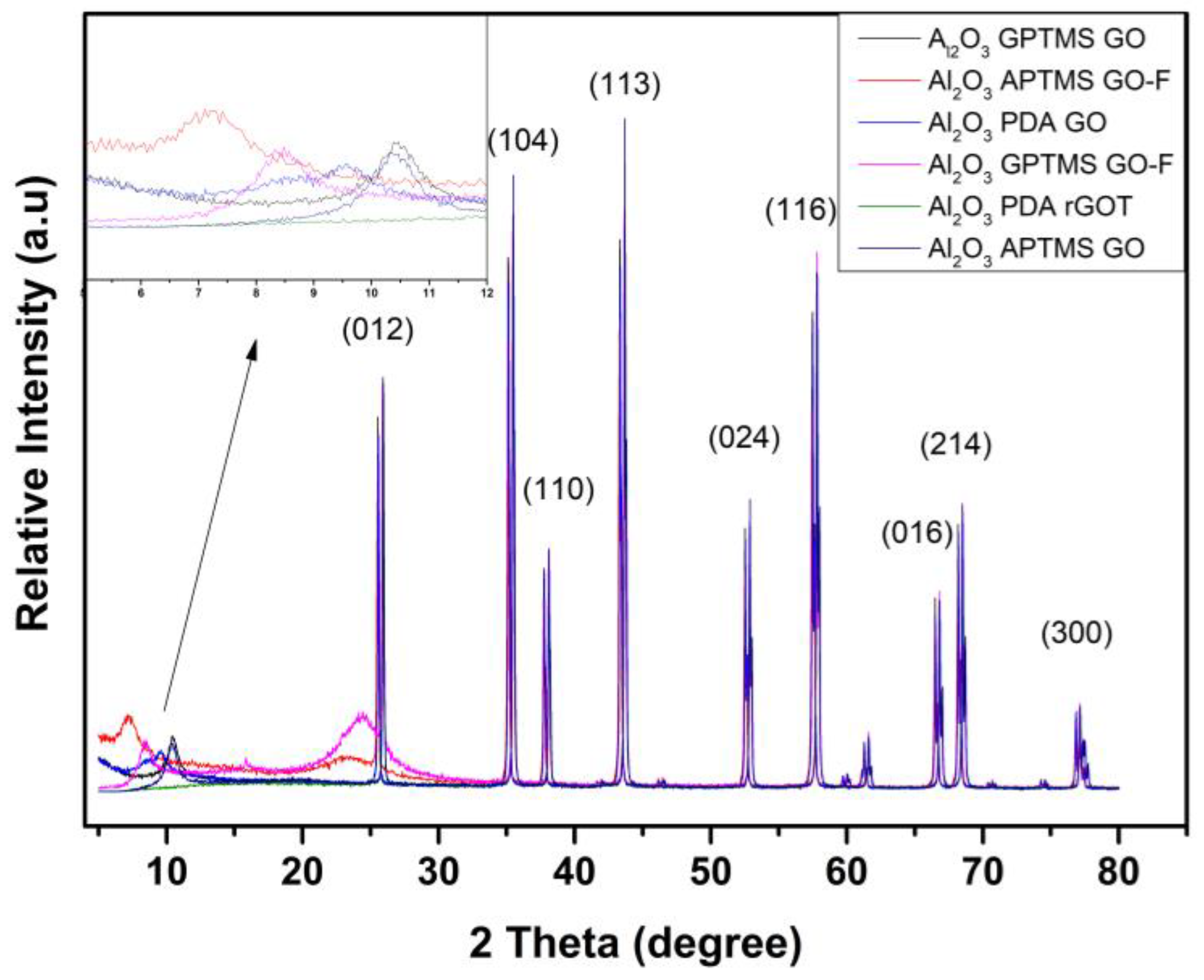
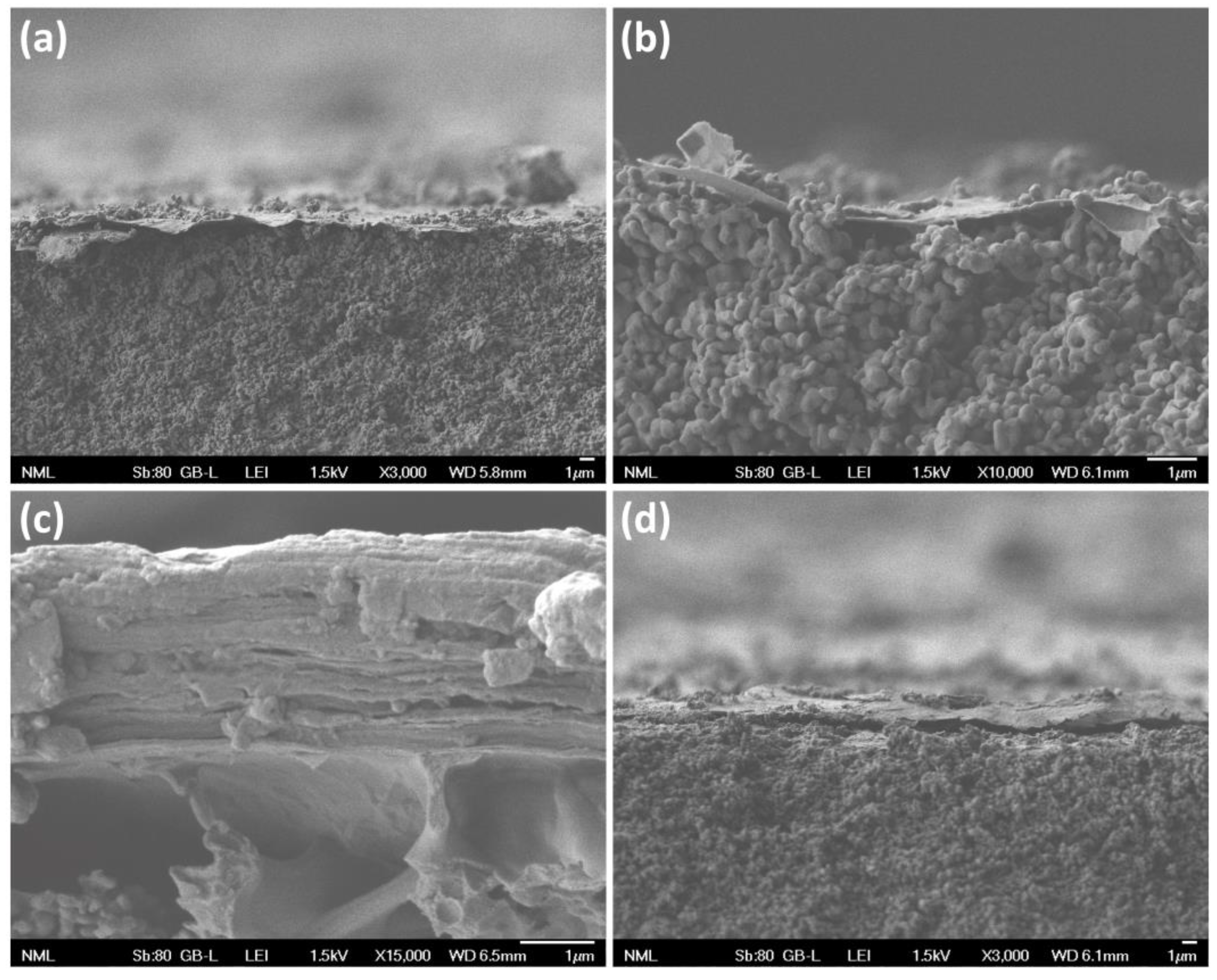
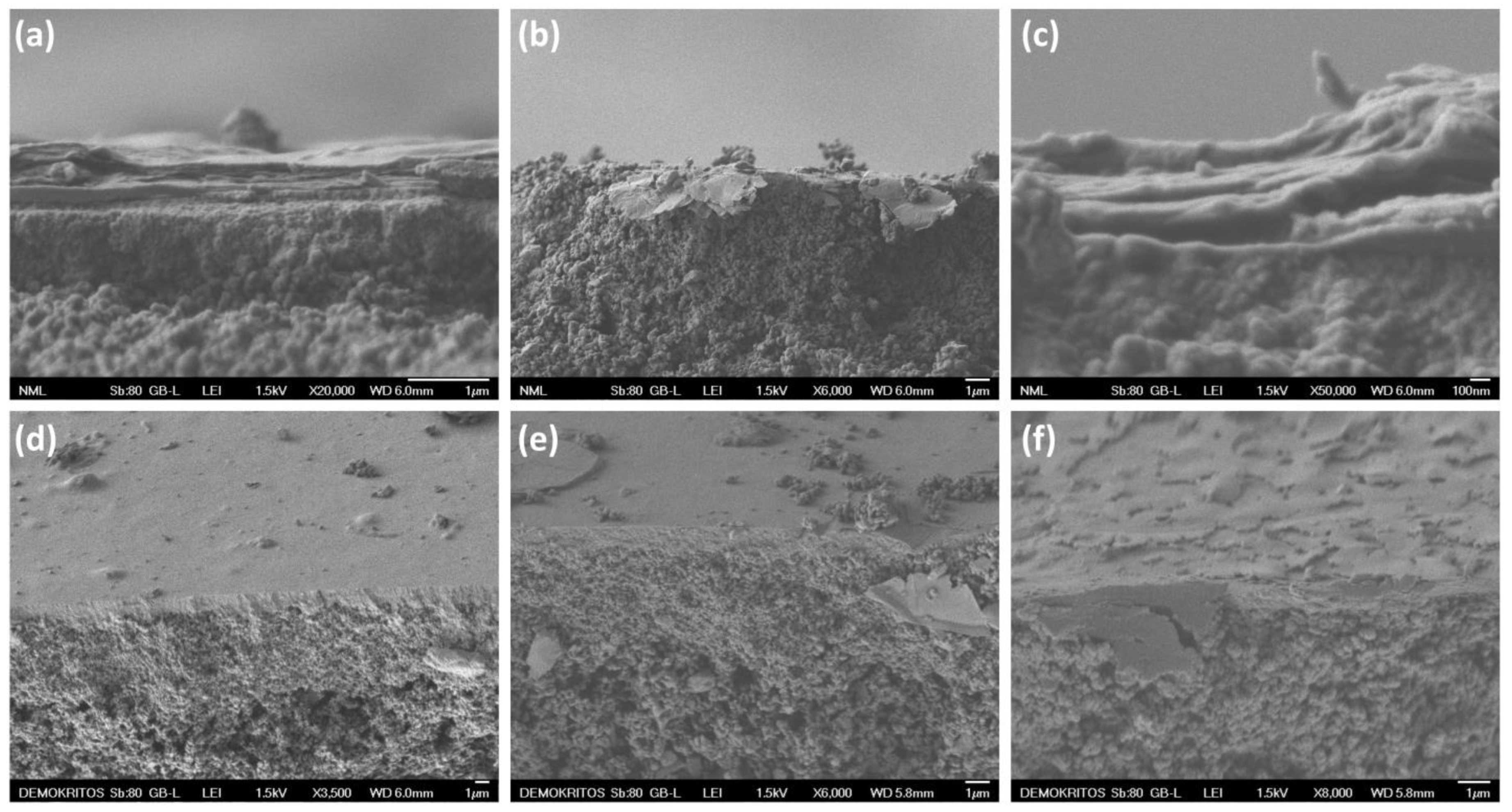
3.1. Structural and Morphological Properties
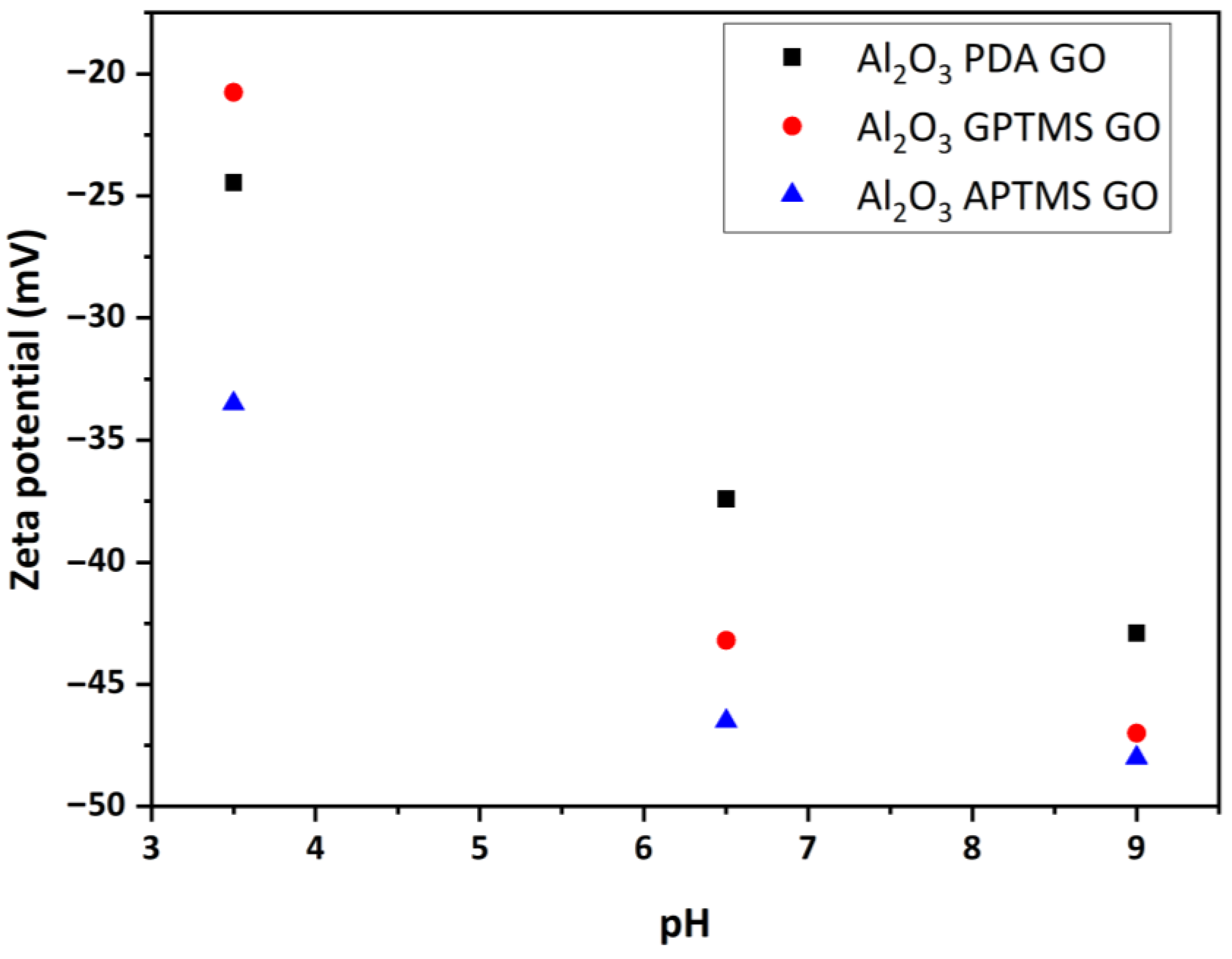
3.2. Surface Properties
3.3. Analysis of He and H2O Permeability
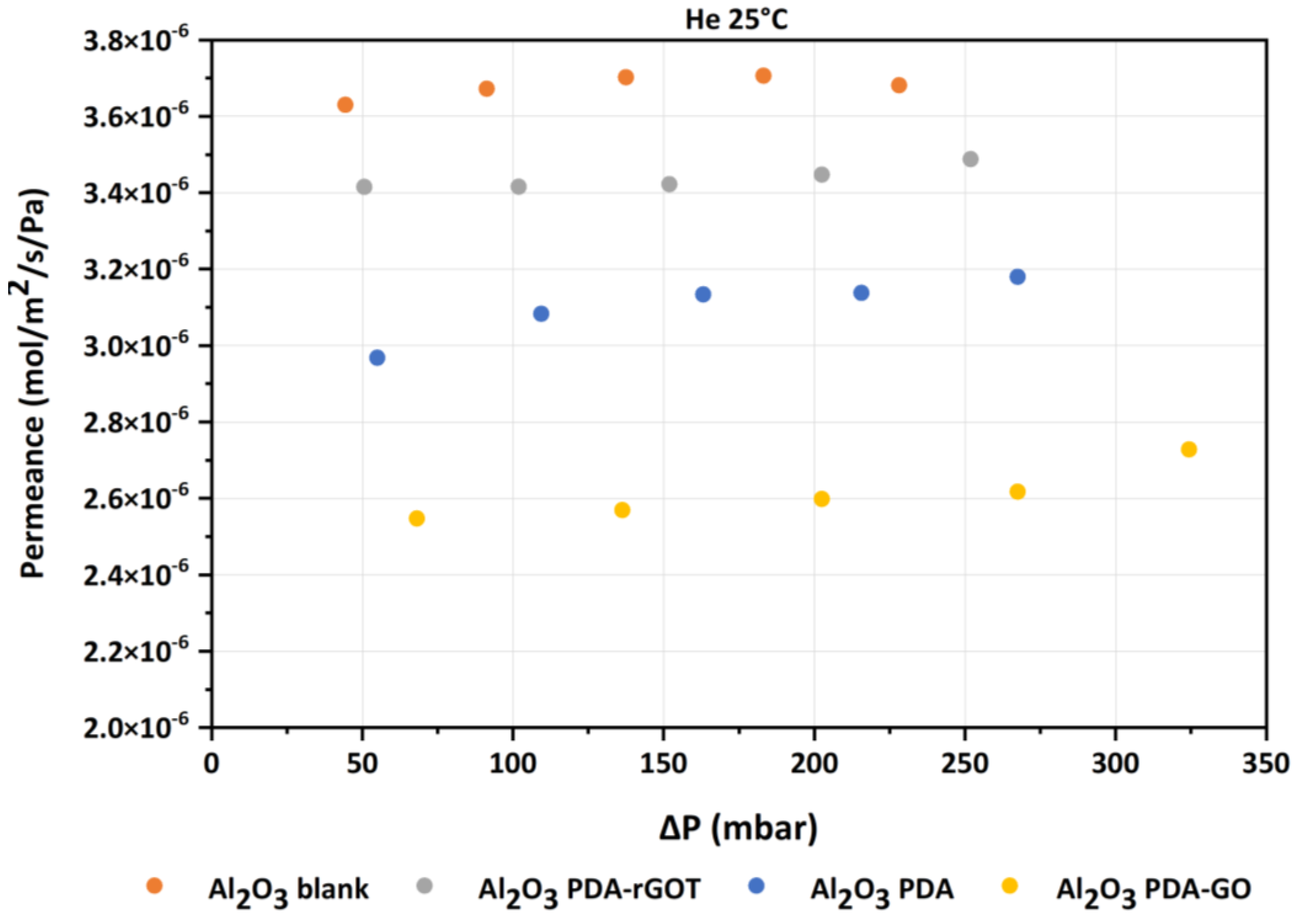
3.3.1. Comparison of the Bare Substrates
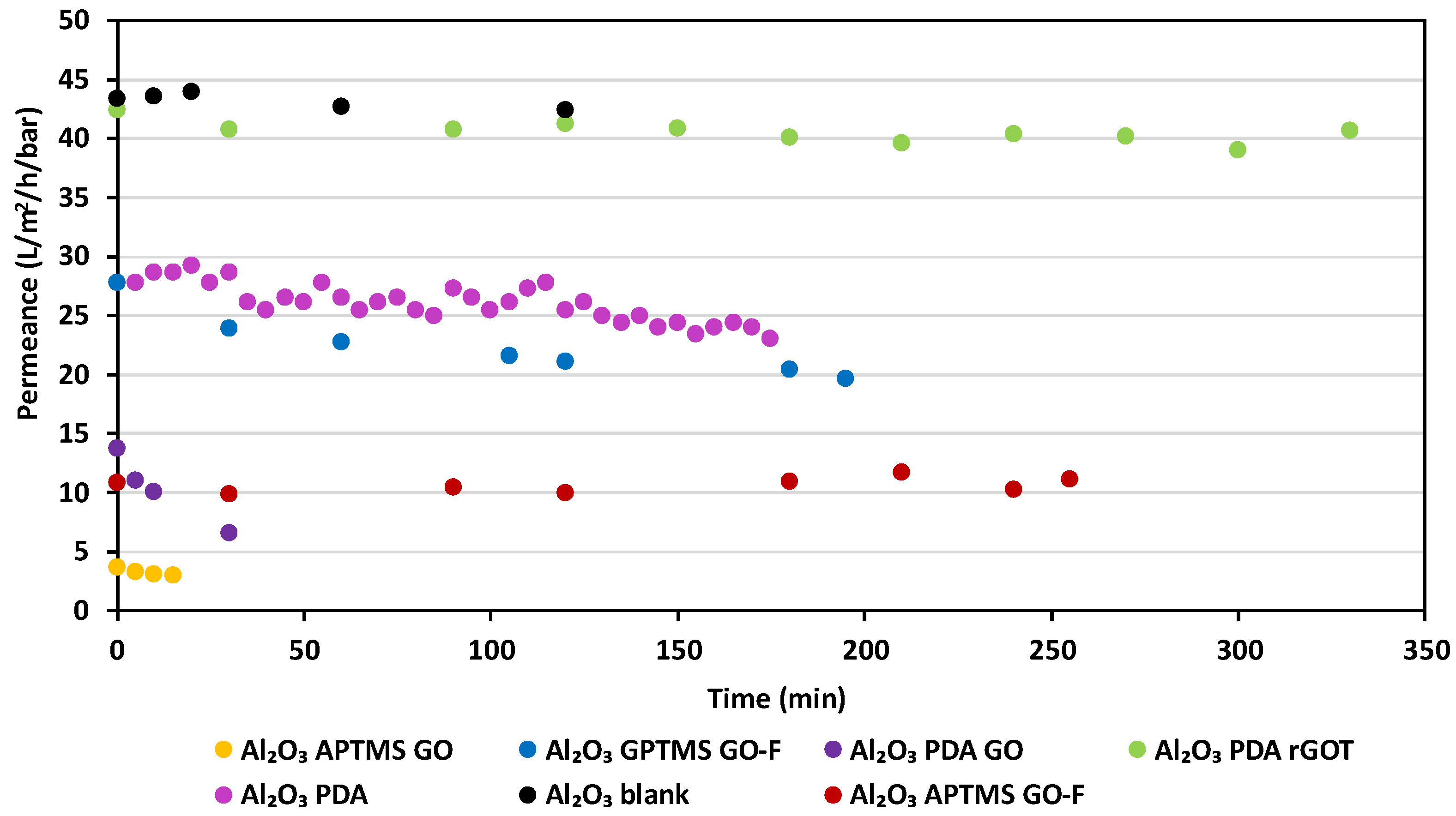
3.3.2. Comparison of the GO–Ceramic Composite Membranes
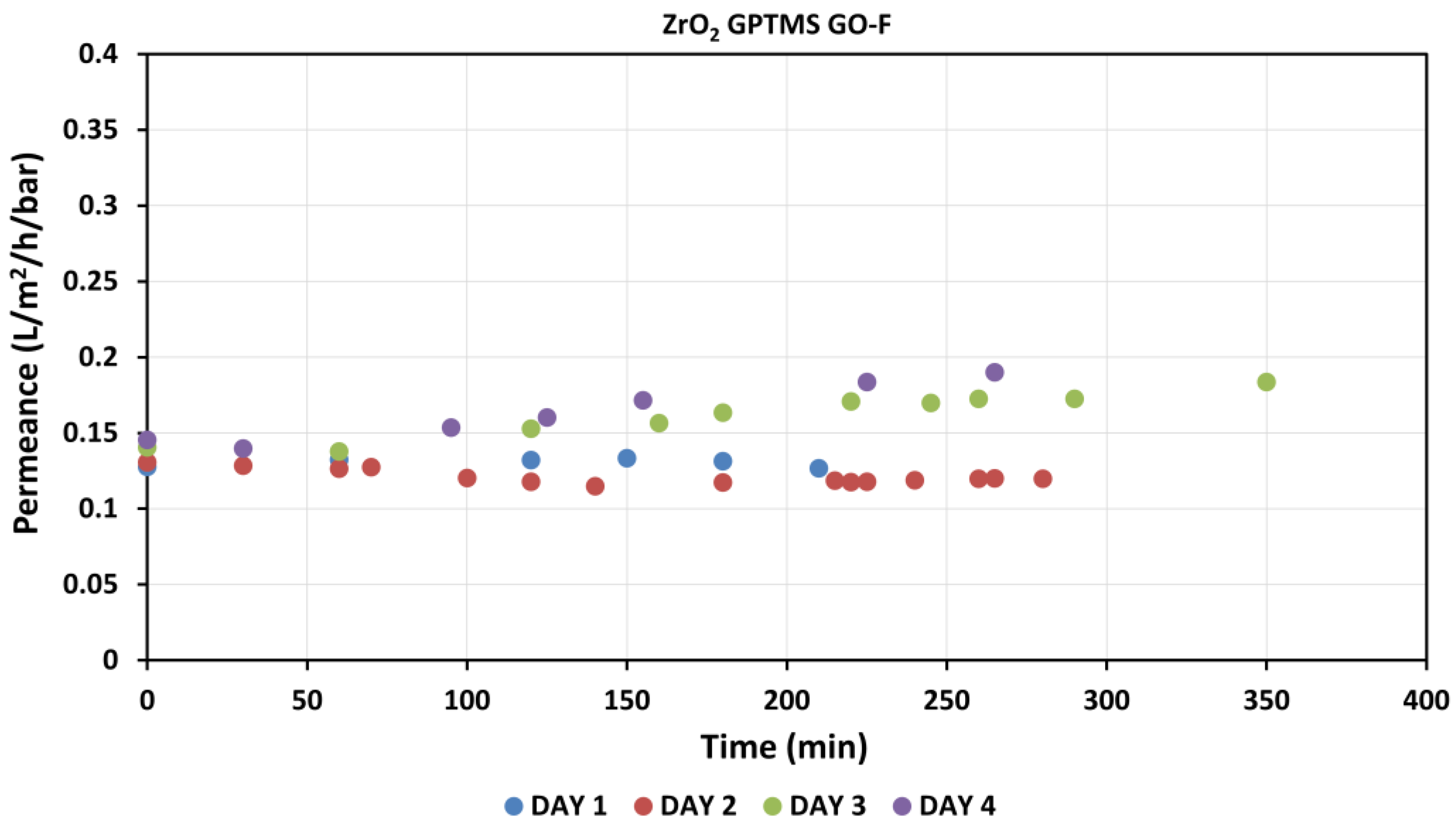
3.3.3. Stability of the GO–Ceramic Composite Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hung, W.-S.; Tsou, C.-H.; Guzman, M.D.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-Linking with Diamine Monomers To Prepare Composite Graphene Oxide-Framework Membranes with Varying d Spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Pedico, A.; Baudino, L.; Aixalà-Perelló, A.; Lamberti, A. Green Methods for the Fabrication of Graphene Oxide Membranes: From Graphite to Membranes. Membranes 2023, 13, 429. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A.; Abussaud, B.; Aljundi, I.H. A Simple Approach to Fabricate Composite Ceramic Membranes Decorated with Functionalized Carbide-Derived Carbon for Oily Wastewater Treatment. Membranes 2022, 12, 394. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Feng, B.; Zhou, C.; Huang, A. Synthesis of highly stable graphene oxide membranes on polydopamine functionalized supports for seawater desalination. Chem. Eng. Sci. 2016, 146, 159–165. [Google Scholar] [CrossRef]
- Zhang, M.; Mao, Y.; Liu, G.; Liu, G.; Fan, Y.; Jin, W. Molecular Bridges Stabilize Graphene Oxide Membranes in Water. Angew. Chem. 2019, 59, 1689–1695. [Google Scholar] [CrossRef]
- Karim, A.; Achiou, B.; Bouazizi, A.; Aaddane, A.; Ouammou, M.; Bouziane, M.; Bennazha, J. Development of reduced graphene oxide membrane on flat Moroccan ceramic pozzolan support. Application for soluble dyes removal. J. Environ. Chem. Eng. 2018, 6, 1475–1485. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, G.; Liu, S.; Shen, J.; Jin, W. A facile way to prepare ceramic-supported graphene oxide composite membrane via silane-graft modification. Appl. Surf. Sci. 2014, 307, 631–637. [Google Scholar] [CrossRef]
- Gu, Q.; Ng, T.C.A.; Zain, I.; Liu, X.; Zhang, L.; Zhang, Z.; Lyu, Z.; He, Z.; Ng, H.Y.; Wang, J. Chemical-grafting of graphene oxide quantum dots (GOQDs) onto ceramic microfiltration membranes for enhanced water permeability and antiorganic fouling potential. Appl. Surf. Sci. 2020, 502, 144128. [Google Scholar] [CrossRef]
- Galata, E.; Veziri, C.M.; Theodorakopoulos, G.V.; Romanos, G.E.; Pavlatou, E.A. Composite GO/Ceramic Membranes Prepared via Chemical Attachment: Characterisation and Gas Permeance Properties. Membranes 2022, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M. Basic Principles of Membrane Technology; Center for Membrane Science and Technology, University of Twente: Enschede, The Netherlands; Springer Science+Business Media: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Lai, Z.; Tsapatsis, M.; Nicolich, J.P. Siliceous ZSM-5 Membranes by Secondary Growth of b-Oriented Seed Layers. Adv. Funct. Mater. 2004, 14, 716–729. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, N.; Caro, J.; Huang, A. Bio-Inspired Polydopamine: A Versatile and Powerful Platform for Covalent Synthesis of Molecular Sieve Membranes. J. Am. Soc. 2013, 135, 17679–17682. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, F.; Feng, S.; Wang, J.; Sun, Z.; Li, B.; Li, Y.; Yang, J.; Elzatahry, A.A.; Xia, Y.; et al. Sol−Gel Design Strategy for Ultradispersed TiO2 Nanoparticles on Graphene for High-Performance Lithium Ion Batteries. J. Am. Chem. Soc. 2013, 135, 18300–18303. [Google Scholar] [CrossRef]
- Zhou, F.; Tien, H.N.; Dong, Q.; Xu, W.L.; Li, H.; Li, S.; Yu, M. Ultrathin, ethylenediamine-functionalized graphene oxide membranes on hollow fibers for CO2 capture. J. Membr. Sci. 2019, 573, 184–191. [Google Scholar] [CrossRef]
- Romanos, G.; Pastrana-Martínez, L.M.; Tsoufis, T.; Athanasekou, C.; Galata, E.; Katsaros, F.; Favvas, E.; Beltsios, K.G.; Siranidi, E.; Falaras, P.; et al. A facile approach for the development of fine-tuned self-standing graphene oxide membranes and their gas and vapor separation performance. J. Membr. Sci. 2015, 493, 734–747. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Pedrosa, M.F.; Silva, A.M.; Psycharis, V.P.; Romanos, G.E. Mild temperature-gas separation performance of graphene oxide membranes for extended period: Micropore to meso- and macropore readjustments and the fate of membranes under the influence of dynamic graphene oxide changes. Chem. Eng. J. Adv. 2021, 5, 100066. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Shi, B.; Guo, W.; Jaroniec, M.; Qiao, S.Z. A Regularly Channeled Lamellar Membrane for Unparalleled Water and Organics Permeation. Angewanndte Chem. 2018, 57, 6814–6818. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. The Reflection of X-rays by Crystals; Royal Society of London: London, UK, 1913; Volume A. [Google Scholar]
- Scherrer, P. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen. Math.-Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Vural, S.; Sari, Ö. Synthesis and characterization of SDS assistant α-alumina structures and investigation of the effect of the calcination time on the morphology. Colloid Polym. Sci. 2019, 297, 107–114. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, J.; Jiang, D. Effects of surface modification and cross-linked graphene oxide with ethylenediamine on electrical properties of reduced graphene oxide films. In Proceedings of the 21 International Conference on Composite Materials, Xi’an, China, 20–25 August 2017. [Google Scholar]
- Kashyap, S.; Mishra, S.; Behera, S.K. Aqueous Colloidal Stability of Graphene Oxide and Chemically Converted Graphene. J. Nanoparticles 2014, 2014, 640281. [Google Scholar] [CrossRef]
- Feng, Y.; Huynh, K.A.; Xie, Z.; Liu, G.; Gao, S. Heteroaggregation and sedimentation of graphene oxide with hematite colloids: Influence of water constituents and impact on tetracycline adsorption. Sci. Total Environ. 2018, 647, 708–715. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, G.K. Pre-post redox electron transfer regioselectivity at the alanine modified nano graphene electrode interface. Chem. Phys. Lett. 2022, 789, 139295. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Albetran, H.M.; Abdelrahman, B.H.; Al-Yaseri, A.; Yekeen, N.; Low, I.M. Wettability of Nanostructured Transition-Metal Oxide (Al2O3, CeO2, and AlCeO3) Powder Surfaces. Materials 2022, 15, 5485. [Google Scholar] [CrossRef]
- Chau, T.T.; Bruckard, W.J.; Koh, P.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef]
- Huizenga, D.G.; Smith, D.M. Knudesen diffusion in random assemblages of uniform spheres. AlChe J. 1986, 1, 1–6. [Google Scholar] [CrossRef]
- Chee, D.N.A.; Ismail, A.F.; Aziz, F.; Amin, M.A.M.; Abdullah, N. The influence of alumina particle size on the properties and performance of alumina hollow fiber as support membrane for protein separation. Sep. Purif. Technol. 2020, 250, 117–147. [Google Scholar]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123–513. [Google Scholar] [CrossRef]
- Wen, X.; Foller, T.; Jin, X.; Musso, T.; Kumar, P.; Joshi, R. Understanding water transport through graphene-based nanochannels via experimental control of slip length. Nat. Commun. 2022, 13, 5690. [Google Scholar] [CrossRef]
- Gong, D.; Liu, X.; Wu, P.; Wang, Y.; Guo, B.; Liu, S.; Chen, H.; Yin, Y.; Liu, G.; Liu, M.; et al. Water pumping effect over the organic ions defined graphene oxide membrane impulses high flux desalination. NPJ Clean Water 2022, 5, 68. [Google Scholar] [CrossRef]

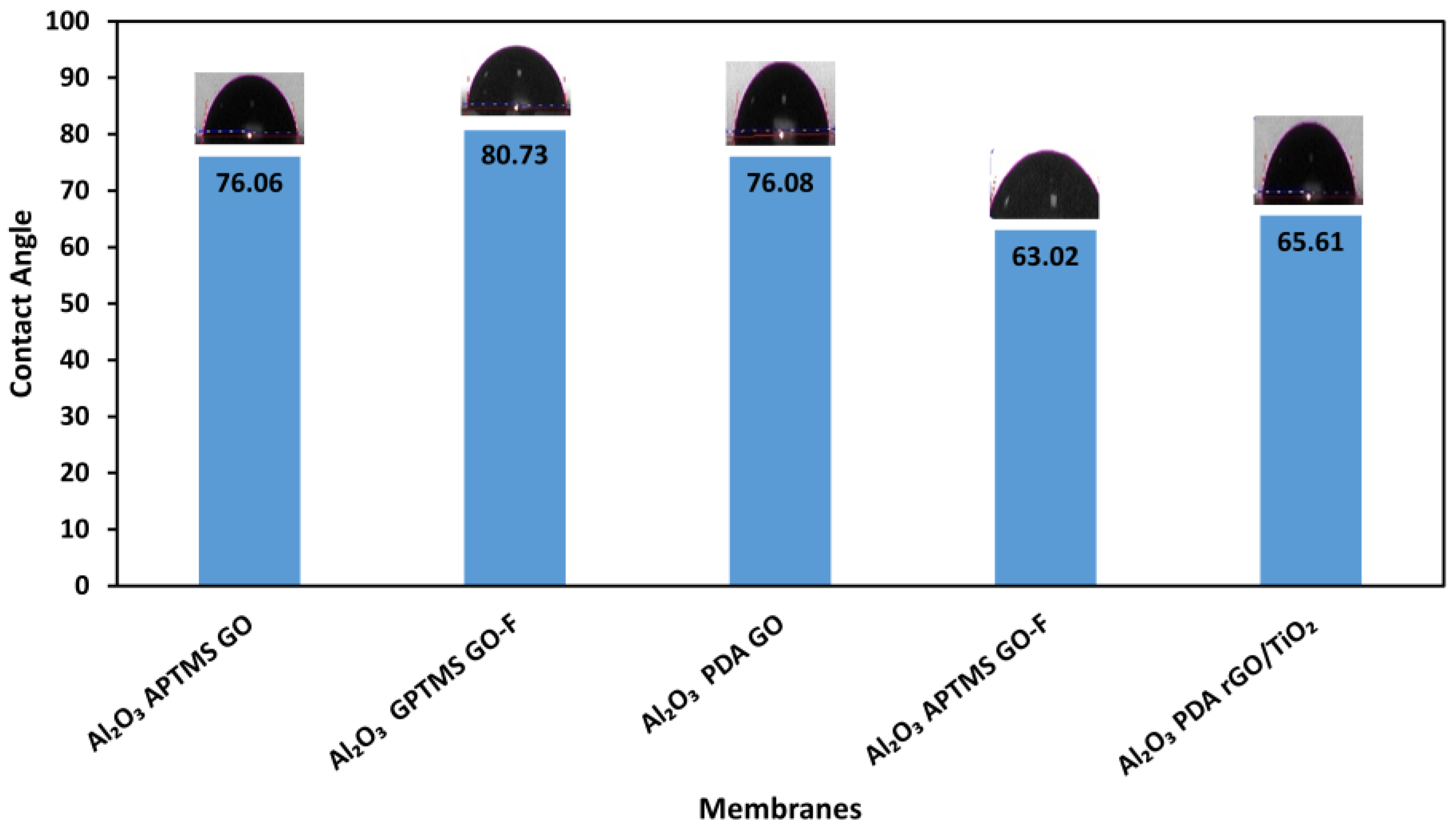



| Composite Membrane | Water Contact Angle (°) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Mean | |
| Al2O3 GPTMS-GO-F | 86.40 | 68.06 | 87.74 | 80.73 |
| Al2O3 PDA-GO | 73.28 | 78.86 | 82.99 | 76.08 |
| Al2O3 APTMS-GO | 77.95 | 72.73 | 77.50 | 76.06 |
| Al2O3 PDA-rGOT | 77.49 | 61.13 | 58.22 | 65.61 |
| Al2O3 APTMS-GO-F | 70.10 | 60.18 | 58.79 | 63.02 |
| Samples | Peg (mol/m2/s/Pa) | Pg (mol·m/m2/s/Pa) | from Gas Permeability | (L·m/m2/h/bar) | (L·m/m2/h/bar) | Thickness (nm) | Pore Dimension (nm) | ||
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 PDA | 2.90 × 10−6 | 5.8 × 10−9 | 1.08 | 25 | 5.00 × 10−2 | 2 × 106 | 61.5 | 1.38 | 1.74 |
| Al2O3 blank | 3.70 × 10−6 | 7.4 × 10−9 | 1.00 | 43.5 | 8.70 × 10−2 | 2 × 106 | 66.7 | 1.00 | 1.00 |
| Al2O3 APTMS-GO | 1.74 × 10−6 | 1.74 × 10−13 | 34.91 | 3.25 | 3.25 × 10−2 | 100 | 1.91 | 1.48 × 106 | 2.68 × 105 |
| Al2O3 PDA-GO | 2.60 × 10−6 | 5.2 × 10−13 | 24.23 | 9.75 | 1.95 × 10−6 | 200 | 2.75 | 3.45 × 105 | 4.46 × 104 |
| Al2O3 GPTMS GO | 4.80 × 10−7 | 1.92 × 10−14 | 72.77 | - | 0.00 | 40 | 0.92 | 2.80 × 107 | - |
| Al2O3 GPTMS GO-F | 1.20 × 10−6 | 5.64 × 10−12 | 10.95 | 28 | 1.32 × 10−4 | 4700 | 6.09 | 1.44 × 104 | 6.61 × 102 |
| Al2O3 APTMS GO-F | 1.20 × 10−6 | 6 × 10−13 | 23.10 | 6 | 3.00 × 10−6 | 500 | 2.89 | 2.85 × 105 | 2.90 × 104 |
| Al2O3 PDA rGOT | 3.42 × 10−6 | 2.4 × 10−13 | 31.38 | 38.7 | 2.71 × 10−6 | 70 | 2.12 | 9.70 × 105 | 3.21 × 104 |
| ZrO2 blank | 1.09 × 10−6 | 1.09 × 10−12 | 1.00 | 4.03 | 4.03 × 10−6 | 1000 | 3.00 | 1.00 | - |
| ZrO2 GPTMS GO | 2.36 × 10−7 | 1.77 × 10−14 | 3.95 | 0.074 | 5.55 × 10−9 | 75 | 0.76 | 2.43 × 102 | 7.26 × 102 |
| ZrO2 GPTMS GO-F | 1.60 × 10−8 | 6.4 × 10−15 | 5.54 | 0.155 | 6.20 × 10−8 | 400 | 0.54 | 9.44 × 102 | 6.50 × 101 |
| He | He | He | He | He | |
|---|---|---|---|---|---|
| T (°C) | 25 | 25 | 25 | 25 | 25 |
| Al2O3 blank P (mbar) | 44.375 | 91.25 | 137.5 | 183.125 | 228.125 |
| Permeance (mol/m2/s/Pa) | 3.63 × 10−6 | 3.67 × 10−6 | 3.70 × 10−6 | 3.70 × 10−6 | 3.68 × 10−6 |
| Al2O3 APTMS-GO | 100 | 149.375 | 198.125 | 246.875 | 295 |
| 1.70 × 10−6 | 1.72 × 10−6 | 1.74 × 10−6 | 1.74 × 10−6 | 1.75 × 10−6 | |
| Al2O3 APTMS-GO-F | 41.875 | 72.5 | 145.625 | 173.125 | 214.375 |
| 1.11 × 10−6 | 1.13 × 10−6 | 1.20 × 10−6 | 1.22 × 10−6 | 1.24 × 10−6 | |
| Al2O3 GPTMS-GO | 170 | 340 | 400 | 495 | 600 |
| 4.63 × 10−7 | 4.87 × 10−7 | 5.01 × 10−7 | 5.07 × 10−7 | 5.65 × 10−7 | |
| Al2O3 GPTMS-GO-F | 70.625 | 143.125 | 210.625 | 279.375 | 346.25 |
| 1.19 × 10−6 | 1.20 × 10−6 | 1.20 × 10−6 | 1.23 × 10−6 | 1.23 × 10−6 | |
| Al2O3 PDA-GO | 68.125 | 136.25 | 202.5 | 268 | 324.375 |
| 2.54 × 10−6 | 2.56 × 10−6 | 2.59 × 10−6 | 2.62 × 10−6 | 2.72 × 10−6 | |
| Al2O3 PDA-rGOT | 50.625 | 101.875 | 151.875 | 202.5 | 251.875 |
| 3.41 × 10−6 | 3.411 × 10−6 | 3.42 × 10−6 | 3.44 × 10−6 | 3.48 × 10−6 | |
| ZrO2 blank | 105.38 | 203.56 | 296.925 | 398.13 | 495.945 |
| 1.09 × 10−6 | 1.07 × 10−6 | 1.31 × 10−6 | 1.47 × 10−6 | 1.60 × 10−6 | |
| ZrO2 APTMS-GO | 109.79 | 207.572 | 319.567 | 393 | 536.495 |
| 1.49 × 10−7 | 1.52 × 10−7 | 1.52 × 10−7 | 1.54 × 10−7 | 1.56 × 10−7 | |
| ZrO2 GPTMS-GO | 104.796 | 202.122 | 294.762 | 395.756 | 491.83 |
| 2.47 × 10−7 | 2.59 × 10−7 | 2.69 × 10−7 | 2.78 × 10−7 | 2.91 × 10−7 | |
| ZrO2 GPTMS-GO-F | 110.25 | 216.392 | 310.645 | 412.711 | 517.852 |
| 1.60 × 10−8 | 1.69 × 10−8 | 1.76 × 10−8 | 1.80 × 10−8 | 1.85 × 10−8 | |
| ZrO2 PDA-rGOT | 119.54 | 213.442 | 313.145 | 400.57 | 522.53 |
| 4.02 × 10−8 | 4.23 × 10−8 | 4.53 × 10−8 | 4.78 × 10−8 | 5.09 × 10−8 | |
| ZrO2 PDA-GO | 127.804 | 217.547 | 313.641 | 416.199 | 522.012 |
| 4.89 × 10−9 | 4.95 × 10−9 | 5.02 × 10−9 | 5.10 × 10−9 | 5.21 × 10−9 | |
| Al2O3 PDA | 55.0 | 109.375 | 163.125 | 215.625 | 267.5 |
| 2.96 × 10−6 | 3.08 × 10−6 | 3.13 × 10−6 | 3.135 × 10−6 | 3.17 × 10−6 |
| Samples | Hydrophilicity Indicator | Pore Dimension (nm) |
|---|---|---|
| Al2O3 PDA | 0.8 | 61.47 |
| Al2O3 blank | 1.0 | 66.67 |
| ZrO2 GPTMS-GO | 2.0 | 0.76 |
| Al2O3 APTMS-GO | 5.5 | 1.91 |
| ZrO2 blank | 6.0 | 3.00 |
| Al2O3 PDA-GO | 7.7 | 2.75 |
| Al2O3 APTMS-GO-F | 9.8 | 2.89 |
| Al2O3 GPTMS-GO-F | 21.7 | 6.09 |
| Al2O3 PDA-rGOT | 30.2 | 2.12 |
| ZrO2 GPTMS-GO-F | 86.5 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galata, E.; Veziri, C.M.; Theodorakopoulos, G.V.; Romanos, G.E.; Pavlatou, E.A. A Combined Gas and Water Permeances Method for Revealing the Deposition Morphology of GO Grafting on Ceramic Membranes. Membranes 2023, 13, 627. https://doi.org/10.3390/membranes13070627
Galata E, Veziri CM, Theodorakopoulos GV, Romanos GE, Pavlatou EA. A Combined Gas and Water Permeances Method for Revealing the Deposition Morphology of GO Grafting on Ceramic Membranes. Membranes. 2023; 13(7):627. https://doi.org/10.3390/membranes13070627
Chicago/Turabian StyleGalata, Evdokia, Charitomeni M. Veziri, George V. Theodorakopoulos, George Em. Romanos, and Evangelia A. Pavlatou. 2023. "A Combined Gas and Water Permeances Method for Revealing the Deposition Morphology of GO Grafting on Ceramic Membranes" Membranes 13, no. 7: 627. https://doi.org/10.3390/membranes13070627
APA StyleGalata, E., Veziri, C. M., Theodorakopoulos, G. V., Romanos, G. E., & Pavlatou, E. A. (2023). A Combined Gas and Water Permeances Method for Revealing the Deposition Morphology of GO Grafting on Ceramic Membranes. Membranes, 13(7), 627. https://doi.org/10.3390/membranes13070627








