Experimental and Modeling Study of the Nanofiltration of Alcohol-Based Molecules and Amino Acids by Commercial Membranes
Abstract
:1. Introduction
2. Theory
3. Materials and Methods
3.1. Experimental Equipment
3.2. Feed Solutions and Analytical Techniques
3.3. Modeling Procedure
- Guess the initial values of and , and calculate the hindrance coefficients according to Equations (12) and (13).
- Calculate the solute intrinsic rejection as a function of experimental permeate flux with Equation (14) and the observed rejection with the experimental permeate flux and the mass transfer coefficient with the following expression:
- Determine the values of ls and d that provided the optimal fit for the experimental solute rejections by minimizing the least-squares objective function, :where m is the number of transmembrane pressures tested for any of the feed solutions.
- Guess the solution permeability coefficient value to calculate the permeate flux. By combining Equations (2) and (4), the permeate flux can be estimated from the calculated observed solute rejection and applied transmembrane pressure with the following expressions:
- 5.
- Determine the value of that provided the optimal fit for the experimental permeate flux by minimizing the least-squares objective function , between experimental and calculated permeate fluxes at each transmembrane pressure:
4. Results and Discussion
4.1. Pure Water Permeate Flux
4.2. Nanofiltration of Alcohol-Based Molecules
4.3. Nanofiltration of Amino Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Micari, M.; Diamantidou, D.; Heijman, B.; Moser, M.; Haidari, A.; Spanjers, H.; Bertsch, V. Experimental and Theoretical Characterization of Commercial Nanofiltration Membranes for the Treatment of Ion Exchange Spent Regenerant. J. Membr. Sci. 2020, 606, 118117. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, L.; Fu, Y.; Zhu, M.; Xue, L. Development of Lower Cost Seawater Desalination Processes Using Nanofiltration Technologies—A Review. Desalination 2015, 376, 109–116. [Google Scholar] [CrossRef]
- Li, Q.; Liao, Z.; Fang, X.; Wang, D.; Xie, J.; Sun, X.; Wang, L.; Li, J. Tannic Acid-Polyethyleneimine Crosslinked Loose Nanofiltration Membrane for Dye/Salt Mixture Separation. J. Membr. Sci. 2019, 584, 324–332. [Google Scholar] [CrossRef]
- Nath, K.; Dave, H.K.; Patel, T.M. Revisiting the Recent Applications of Nanofiltration in Food Processing Industries: Progress and Prognosis. Trends Food Sci. Technol. 2018, 73, 12–24. [Google Scholar] [CrossRef]
- Salehi, F. Current and Future Applications for Nanofiltration Technology in the Food Processing. Food Bioprod. Process. 2014, 92, 161–177. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Geng, N. Effect of Interlayer Construction on TFC Nanofiltration Membrane Performance: A Review from Materials Perspective. Membranes 2023, 13, 497. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of Solution Chemistry on the Surface Charge of Polymeric Reverse Osmosis and Nanofiltration Membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Mänttäri, M.; Pihlajamäki, A.; Nyström, M. Effect of PH on Hydrophilicity and Charge and Their Effect on the Filtration Efficiency of NF Membranes at Different PH. J. Membr. Sci. 2006, 280, 311–320. [Google Scholar] [CrossRef]
- Bruni, L.; Bandini, S. The Role of the Electrolyte on the Mechanism of Charge Formation in Polyamide Nanofiltration Membranes. J. Membr. Sci. 2008, 308, 136–151. [Google Scholar] [CrossRef]
- Wu, D.; Martin, J.; Du, J.R.; Zhang, Y.; Lawless, D.; Feng, X. Effects of Chlorine Exposure on Nanofiltration Performance of Polyamide Membranes. J. Membr. Sci. 2015, 487, 256–270. [Google Scholar] [CrossRef]
- Spiegler, K.S.; Kedem, O. Thermodynamics of Hyperfiltration (Reverse Osmosis): Criteria for Efficient Membranes. Desalination 1966, 1, 311–326. [Google Scholar] [CrossRef]
- Schaep, J.; Vandecasteele, C.; Wahab Mohammad, A.; Richard Bowen, W. Modelling the Retention of Ionic Components for Different Nanofiltration Membranes. Sep. Purif. Technol. 2001, 22–23, 169–179. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W.; Hilal, N. Characterisation of Nanofiltration Membranes for Predictive Purposes—Use of Salts, Uncharged Solutes and Atomic Force Microscopy. J. Membr. Sci. 1997, 126, 91–105. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W. Diafiltration by Nanofiltration: Prediction and Optimization. AIChE J. 1998, 44, 1799–1812. [Google Scholar] [CrossRef]
- Donnan, F.G. Theory of Membrane Equilibria and Membrane Potentials in the Presence of Non-Dialysing Electrolytes. A Contribution to Physical-Chemical Physiology. J. Membr. Sci. 1995, 100, 45–55. [Google Scholar] [CrossRef]
- Zhang, L.; Hamelers, H.V.M.; Biesheuvel, P.M. Modeling Permeate PH in RO Membranes by the Extended Donnan Steric Partitioning Pore Model. J. Membr. Sci. 2020, 613, 118511. [Google Scholar] [CrossRef]
- Marecka-Migacz, A.; Mitkowski, P.T.; Nędzarek, A.; Różański, J.; Szaferski, W. Effect of Ph on Total Volume Membrane Charge Density in the Nanofiltration of Aqueous Solutions of Nitrate Salts of Heavy Metals. Membranes 2020, 10, 235. [Google Scholar] [CrossRef]
- Szymczyk, A.; Fievet, P.; Bandini, S. On the Amphoteric Behavior of Desal DK Nanofiltration Membranes at Low Salt Concentrations. J. Membr. Sci. 2010, 355, 60–68. [Google Scholar] [CrossRef]
- Oatley, D.L.; Llenas, L.; Pérez, R.; Williams, P.M.; Martínez-Lladó, X.; Rovira, M. Review of the Dielectric Properties of Nanofiltration Membranes and Verification of the Single Oriented Layer Approximation. Adv. Colloid Interface Sci. 2012, 173, 1–11. [Google Scholar] [CrossRef]
- Yaroshchuk, A.; Bruening, M.L.; Zholkovskiy, E. Modelling Nanofiltration of Electrolyte Solutions. Adv. Colloid Interface Sci. 2019, 268, 39–63. [Google Scholar] [CrossRef]
- Wang, R.; Lin, S. Pore Model for Nanofiltration: History, Theoretical Framework, Key Predictions, Limitations, and Prospects. J. Membr. Sci. 2021, 620, 118809. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, F.; Zhu, Y.; Li, A.; He, W.; Huang, J.; Li, G. Investigation of Dielectric Constants of Water in a Nano-Confined Pore. RSC Adv. 2020, 10, 8628–8635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Quintana, S.; Carmona, F.J.; Palacio, L.; Hernández, A.; Prádanos, P. Water Viscosity in Confined Nanoporous Media and Flow through Nanofiltration Membranes. Microporous Mesoporous Mater. 2020, 303, 110289. [Google Scholar] [CrossRef]
- Bandini, S.; Morelli, V. Effect of Temperature, pH and Composition on Nanofiltration of Mono/Disaccharides: Experiments and Modeling Assessment. J. Membr. Sci. 2017, 533, 57–74. [Google Scholar] [CrossRef]
- Escoda, A.; Fievet, P.; Lakard, S.; Szymczyk, A.; Déon, S. Influence of Salts on the Rejection of Polyethyleneglycol by an NF Organic Membrane: Pore Swelling and Salting-out Effects. J. Membr. Sci. 2010, 347, 174–182. [Google Scholar] [CrossRef]
- Dechadilok, P.; Deen, W.M. Hindrance Factors for Diffusion and Convection in Pores. Ind. Eng. Chem. Res. 2006, 45, 6953–6959. [Google Scholar] [CrossRef]
- Schock, G.; Miquel, A. Mass Transfer and Pressure Loss in Spiral Wound Modules. Desalination 1987, 64, 339–352. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Yiantsios, S.G.; Karabelas, A.J. A Numerical and Experimental Study of Mass Transfer in Spacer-Filled Channels: Effects of Spacer Geometrical Characteristics and Schmidt Number. J. Membr. Sci. 2009, 326, 234–251. [Google Scholar] [CrossRef]
- Geraldes, V.; Alfonso, M.D. Generalized Mass-Transfer Correction Factor for Nanofiltration and Reverse Osmosis. AIChE J. 2006, 52, 3353–3362. [Google Scholar] [CrossRef]
- Sabaté, J.; Labanda, J.; Llorens, J. Influence of Coion and Counterion Size on Multi-Ionic Solution Nanofiltration. J. Membr. Sci. 2009, 345, 298–304. [Google Scholar] [CrossRef]
- Dalwani, M.; Benes, N.E.; Bargeman, G.; Stamatialis, D.; Wessling, M. Effect of PH on the Performance of Polyamide/Polyacrylonitrile Based Thin Film Composite Membranes. J. Membr. Sci. 2011, 372, 228–238. [Google Scholar] [CrossRef]
- Oatley, D.L.; Llenas, L.; Aljohani, N.H.M.; Williams, P.M.; Martínez-Lladó, X.; Rovira, M.; de Pablo, J. Investigation of the Dielectric Properties of Nanofiltration Membranes. Desalination 2013, 315, 100–106. [Google Scholar] [CrossRef]
- Sabaté, J.; Labanda, J.; Llorens, J. Nanofiltration of Biogenic Amines in Acidic Conditions: Influence of Operation Variables and Modeling. J. Membr. Sci. 2008, 310, 594–601. [Google Scholar] [CrossRef]
- Bowen, W.R.; Welfoot, J.S. Modelling the Performance of Membrane Nanofiltration-Critical Assessment and Model Development. Chem. Eng. Sci. 2002, 57, 1121–1137. [Google Scholar] [CrossRef]
- Koops, G.H.; Yamada, S.; Nakao, S.I. Separation of Linear Hydrocarbons and Carboxylic Acids from Ethanol and Hexane Solutions by Reverse Osmosis. J. Membr. Sci. 2001, 189, 241–254. [Google Scholar] [CrossRef]
- Geens, J.; Peeters, K.; Van Der Bruggen, B.; Vandecasteele, C. Polymeric Nanofiltration of Binary Water-Alcohol Mixtures: Influence of Feed Composition and Membrane Properties on Permeability and Rejection. J. Membr. Sci. 2005, 255, 255–264. [Google Scholar] [CrossRef]
- Labanda, J.; Sabaté, J.; Llorens, J. Permeation of Organic Solutes in Water-Ethanol Mixtures with Nanofiltration Membranes. Desalination 2013, 315, 83–90. [Google Scholar] [CrossRef]
- Kwiatkowski, J.; Cheryan, M. Performance of Nanofiltration Membranes in Ethanol. Sep. Sci. Technol. 2005, 40, 2651–2662. [Google Scholar] [CrossRef]
- Kovacs, Z.; Samhaber, W. Nanofiltration of Concentrated Amino Acid Solutions. Desalination 2009, 240, 78–88. [Google Scholar] [CrossRef]
- Labban, O.; Liu, C.; Chong, T.H.; Lienhard V, J.H. Fundamentals of Low-Pressure Nanofiltration: Membrane Characterization, Modeling, and Understanding the Multi-Ionic Interactions in Water Softening. J. Membr. Sci. 2017, 521, 18–32. [Google Scholar] [CrossRef]
- Kovács, Z.; Discacciati, M.; Samhaber, W. Modeling of Amino Acid Nanofiltration by Irreversible Thermodynamics. J. Membr. Sci. 2009, 332, 38–49. [Google Scholar] [CrossRef]
- Almazán, J.E.; Romero-Dondiz, E.M.; Rajal, V.B.; Castro-Vidaurre, E.F. Nanofiltration of Glucose: Analysis of Parameters and Membrane Characterization. Chem. Eng. Res. Des. 2015, 94, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Bargeman, G.; Vollenbroek, J.M.; Straatsma, J.; Schroën, C.G.P.H.; Boom, R.M. Nanofiltration of Multi-Component Feeds. Interactions between Neutral and Charged Components and Their Effect on Retention. J. Membr. Sci. 2005, 247, 11–20. [Google Scholar] [CrossRef]
- Straatsma, J.; Bargeman, G.; van der Horst, H.C.; Wesselingh, J.A. Can Nanofiltration Be Fully Predicted by a Model? J. Membr. Sci. 2002, 198, 273–284. [Google Scholar] [CrossRef]
- Gaglianò, M.; Conidi, C.; De Luca, G.; Cassano, A. Partial Removal of Sugar from Apple Juice by Nanofiltration and Discontinuous Diafiltration. Membranes 2022, 12, 712. [Google Scholar] [CrossRef]
- Rathnayake, B.; Valkama, H.; Ohenoja, M.; Haverinen, J.; Keiski, R.L. Evaluation of Nanofiltration Membranes for the Purification of Monosaccharides: Influence of PH, Temperature, and Sulfates on the Solute Retention and Fouling. Membranes 2022, 12, 1210. [Google Scholar] [CrossRef]
- Tonova, K.; Lazarova, M.; Dencheva-Zarkova, M.; Genova, J. Nanofiltration of Aquatic Weed Hydrolysate: Diafiltration versus Concentration Mode for Separating Saccharides from Phenolics. Chem. Eng. Res. Des. 2022, 182, 360–370. [Google Scholar] [CrossRef]
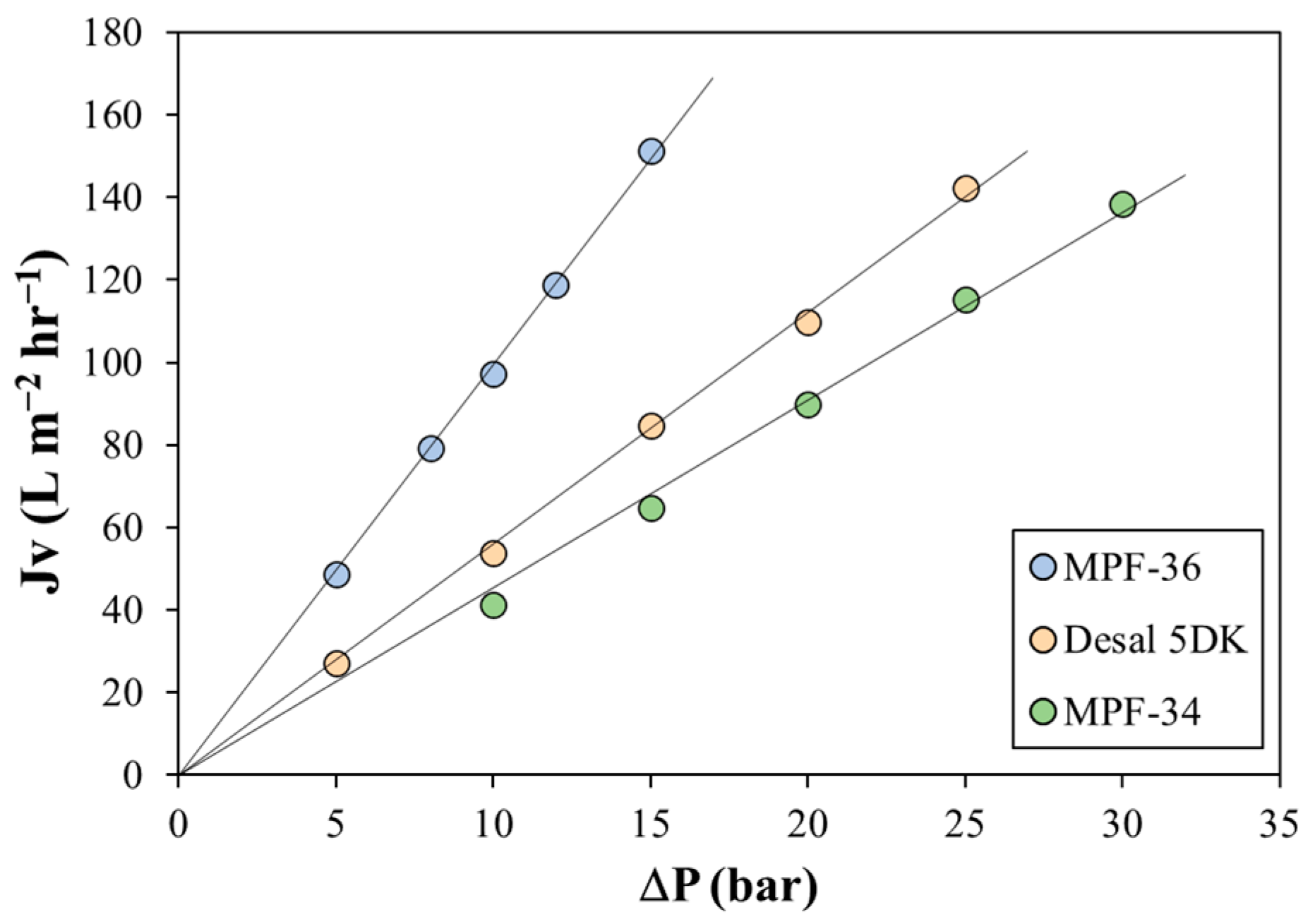

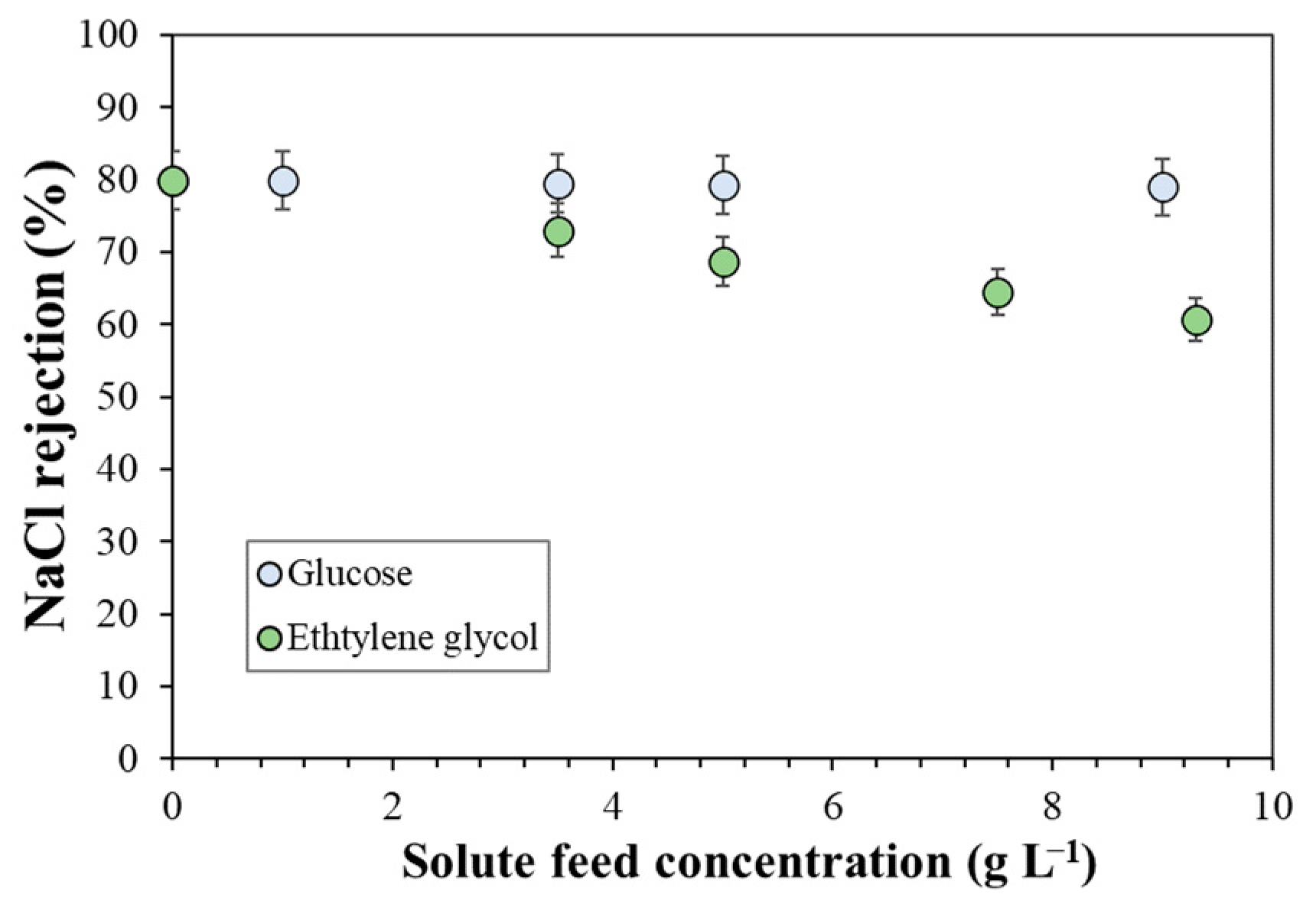



| Species | MW (g mol−1) | pka1 | pka2 | rs b (nm) | Ds (109 m2 s−1) |
|---|---|---|---|---|---|
| Glucose | 180.2 | - | - | 0.365 | 0.586 |
| Glycerol | 92.1 | - | - | 0.260 | 0.950 |
| Ethylene glycol | 62.07 | - | - | 0.211 | 1.014 |
| Triglycine | 189.2 | 3.23 | 8.09 | 0.375 a | 0.571 |
| Glycine | 75.0 | 2.37 | 9.60 | 0.245 | 0.873 |
| MPF-36 Membrane | ||||||
|---|---|---|---|---|---|---|
| Solute | λ (-) | δ (μm) | rp b (nm) | LSR,s | Lp (L m−2 hr−1 bar−1) | LSJv,s |
| Glucose | 0.430 | 3.96 | 0.848 | 0.696 | 9.97 | 0.162 |
| Glycerol | 0.302 | 3.88 | 0.961 | 0.256 | 10.1 | 0.233 |
| Ethylene glycol | 0.237 | 4.68 | 0.890 | 0.520 | 10.4 | 0.364 |
| Glycine | 0.285 | 3.86 | 0.859 | 0.932 | 10.1 | 0.321 |
| Triglycine | 0.451 | 4.12 | 0.832 | 0.311 | 10.0 | 0.248 |
| MPF-34 membrane | ||||||
| λ (-) | δ (μm) | rp b (nm) | LSR,s | Lp (L m−2 hr−1 bar−1) | LSJv,s | |
| Glucose | 0.892 | 2.01 | 0.409 | 0.135 | 4.57 | 0.138 |
| Glycerol | 0.636 | 1.88 | 0.409 | 0.206 | 4.61 | 0.214 |
| Ethylene glycol a | 0.512–0.501 | 1.49–1.48 | 0.412–0.421 | 0.366–0.308 | 4.43–4.25 | 0.219–0.283 |
| Glycine | 0.634 | 2.09 | 0.386 | 0.211 | 4.61 | 0.206 |
| Triglycine | 0.938 | 1.92 | 0.399 | 0.0891 | 4.58 | 0.200 |
| Desal 5DK membrane | ||||||
| λ (-) | δ (μm) | rp b (nm) | LSR,s | Lp (L m−2 hr−1 bar−1) | LSJv,s | |
| Glucose | 0.807 | 2.14 | 0.452 | 0.250 | 5.69 | 0.215 |
| Glycerol | 0.573 | 2.43 | 0.453 | 0.570 | 5.47 | 0.261 |
| Ethylene glycol a | 0.455–0.449 | 1.97–1.91 | 0.463–0.470 | 0.393–0.489 | 5.22–5.08 | 0.108–0.153 |
| Glycine | 0.580 | 2.20 | 0.422 | 0.705 | 5.62 | 0.120 |
| Triglycine | 0.904 | 2.12 | 0.415 | 0.0435 | 5.47 | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahgodari, S.; Labanda, J.; Llorens, J. Experimental and Modeling Study of the Nanofiltration of Alcohol-Based Molecules and Amino Acids by Commercial Membranes. Membranes 2023, 13, 631. https://doi.org/10.3390/membranes13070631
Shahgodari S, Labanda J, Llorens J. Experimental and Modeling Study of the Nanofiltration of Alcohol-Based Molecules and Amino Acids by Commercial Membranes. Membranes. 2023; 13(7):631. https://doi.org/10.3390/membranes13070631
Chicago/Turabian StyleShahgodari, Shirin, Jordi Labanda, and Joan Llorens. 2023. "Experimental and Modeling Study of the Nanofiltration of Alcohol-Based Molecules and Amino Acids by Commercial Membranes" Membranes 13, no. 7: 631. https://doi.org/10.3390/membranes13070631
APA StyleShahgodari, S., Labanda, J., & Llorens, J. (2023). Experimental and Modeling Study of the Nanofiltration of Alcohol-Based Molecules and Amino Acids by Commercial Membranes. Membranes, 13(7), 631. https://doi.org/10.3390/membranes13070631







