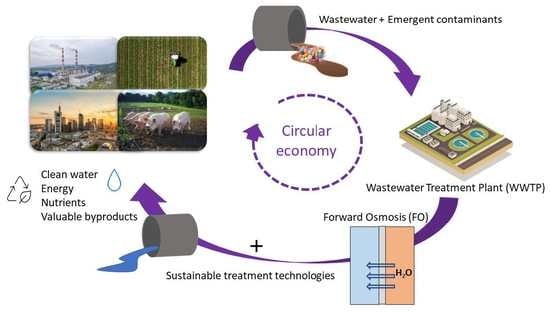
Forward Osmosis Application for the Removal of Emerging Contaminants from Municipal Wastewater: A Review
Abstract
1. Introduction
2. Problems in Wastewater Treatment
3. Membrane Technologies
4. Forward Osmosis Development
4.1. Background of FO
4.2. Types of FO Membranes
4.3. Main Manufacturers of FO Modules
4.4. Important Factors
4.4.1. Draw Solution
4.4.2. Reverse Salt Flow or Reverse Solute Diffusion
4.4.3. Concentration Polarization
4.4.4. Membrane Fouling
5. Wastewater Contamination
5.1. Contaminants of Emerging Concern or Micropollutants
5.1.1. Environmental Effects
5.1.2. Ecotoxicological Risk Evaluation
- Amount of the substance present in the environment (for example, soil, water, or air).
- Exposure time of the receptor with the contaminated environment.
- The inherent toxicity of the substance.
- –
- Median effective concentration (EC50): Concentration obtained statistically or graphically estimated that causes a given effect in 50% of the group of organisms, under specified conditions.
- –
- Median lethal concentration (LC50): Statistically derived or graphically estimated concentration (in air or water) that causes death, during exposure or within a defined period after exposure, of 50% of the group of organisms during a given period and other specific conditions. LC50 is generally expressed in mg/L.
- –
- Median lethal dose (LD50): Individual dose of a substance that is statistically or graphically estimated to be lethal to 50% of the group of organisms under specified conditions. Generally, LD50 is expressed in mg/kg of body weight.
- –
- No observable effect level (NOEL): The highest concentration or amount of a substance found experimentally or by observation that does not cause alterations in the morphology, functional capacity, growth, development, or life span of organisms, distinguishable from those observed in organisms normal (control) samples of the same species and strain, under conditions identical to those of exposure.
5.2. Options to Contaminants of Emerging Concern Removal in Wastewater
- –
- Physicochemical treatments: The coagulation–flocculation process has been found to be unable to remove contaminants, in addition to existing techniques in WWTPs such as grit chambers or sedimentation tanks to remove solid particles, ash, and other suspended solids [147]. This group of physiochemical treatments also includes processes such as activated carbon (AC) adsorption or ultraviolet (UV) irradiation. Another example in this field involves advanced oxidation technologies that can eliminate some of these microcontaminants from residual waters such as ozonation. Although oxidation is a promising process for removing pollutants from wastewater, especially using chlorine or ozone, the reaction of these chemicals produces byproducts, and the effects of these byproducts are unknown. Therefore, special care must be taken when using these chemicals for wastewater treatment [148].
- –
- Biological treatments: Activated sludge can convert organic compounds into biomass, among other compounds. However, while this is a great achievement, not all compounds are completely broken down into biomass in this process. Biological treatment is a common method for wastewater treatment that uses microorganisms to remove pollutants. However, it is only capable of removing a part of a wide range of emerging pollutants [149].
- –
- Membrane treatments: These include membrane bioreactors (MBR) and membrane filtration processes [150,151]. Pressure-driven membrane techniques such as microfiltration (MF), ultrafiltration (UF), reverse osmosis (RO), and nanofiltration (NF) have also been used to treat water contaminated with micropollutants [152]. Both NF and RO can remove contaminants such as suspended and dissolved solids, organic matter, viruses, and bacteria, but RO is additionally capable of eliminating smaller molecules such as ions. However, these processes, due to membrane concentration polarization and the high hydraulic pressures required, have high costs and are difficult to scale [92]. A possible alternative to overcome the disadvantages of pressure-driven membrane techniques could be the use of FO processes [153]. In the forward osmosis process, the driving force is the osmotic gradient rather than the pressure-driven force, which could be an important advantage with respect to membrane fouling, as already mentioned. In this process, the osmotic pressure gradient facilitates the passage of water across a semipermeable membrane between a concentrated extraction solution and a less concentrated feed solution, while retaining other solutes. This leads to dilution of the extraction solution, while the solutes in the feed stream become concentrated [43,154].
Forward Osmosis in the Removal of Contaminants of Emerging Concern from Wastewater
6. Concluding Remarks and Future Perspectives
- –
- Focusing on optimizing FO systems by developing advanced membrane materials, exploring innovative fouling mitigation strategies, and investigating novel approaches for the recovery of draw solutions, as well as working with real wastewater to work in real conditions.
- –
- Process integration and hybrid systems by exploring the integration of FO with other water treatment processes, e.g., reverse osmosis or electrochemical processes, to improve overall treatment efficiency. Hybrid systems that combine FO with other technologies may offer unique advantages for specific applications.
- –
- Scaling up and commercialization by advancing FO from laboratory-scale to large-scale implementation, which will require addressing engineering challenges and optimizing system designs.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, W.; Dong, Y.; Li, C.; Han, X.; Liu, G.; Liu, J.; Feng, Y. Field Tests of Cubic-Meter Scale Microbial Electrochemical System in a Municipal Wastewater Treatment Plant. Water Res. 2019, 155, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward Osmosis for Application in Wastewater Treatment: A Review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S.M. The State of Desalination and Brine Production: A Global Outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Herrera-Navarrete, R.; Colín-Cruz, A.; Arellano-Wences, H.J.; Sampedro-Rosas, M.L.; Rosas-Acevedo, J.L.; Rodríguez-Herrera, A.L. Municipal Wastewater Treatment Plants: Gap, Challenges, and Opportunities in Environmental Management. Environ. Manag. 2022, 69, 75–88. [Google Scholar] [CrossRef]
- Yang, L.; Wen, Q.; Chen, Z.; Duan, R.; Yang, P. Impacts of Advanced Treatment Processes on Elimination of Antibiotic Resistance Genes in a Municipal Wastewater Treatment Plant. Front. Environ. Sci. Eng. 2019, 13, 32. [Google Scholar] [CrossRef]
- Piao, W.; Kim, Y.; Kim, H.; Kim, M.; Kim, C. Life Cycle Assessment and Economic Efficiency Analysis of Integrated Management of Wastewater Treatment Plants. J. Clean. Prod. 2016, 113, 325–337. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Oulego, P.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Towards the Implementation of Circular Economy in the Wastewater Sector: Challenges and Opportunities. Water 2020, 12, 1431. [Google Scholar] [CrossRef]
- Salamanca, M.; López-Serna, R.; Palacio, L.; Hernandez, A.; Prádanos, P.; Peña, M. Ecological Risk Evaluation and Removal of Emerging Pollutants in Urban Wastewater by a Hollow Fiber Forward Osmosis Membrane. Membranes 2022, 12, 293. [Google Scholar] [CrossRef]
- Ghimire, U.; Sarpong, G.; Gude, V.G. Transitioning Wastewater Treatment Plants toward Circular Economy and Energy Sustainability. ACS Omega 2021, 6, 11794–11803. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Wastewater Treatment: An Overview. In Green Adsorbents for Pollutant Removal. Environmental Chemistry for a Sustainable World; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 18, pp. 1–21. [Google Scholar] [CrossRef]
- Roy, M.; Saha, R. Dyes and Their Removal Technologies from Wastewater: A Critical Review. In Intelligent Environmental Data Monitoring for Pollution Management; Siddhartha Bhattacharyya, S., Mondal, N.K., Platos, J., Snášel, V., Krömer, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 127–160. [Google Scholar] [CrossRef]
- Pan, Z.; Song, C.; Li, L.; Wang, H.; Pan, Y.; Wang, C.; Li, J.; Wang, T.; Feng, X. Membrane Technology Coupled with Electrochemical Advanced Oxidation Processes for Organic Wastewater Treatment: Recent Advances and Future Prospects. Chem. Eng. J. 2019, 376, 120909. [Google Scholar] [CrossRef]
- Katiyar, J.; Bargole, S.; George, S.; Bhoi, R.; Saharan, V.K. Advanced Technologies for Wastewater Treatment: New Trends. In Handbook of Nanomaterials for Wastewater Treatment: Fundamentals and Scale up Issues; Bhanvase, B., Sonawane, S., Pawade, V., Pandit, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 85–133. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Cornejo, P.K.; Zhang, Q.; Mihelcic, J.R. How Does Scale of Implementation Impact the Environmental Sustainability of Wastewater Treatment Integrated with Resource Recovery? Environ. Sci. Technol. 2016, 50, 6680–6689. [Google Scholar] [CrossRef]
- Metcalf & Eddy. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Awad, H.; Gar Alalm, M.; El-Etriby, H.K. Environmental and Cost Life Cycle Assessment of Different Alternatives for Improvement of Wastewater Treatment Plants in Developing Countries. Sci. Total Environ. 2019, 660, 57–68. [Google Scholar] [CrossRef]
- Sabeen, A.H.; Noor, Z.Z.; Ngadi, N.; Almuraisy, S.; Raheem, A.B. Quantification of Environmental Impacts of Domestic Wastewater Treatment Using Life Cycle Assessment: A Review. J. Clean. Prod. 2018, 190, 221–233. [Google Scholar] [CrossRef]
- Ferrari, F. Combining Forward Osmosis and Anaerobic Membrane Bioreactor Technologies for Raw Municipal Wastewater Treatment. Ph.D. Thesis, Institut Català de Recerca de l’Aigua (ICRA), Universitat de Girona, Girona, Spain, 2020. Available online: http://hdl.handle.net/10256/18810 (accessed on 10 June 2023).
- Lorenzo-Toja, Y.; Vázquez-Rowe, I.; Amores, M.J.; Termes-Rifé, M.; Marín-Navarro, D.; Moreira, M.T.; Feijoo, G. Benchmarking Wastewater Treatment Plants under an Eco-Efficiency Perspective. Sci. Total Environ. 2016, 566–567, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.; Concepción, H.; Vrecko, D.; Vilanova, R. Life Cycle Assessment as an Environmental Evaluation Tool for Control Strategies in Wastewater Treatment Plants. J. Clean. Prod. 2015, 107, 653–661. [Google Scholar] [CrossRef]
- Low, E.W.; Chase, H.A. Reducing production of excess biomass during wastewater treatment. Water Res. 1999, 33, 1119–1132. [Google Scholar] [CrossRef]
- Ma, H.; Guo, Y.; Qin, Y.; Li, Y.Y. Nutrient Recovery Technologies Integrated with Energy Recovery by Waste Biomass Anaerobic Digestion. Bioresour. Technol. 2018, 269, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Kazimierowicz, J.; Dębowski, M. Advantages and Limitations of Anaerobic Wastewater Treatment—Technological Basics, Development Directions, and Technological Innovations. Energies 2023, 16, 83. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Ngo, H.H.; Guo, W.; Nghiem, L.D. Assessing the Integration of Forward Osmosis and Anaerobic Digestion for Simultaneous Wastewater Treatment and Resource Recovery. Bioresour. Technol. 2018, 260, 221–226. [Google Scholar] [CrossRef]
- Ozgun, H.; Dereli, R.K.; Ersahin, M.E.; Kinaci, C.; Spanjers, H.; Van Lier, J.B. A Review of Anaerobic Membrane Bioreactors for Municipal Wastewater Treatment: Integration Options, Limitations and Expectations. Sep. Purif. Technol. 2013, 118, 89–104. [Google Scholar] [CrossRef]
- Jin, Z.; Meng, F.; Gong, H.; Wang, C.; Wang, K. Improved Low-Carbon-Consuming Fouling Control in Long-Term Membrane-Based Sewage Pre-Concentration: The Role of Enhanced Coagulation Process and Air Backflushing in Sustainable Sewage Treatment. J. Membr. Sci. 2017, 529, 252–262. [Google Scholar] [CrossRef]
- Wan, J.; Gu, J.; Zhao, Q.; Liu, Y. COD Capture: A Feasible Option towards Energy Self-Sufficient Domestic Wastewater Treatment. Sci. Rep. 2016, 6, 25054. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, Y.; Cao, C.; Zhang, J.; Ng, J.W.; Tang, C. Performance of a Submerged Anaerobic Membrane Bioreactor with Forward Osmosis Membrane for Low-Strength Wastewater Treatment. Water Res. 2014, 50, 114–123. [Google Scholar] [CrossRef]
- Sikosana, M.L.; Sikhwivhilu, K.; Moutloali, R.; Madyira, D.M. Municipal Wastewater Treatment Technologies: A Review. Procedia Manuf. 2019, 35, 1018–1024. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Love, N.G.; Skerlos, S.J.; Raskin, L. Perspectives on Anaerobic Membrane Bioreactor Treatment of Domestic Wastewater: A Critical Review. Bioresour. Technol. 2012, 122, 149–159. [Google Scholar] [CrossRef]
- Korenak, J.; Basu, S.; Balakrishnan, M.; Hélix-Nielsen, C.; Petrinic, I. Forward Osmosis in Wastewater Treatment Processes. Acta Chim. Slov. 2017, 64, 83–94. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward Osmosis as a Platform for Resource Recovery from Municipal Wastewater—A Critical Assessment of the Literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef]
- Bansod, P.G.; Bhasarkar, J.; Dharaskar, S.; Kodape, S.M. Review of Membrane Technology Applications in Wastewater Treatment and Biofuels. Mater. Today Proc. 2022, 61, 379–385. [Google Scholar] [CrossRef]
- Gao, T.; Xiao, K.; Zhang, J.; Zhang, X.; Wang, X.; Liang, S.; Sun, J.; Meng, F.; Huang, X. Cost-Benefit Analysis and Technical Efficiency Evaluation of Full-Scale Membrane Bioreactors for Wastewater Treatment Using Economic Approaches. J. Clean. Prod. 2021, 301, 126984. [Google Scholar] [CrossRef]
- Hilal, N.; Wright, C.J. Exploring the Current State of Play for Cost-Effective Water Treatment by Membranes. NPJ Clean Water 2018, 1, 8. [Google Scholar] [CrossRef]
- Selatile, M.K.; Ray, S.S.; Ojijo, V.; Sadiku, R. Recent Developments in Polymeric Electrospun Nanofibrous Membranes for Seawater Desalination. RSC Adv. 2018, 8, 37915–37938. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Du, H.; Kommalapati, R.R. A Sequential Membrane Process of Ultrafiltration Forward Osmosis and Reverse Osmosis for Poultry Slaughterhouse Wastewater Treatment and Reuse. Membranes 2023, 13, 296. [Google Scholar] [CrossRef]
- Li, X.; Chou, S.; Wang, R.; Shi, L.; Fang, W.; Chaitra, G.; Tang, C.Y.; Torres, J.; Hu, X.; Fane, A.G. Nature Gives the Best Solution for Desalination: Aquaporin-Based Hollow Fiber Composite Membrane with Superior Performance. J. Membr. Sci. 2015, 494, 68–77. [Google Scholar] [CrossRef]
- Bao, X.; Wu, Q.; Tian, J.; Shi, W.; Wang, W.; Zhang, Z.; Zhang, R.; Zhang, B.; Guo, Y.; Shu, S.; et al. Fouling Mechanism of Forward Osmosis Membrane in Domestic Wastewater Concentration: Role of Substrate Structures. Chem. Eng. J. 2019, 370, 262–273. [Google Scholar] [CrossRef]
- Lateef, S.K.; Soh, B.Z.; Kimura, K. Direct Membrane Filtration of Municipal Wastewater with Chemically Enhanced Backwash for Recovery of Organic Matter. Bioresour. Technol. 2013, 150, 149–155. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane Fouling in Osmotically Driven Membrane Processes: A Review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward Osmosis: Principles, Applications, and Recent Developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Power Generation with Pressure Retarded Osmosis: An Experimental and Theoretical Investigation. J. Membr. Sci. 2009, 343, 42–52. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; McCutcheon, J.R.; Elimelech, M. Performance Evaluation of Sucrose Concentration Using Forward Osmosis. J. Membr. Sci. 2009, 338, 61–66. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent Developments in Forward Osmosis: Opportunities and Challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Cornelissen, E.R.; Harmsen, D.; de Korte, K.F.; Ruiken, C.J.; Qin, J.J.; Oo, H.; Wessels, L.P. Membrane Fouling and Process Performance of Forward Osmosis Membranes on Activated Sludge. J. Membr. Sci. 2008, 319, 158–168. [Google Scholar] [CrossRef]
- Alsvik, I.L.; Hägg, M.B. Pressure Retarded Osmosis and Forward Osmosis Membranes: Materials and Methods. Polymers 2013, 5, 303–327. [Google Scholar] [CrossRef]
- Qin, J.J.; Lay, W.C.L.; Kekre, K.A. Recent Developments and Future Challenges of Forward Osmosis for Desalination: A Review. Desalination Water Treat. 2012, 39, 123–136. [Google Scholar] [CrossRef]
- Ng, D.Y.F.; Chen, Y.; Dong, Z.; Wang, R. Membrane Compaction in Forward Osmosis Process. Desalination 2019, 468, 114067. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X. Forward Osmosis Technology for Water Treatment: Recent Advances and Future Perspectives. J. Clean. Prod. 2021, 280, 124354. [Google Scholar] [CrossRef]
- Gerstandt, K.; Peinemann, K.V.; Skilhagen, S.E.; Thorsen, T.; Holt, T. Membrane Processes in Energy Supply for an Osmotic Power Plant. Desalination 2008, 224, 64–70. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of Membrane Support Layer Hydrophobicity on Water Flux in Osmotically Driven Membrane Processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- Thorsen, T.; Holt, T. The Potential for Power Production from Salinity Gradients by Pressure Retarded Osmosis. J. Membr. Sci. 2009, 335, 103–110. [Google Scholar] [CrossRef]
- Wang, Y.; Wicaksana, F.; Tang, C.Y.; Fane, A.G. Direct Microscopic Observation of Forward Osmosis Membrane Fouling. Environ. Sci. Technol. 2010, 44, 7102–7109. [Google Scholar] [CrossRef]
- Vinardell, S.; Blandin, G.; Ferrari, F.; Lesage, G.; Mata-Alvarez, J.; Dosta, J.; Astals, S. Techno-Economic Analysis of Forward Osmosis Pre-Concentration before an Anaerobic Membrane Bioreactor: Impact of Draw Solute and Membrane Material. J. Clean. Prod. 2022, 356, 131776. [Google Scholar] [CrossRef]
- Kim, M.K.; Chang, J.W.; Park, K.; Yang, D.R. Comprehensive Assessment of the Effects of Operating Conditions on Membrane Intrinsic Parameters of Forward Osmosis (FO) Based on Principal Component Analysis (PCA). J. Membr. Sci. 2022, 641, 119909. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Chen, Z.; Zhu, J.; Chen, G. A Comprehensive Review on Forward Osmosis Water Treatment: Recent Advances and Prospects of Membranes and Draw Solutes. Int. J. Environ. Res. Public Health 2022, 19, 8215. [Google Scholar] [CrossRef] [PubMed]
- Akther, N.; Sodiq, A.; Giwa, A.; Daer, S.; Arafat, H.A.; Hasan, S.W. Recent Advancements in Forward Osmosis Desalination: A Review. Chem. Eng. J. 2015, 281, 502–522. [Google Scholar] [CrossRef]
- Suwaileh, W.A.; Johnson, D.J.; Sarp, S.; Hilal, N. Advances in Forward Osmosis Membranes: Altering the Sub-Layer Structure via Recent Fabrication and Chemical Modification Approaches. Desalination 2018, 436, 176–201. [Google Scholar] [CrossRef]
- Dabaghian, Z.; Rahimpour, A. Carboxylated Carbon Nanofibers as Hydrophilic Porous Material to Modification of Cellulosic Membranes for Forward Osmosis Desalination. Chem. Eng. Res. Des. 2015, 104, 647–657. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.M.; Chen, G.; Chung, W.J.; Shon, H.K. Graphene Oxide Incorporated Polysulfone Substrate for the Fabrication of Flat-Sheet Thin-Film Composite Forward Osmosis Membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Liao, R.; Tang, C.Y. Zeolite-Polyamide Thin Film Nanocomposite Membranes: Towards Enhanced Performance for Forward Osmosis. J. Membr. Sci. 2012, 405–406, 149–157. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward Osmosis Membranes and Processes: A Comprehensive Review of Research Trends and Future Outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Kahrizi, M.; Gonzales, R.R.; Kong, L.; Matsuyama, H.; Lu, P.; Lin, J.; Zhao, S. Significant Roles of Substrate Properties in Forward Osmosis Membrane Performance: A Review. Desalination 2022, 528, 115615. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Selection of Inorganic-Based Draw Solutions for Forward Osmosis Applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Ge, Q.; Ling, M.; Chung, T.S. Draw Solutions for Forward Osmosis Processes: Developments, Challenges, and Prospects for the Future. J. Membr. Sci. 2013, 442, 225–237. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Sarp, S.; Bucs, S.S.; Amy, G.; Vrouwenvelder, J.S. Forward Osmosis Niches in Seawater Desalination and Wastewater Reuse. Water Res. 2014, 66, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S. A Novel Low Energy Fertilizer Driven Forward Osmosis Desalination for Direct Fertigation: Evaluating the Performance of Fertilizer Draw Solutions. J. Membr. Sci. 2011, 375, 172–181. [Google Scholar] [CrossRef]
- Dutta, S.; Nath, K. Dewatering of Brackish Water and Wastewater by an Integrated Forward Osmosis and Nanofiltration System for Direct Fertigation. Arab. J. Sci. Eng. 2019, 44, 9977–9986. [Google Scholar] [CrossRef]
- Yang, S.; Lee, S.; Hong, S. Enhancing the Applicability of Forward Osmosis Membrane Process Utilizing Food Additives as Draw Solutes. J. Membr. Sci. 2021, 638, 119705. [Google Scholar] [CrossRef]
- Tayel, A.; Nasr, P.; Sewilam, H. Forward Osmosis Desalination Using Pectin-Coated Magnetic Nanoparticles as a Draw Solution. Clean Technol. Environ. Policy 2019, 21, 1617–1628. [Google Scholar] [CrossRef]
- Razmjou, A.; Simon, G.P.; Wang, H. Effect of Particle Size on the Performance of Forward Osmosis Desalination by Stimuli-Responsive Polymer Hydrogels as a Draw Agent. Chem. Eng. J. 2013, 215–216, 913–920. [Google Scholar] [CrossRef]
- Duan, J.; Litwiller, E.; Choi, S.H.; Pinnau, I. Evaluation of Sodium Lignin Sulfonate as Draw Solute in Forward Osmosis for Desert Restoration. J. Membr. Sci. 2014, 453, 463–470. [Google Scholar] [CrossRef]
- Bowden, K.S.; Achilli, A.; Childress, A.E. Organic Ionic Salt Draw Solutions for Osmotic Membrane Bioreactors. Bioresour. Technol. 2012, 122, 207–216. [Google Scholar] [CrossRef]
- Hau, N.T.; Chen, S.S.; Nguyen, N.C.; Huang, K.Z.; Ngo, H.H.; Guo, W. Exploration of EDTA Sodium Salt as Novel Draw Solution in Forward Osmosis Process for Dewatering of High Nutrient Sludge. J. Membr. Sci. 2014, 455, 305–311. [Google Scholar] [CrossRef]
- Gadelha, G.; Nawaz, M.S.; Hankins, N.P.; Khan, S.J.; Wang, R.; Tang, C.Y. Assessment of Micellar Solutions as Draw Solutions for Forward Osmosis. Desalination 2014, 354, 97–106. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, D.; She, Q.; Tang, C.Y. Gypsum Scaling in Pressure Retarded Osmosis: Experiments, Mechanisms and Implications. Water Res. 2014, 48, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. A Natural Driven Membrane Process for Brackish and Wastewater Treatment: Photovoltaic Powered ED and FO Hybrid System. Environ. Sci. Technol. 2013, 47, 10548–10555. [Google Scholar] [CrossRef] [PubMed]
- Alnaizy, R.; Aidan, A.; Qasim, M. Copper Sulfate as Draw Solute in Forward Osmosis Desalination. J. Environ. Chem. Eng. 2013, 1, 424–430. [Google Scholar] [CrossRef]
- Stone, M.L.; Rae, C.; Stewart, F.F.; Wilson, A.D. Switchable Polarity Solvents as Draw Solutes for Forward Osmosis. Desalination 2013, 312, 124–129. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Selection of Forward Osmosis Draw Solutes for Subsequent Integration with Anaerobic Treatment to Facilitate Resource Recovery from Wastewater. Bioresour. Technol. 2015, 191, 30–36. [Google Scholar] [CrossRef]
- Zou, S.; Qin, M.; He, Z. Tackle Reverse Solute Flux in Forward Osmosis towards Sustainable Water Recovery: Reduction and Perspectives. Water Res. 2019, 149, 362–374. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.; Hong, S. A Novel Analysis of Reverse Draw and Feed Solute Fluxes in Forward Osmosis Membrane Process. Desalination 2014, 352, 128–135. [Google Scholar] [CrossRef]
- Zou, S.; Gu, Y.; Xiao, D.; Tang, C.Y. The Role of Physical and Chemical Parameters on Forward Osmosis Membrane Fouling during Algae Separation. J. Membr. Sci. 2011, 366, 356–362. [Google Scholar] [CrossRef]
- Suh, C.; Lee, S. Modeling Reverse Draw Solute Flux in Forward Osmosis with External Concentration Polarization in Both Sides of the Draw and Feed Solution. J. Membr. Sci. 2013, 427, 365–374. [Google Scholar] [CrossRef]
- Hancock, N.T.; Cath, T.Y. Solute Coupled Diffusion in Osmotically Driven Membrane Processes. Environ. Sci. Technol. 2009, 43, 6769–6775. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Mulcahy, D. Effects of Membrane Orientation on Process Performance in Forward Osmosis Applications. J. Membr. Sci. 2011, 382, 308–315. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Liang, P.; Zhang, X.; Qiu, Y.; Kimura, K.; Huang, X. Anaerobic Digestion Performance of Concentrated Municipal Sewage by Forward Osmosis Membrane: Focus on the Impact of Salt and Ammonia Nitrogen. Bioresour. Technol. 2019, 276, 204–210. [Google Scholar] [CrossRef]
- Salamanca, M.; Palacio, L.; Hernandez, A.; Peña, M.; Prádanos, P. Evaluation of Forward Osmosis and Low-Pressure Reverse Osmosis with a Tubular Membrane for the Concentration of Municipal Wastewater and the Production of Biogas. Membranes 2023, 13, 266. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of Concentrative and Dilutive Internal Concentration Polarization on Flux Behavior in Forward Osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Sablani, S.S.; Goosena, F.A.; Al-Belushi, R.; Wilf, M. Concentration Polarization in Ultrafiltration and Reverse Osmosis: A Critical Review. Desalination 2001, 141, 269–289. [Google Scholar] [CrossRef]
- Salamanca, M.; López-Serna, R.; Palacio, L.; Hernández, A.; Prádanos, P.; Peña, M. Study of the Rejection of Contaminants of Emerging Concern by a Biomimetic Aquaporin Hollow Fiber Forward Osmosis Membrane. J. Water Process Eng. 2021, 40, 101914. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling Mitigation in Forward Osmosis and Membrane Distillation for Desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Yangali-Quintanilla, V.; Li, Z.; Amy, G. Rejection of Micropollutants by Clean and Fouled Forward Osmosis Membrane. Water Res. 2011, 45, 6737–6744. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of Fouling Behavior in Forward Osmosis (FO) and Reverse Osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Alhawari, A.; Alanezi, A.A.; Altaee, A. A Review of Fouling Mechanisms, Control Strategies and Real-Time Fouling Monitoring Techniques in Forward Osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Wicaksana, F.; Tang, C.; Al-Rabiah, A.A.; Al-Zahrani, S.M.; Wang, R. Combined Organic-Inorganic Fouling of Forward Osmosis Hollow Fiber Membranes. Water Res. 2012, 46, 6329–6338. [Google Scholar] [CrossRef]
- Boo, C.; Lee, S.; Elimelech, M.; Meng, Z.; Hong, S. Colloidal Fouling in Forward Osmosis: Role of Reverse Salt Diffusion. J. Membr. Sci. 2012, 390–391, 277–284. [Google Scholar] [CrossRef]
- Hancock, N.T.; Xu, P.; Heil, D.M.; Bellona, C.; Cath, T.Y. Comprehensive Bench- and Pilot-Scale Investigation of Trace Organic Compounds Rejection by Forward Osmosis. Environ. Sci. Technol. 2011, 45, 8483–8490. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A Novel Thin Film Composite Forward Osmosis Membrane Prepared from PSf-TiO2 Nanocomposite Substrate for Water Desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Park, M.J.; Gonzales, R.R.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic Polyvinyl Alcohol Coating on Hydrophobic Electrospun Nanofiber Membrane for High Performance Thin Film Composite Forward Osmosis Membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Niksefat, N.; Jahanshahi, M.; Rahimpour, A. The Effect of SiO2 Nanoparticles on Morphology and Performance of Thin Film Composite Membranes for Forward Osmosis Application. Desalination 2014, 343, 140–146. [Google Scholar] [CrossRef]
- Wu, W.; Shi, Y.; Liu, G.; Fan, X.; Yu, Y. Recent Development of Graphene Oxide Based Forward Osmosis Membrane for Water Treatment: A Critical Review. Desalination 2020, 491, 114452. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Tang, C.Y.; Wang, Z.; Gao, C. Fabrication of Carbon Nanotubes Incorporated Double-Skinned Thin Film Nanocomposite Membranes for Enhanced Separation Performance and Antifouling Capability in Forward Osmosis Process. Desalination 2015, 369, 1–9. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Cornelissen, E.R.; Harmsen, D.J.H.; Post, J.W.; Lampi, K.; Ramaekers, H.; Rietveld, L.C.; Roest, K. Water Recovery from Sewage Using Forward Osmosis. Water Sci. Technol. 2011, 64, 1443–1449. [Google Scholar] [CrossRef]
- Yang, S.; Gao, B.; Jang, A.; Shon, H.K.; Yue, Q. Municipal Wastewater Treatment by Forward Osmosis Using Seawater Concentrate as Draw Solution. Chemosphere 2019, 237, 124485. [Google Scholar] [CrossRef]
- Blandin, G.; Rosselló, B.; Monsalvo, V.M.; Batlle-Vilanova, P.; Viñas, J.M.; Rogalla, F.; Comas, J. Volatile Fatty Acids Concentration in Real Wastewater by Forward Osmosis. J. Membr. Sci. 2019, 575, 60–70. [Google Scholar] [CrossRef]
- Chun, Y.; Mulcahy, D.; Zou, L.; Kim, I.S. A Short Review of Membrane Fouling in Forward Osmosis Processes. Membranes 2017, 7, 30. [Google Scholar] [CrossRef]
- Xiao, F.; Xiao, P.; Zhang, W.J.; Wang, D.S. Identification of Key Factors Affecting the Organic Fouling on Low-Pressure Ultrafiltration Membranes. J. Membr. Sci. 2013, 447, 144–152. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic Fouling of Forward Osmosis Membranes: Fouling Reversibility and Cleaning without Chemical Reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Gavrilescu, M. Water, Soil, and Plants Interactions in a Threatened Environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Pastorino, P.; Ginebreda, A. Contaminants of Emerging Concern (CECs): Occurrence and Fate in Aquatic Ecosystems. Int. J. Environ. Res. Public Health 2021, 18, 13401. [Google Scholar] [CrossRef]
- Tran, N.H.; Gin, K.Y.H. Occurrence and Removal of Pharmaceuticals, Hormones, Personal Care Products, and Endocrine Disrupters in a Full-Scale Water Reclamation Plant. Sci. Total Environ. 2017, 599–600, 1503–1516. [Google Scholar] [CrossRef]
- Commission implementing decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for union-wide monitoring in the field of water policypursuant to directive 2008/105/EC of the European Parliament and of the council. Off. J. Eur. Union 2015, L78, 40–42.
- Commission implementing decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for union-wide monitoring in the field of water policy pursuant to directive 2008/105/EC of the European Parliament and of the council and repealing commission implementing decision (EU) 2015/495. Off. J. Eur. Union 2018, L141, 9–12.
- Carere, M.; Polesello, S.; Kase, R.; Gawlik, B.M. The Emerging Contaminants in the Context of the EU Water Framework Directive. In Emerging Contaminants in River Ecosystems; The Handbook of Environmental Chemistry; Petrovic, M., Sabater, S., Elosegi, A., Barceló, D., Eds.; Springer: Cham, Switzerland, 2016; Volume 46. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Becker, J.A. A Review of Emerging Contaminants in Water: Classification, Sources, and Potential Risks. In Impact of Water Pollution on Human Health and Environmental Sustainability; McKeown, A.E., Bugyi, G., Eds.; IGI Global Publisher: Hershey, PA, USA, 2016; pp. 55–80. [Google Scholar] [CrossRef]
- Burkhardt-Holm, P. Linking Water Quality to Human Health and Environment: The Fate of Micropollutants; Institute of Water Policy, National University of Singapore: Singapore, 2011; p. 62. [Google Scholar]
- Fatta-Kassinos, D.; Manaia, C.; Berendonk, T.U.; Cytryn, E.; Bayona, J.; Chefetz, B.; Slobodnik, J.; Kreuzinger, N.; Rizzo, L.; Malato, S.; et al. COST Action ES1403: New and Emerging Challenges and Opportunities in Wastewater REUSe (NEREUS). Environ. Sci. Pollut. Res. 2015, 22, 7183–7186. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for the Release of Antibiotics in the Environment: A Review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and Personal Care Products (PPCPs) in the Freshwater Aquatic Environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, Endocrine Disruptors, Personal Care Products, Nanomaterials and Perfluorinated Pollutants: A Review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, Fate and Transformation of Emerging Contaminants in Water: An Overarching Review of the Field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent Developments in Hazardous Pollutants Removal from Wastewater and Water Reuse within a Circular Economy. NPJ Clean Water 2022, 5, 12. [Google Scholar] [CrossRef]
- Kumar, M.; Ngasepam, J.; Dhangar, K.; Mahlknecht, J.; Manna, S. Critical Review on Negative Emerging Contaminant Removal Efficiency of Wastewater Treatment Systems: Concept, Consistency and Consequences. Bioresour. Technol. 2022, 352, 127054. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K. Emerging Contaminants Effect on Aquatic Ecosystem: Human Health Risks. Agric. Res. Technol. 2019, 19, 556104. [Google Scholar] [CrossRef]
- Kar, S.; Sangem, P.; Anusha, N.; Senthilkumaran, B. Endocrine Disruptors in Teleosts: Evaluating Environmental Risks and Biomarkers. Aquac. Fish. 2021, 6, 1–26. [Google Scholar] [CrossRef]
- Archer, E.; Volschenk, M.; Brocker, L.; Wolfaardt, G.M. A Two-Year Study of Emerging Micro-Pollutants and Drugs of Abuse in Two Western Cape Wastewater Treatment Works (South Africa). Chemosphere 2021, 285, 131460. [Google Scholar] [CrossRef]
- Memmert, U.; Peither, A.; Burri, R.; Weber, K.; Schmidt, T.; Sumpter, J.P.; Hartmann, A. Diclofenac: New Data on Chronic Toxicity and Bioconcentration in Fish. Environ. Toxicol. Chem. 2013, 32, 442–452. [Google Scholar] [CrossRef]
- OECD. Considerations for Assessing the Risks of Combined Exposure to Multiple Chemicals; Series on Testing and Assessment No. 296; Environment, Health and Safety Division, Environment Directorate: Paris, France, 2018. [Google Scholar]
- Arbeláez Salazar, P.A. Contaminantes Emergentes en Aguas Residuales y de río y Fangos de Depuradora. Ph.D. Thesis, Universitat Rovira i Virgili, Tarragona, Spain, 2015. Available online: http://hdl.handle.net/10803/334397 (accessed on 10 June 2023).
- Cote, I.; Vandenberg, J.J.; Druwe, I.L.; Angrish, M.M. Incorporating Epigenetics into a Risk Assessment Framework. In Toxicoepigenetics: Core Principles and Applications; McCullough, S.D., Dolinoy, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 289–310. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Ecological Risk. In Guidelines for Ecological Risk; Environmental Protection Agency (EPA): Washington, DC, USA, 1998. [Google Scholar]
- Schuijt, L.M.; Peng, F.J.; van den Berg, S.J.P.; Dingemans, M.M.L.; Van den Brink, P.J. (Eco)Toxicological Tests for Assessing Impacts of Chemical Stress to Aquatic Ecosystems: Facts, Challenges, and Future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef]
- Mitchell, E.J.A.K.; Burgess, J.E.; Stuetz, R.M. Developments in Ecotoxicity Testing. Rev. Environ. Sci. Biotechnol. 2002, 1, 169–198. [Google Scholar] [CrossRef]
- DIRECTIVE 2000/60/EC. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, 327, 1–72. [Google Scholar]
- Guo, F.; Wang, Y.; Peng, J.; Huang, H.; Tu, X.; Zhao, H.; Zhan, N.; Rao, Z.; Zhao, G.; Yang, H. Occurrence, Distribution, and Risk Assessment of Antibiotics in the Aquatic Environment of the Karst Plateau Wetland of Yangtze River Basin, Southwestern China. Int. J. Environ. Res. Public Health 2022, 19, 7211. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental Risk Assessment of Pharmaceutical Residues in Wastewater Effluents, Surface Waters and Sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Matamoros, V.; Rodríguez, Y.; Albaigés, J. A Comparative Assessment of Intensive and Extensive Wastewater Treatment Technologies for Removing Emerging Contaminants in Small Communities. Water Res. 2016, 88, 777–785. [Google Scholar] [CrossRef]
- Gosset, A.; Polomé, P.; Perrodin, Y. Ecotoxicological Risk Assessment of Micropollutants from Treated Urban Wastewater Effluents for Watercourses at a Territorial Scale: Application and Comparison of Two Approaches. Int. J. Hyg. Environ. Health 2020, 224, 113437. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging Pollutants in the Urban Water Cycle in Latin America: A Review of the Current Literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Martínez-Pachón, D.; Moncayo-Lasso, A.; Ibáñez, M.; Hernández, F.; Torres-Palma, R.A. Degradation of Seventeen Contaminants of Emerging Concern in Municipal Wastewater Effluents by Sonochemical Advanced Oxidation Processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A Review of the Effects of Emerging Contaminants in Wastewater and Options for Their Removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent Developments in Physical, Biological, Chemical, and Hybrid Treatment Techniques for Removing Emerging Contaminants from Wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Huber, M.M.; Göbel, A.; Joss, A.; Hermann, N.; Löffler, D.; McArdell, C.S.; Ried, A.; Siegrist, H.; Ternes, T.A.; Von Gunten, U. Oxidation of Pharmaceuticals during Ozonation of Municipal Wastewater Effluents: A Pilot Study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef]
- Petrović, M.; Gonzalez, S.; Barceló, D. Analysis and Removal of Emerging Contaminants in Wastewater and Drinking Water. TrAC-Trends Anal. Chem. 2003, 22, 685–696. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent Advances in Membrane Bioreactors (MBRs): Membrane Fouling and Membrane Material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, Y.; Mamrol, N.; Ren, L.; Li, X.; Shao, J.; Yang, X.; van der Bruggen, B. Membrane Bioreactors for Hospital Wastewater Treatment: Recent Advancements in Membranes and Processes. Front. Chem. Sci. Eng. 2022, 16, 634–660. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Ibrahim, H.A.H.; Amer, M.S.; Ali, E.M. Wastewater Treatment by Membrane Bioreactor as Potent and Advanced Technology. In Membrane-Based Hybrid Processes for Wastewater Treatment; Shah, M.P., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 45–72. [Google Scholar] [CrossRef]
- Blandin, G.; Ferrari, F.; Lesage, G.; Le-Clech, P.; Héran, M.; Martinez-Lladó, X. Forward Osmosis as Concentration Process: Review of Opportunities and Challenges. Membranes 2020, 10, 284. [Google Scholar] [CrossRef]
- Madsen, H.T.; Bajraktari, N.; Hélix-Nielsen, C.; Van der Bruggen, B.; Søgaard, E.G. Use of Biomimetic Forward Osmosis Membrane for Trace Organics Removal. J. Membr. Sci. 2015, 476, 469–474. [Google Scholar] [CrossRef]
- Engelhardt, S.; Sadek, A.; Duirk, S. Rejection of Trace Organic Water Contaminants by an Aquaporin-Based Biomimetic Hollow Fiber Membrane. Sep. Purif. Technol. 2018, 197, 170–177. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, J.; Yoon, Y. Removal of Contaminants of Emerging Concern by Membranes in Water and Wastewater: A Review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Cartinella, J.L.; Cath, T.Y.; Flynn, M.T.; Miller, G.C.; Hunter, K.W.; Childress, A.E. Removal of Natural Steroid Hormones from Wastewater Using Membrane Contactor Processes. Environ. Sci. Technol. 2006, 40, 7381–7386. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Relating Rejection of Trace Organic Contaminants to Membrane Properties in Forward Osmosis: Measurements, Modelling and Implications. Water Res. 2014, 49, 265–274. [Google Scholar] [CrossRef]
- Huang, M.; Chen, Y.; Huang, C.H.; Sun, P.; Crittenden, J. Rejection and Adsorption of Trace Pharmaceuticals by Coating a Forward Osmosis Membrane with TiO2. Chem. Eng. J. 2015, 279, 904–911. [Google Scholar] [CrossRef]
- Jin, X.; Shan, J.; Wang, C.; Wei, J.; Tang, C.Y. Rejection of Pharmaceuticals by Forward Osmosis Membranes. J. Hazard. Mater. 2012, 227–228, 55–61. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Feng, Y.; Shen, C.; Yang, F. Integrating Electrochemical Oxidation into Forward Osmosis Process for Removal of Trace Antibiotics in Wastewater. J. Hazard. Mater. 2015, 296, 248–255. [Google Scholar] [CrossRef]
- Heo, J.; Boateng, L.K.; Flora, J.R.V.; Lee, H.; Her, N.; Park, Y.G.; Yoon, Y. Comparison of Flux Behavior and Synthetic Organic Compound Removal by Forward Osmosis and Reverse Osmosis Membranes. J. Membr. Sci. 2013, 443, 69–82. [Google Scholar] [CrossRef]
- D’Haese, A.; Le-Clech, P.; Van Nevel, S.; Verbeken, K.; Cornelissen, E.R.; Khan, S.J.; Verliefde, A.R.D. Trace Organic Solutes in Closed-Loop Forward Osmosis Applications: Influence of Membrane Fouling and Modeling of Solute Build-up. Water Res. 2013, 47, 5232–5244. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Comparison of the Removal of Hydrophobic Trace Organic Contaminants by Forward Osmosis and Reverse Osmosis. Water Res. 2012, 46, 2683–2692. [Google Scholar] [CrossRef]
- Kong, F.X.; Yang, H.W.; Wu, Y.Q.; Wang, X.M.; Xie, Y.F. Rejection of Pharmaceuticals during Forward Osmosis and Prediction by Using the Solution-Diffusion Model. J. Membr. Sci. 2015, 476, 410–420. [Google Scholar] [CrossRef]
- Xiao, T.; Dou, P.; Wang, J.; Song, J.; Wang, Y.; Li, X.M.; He, T. Concentrating Greywater Using Hollow Fiber Thin Film Composite Forward Osmosis Membranes: Fouling and Process Optimization. Chem. Eng. Sci. 2018, 190, 140–148. [Google Scholar] [CrossRef]
- Petrinic, I.; Bukšek, H.; Galambos, I.; Gerencsér-Berta, R.; Sheldon, M.S.; Helix-Nielsen, C. Removal of Naproxen and Diclofenac Using an Aquaporin Hollow Fiber Forward Osmosis Module. Desalination Water Treat. 2020, 192, 415–423. [Google Scholar] [CrossRef]
- Engelhardt, S.; Vogel, J.; Duirk, S.E.; Moore, F.B.; Barton, H.A. Urea and Ammonium Rejection by an Aquaporin-Based Hollow Fiber Membrane. J. Water Process Eng. 2019, 32, 100903. [Google Scholar] [CrossRef]
- Nikbakht Fini, M.; Madsen, H.T.; Sørensen, J.L.; Muff, J. Moving from Lab to Pilot Scale in Forward Osmosis for Pesticides Rejection Using Aquaporin Membranes. Sep. Purif. Technol. 2020, 240, 116616. [Google Scholar] [CrossRef]
- Sanahuja-Embuena, V.; Frauholz, J.; Oruc, T.; Trzaskus, K.; Hélix-Nielsen, C. Transport Mechanisms behind Enhanced Solute Rejection in Forward Osmosis Compared to Reverse Osmosis Mode. J. Membr. Sci. 2021, 636, 119561. [Google Scholar] [CrossRef]
- Li, R.; Braekevelt, S.; De Carfort, J.L.N.; Hussain, S.; Bollmann, U.E.; Bester, K. Laboratory and Pilot Evaluation of Aquaporin-Based Forward Osmosis Membranes for Rejection of Micropollutants. Water Res. 2021, 194, 116924. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Luo, W.; Guo, H.; Nghiem, L.D.; Tang, C.Y.; Gray, S.R. Trace Organic Contaminant Rejection by Aquaporin Forward Osmosis Membrane: Transport Mechanisms and Membrane Stability. Water Res. 2018, 132, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Coday, B.D.; Yaffe, B.G.M.; Xu, P.; Cath, T.Y. Rejection of Trace Organic Compounds by Forward Osmosis Membranes: A Literature Review. Environ. Sci. Technol. 2014, 48, 3612–3624. [Google Scholar] [CrossRef] [PubMed]
- Alturki, A.A.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D.; Elimelech, M. Removal of Trace Organic Contaminants by the forward Osmosis Process. Sep. Purif. Technol. 2013, 103, 258–266. [Google Scholar] [CrossRef]
- Huang, J.J.; Chen, S.; Liao, Y.; Chen, Y.; You, X.; Wang, R. Performance, Fouling and Cleaning of a Thin Film Composite Hollow Fiber Membrane during Fertiliser-Drawn Forward Osmosis Process for Micro-Polluted Water. Environ. Sci. Water Res. Technol. 2021, 7, 1279–1291. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. A Forward Osmosis-Membrane Distillation Hybrid Process for Direct Sewer Mining: System Performance and Limitations. Environ. Sci. Technol. 2013, 47, 13486–13493. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, Z.; Wang, D.K.; Diniz da Costa, J.C. Processing Municipal Wastewaters by Forward Osmosis Using CTA Membrane. J. Membr. Sci. 2014, 468, 269–275. [Google Scholar] [CrossRef]
- Xue, W.; Tobino, T.; Nakajima, F.; Yamamoto, K. Seawater-Driven Forward Osmosis for Enriching Nitrogen and Phosphorous in Treated Municipal Wastewater: Effect of Membrane Properties and Feed Solution Chemistry. Water Res. 2015, 69, 120–130. [Google Scholar] [CrossRef]
- Ortega-Bravo, J.C.; Ruiz-Filippi, G.; Donoso-Bravo, A.; Reyes-Caniupán, I.E.; Jeison, D. Forward Osmosis: Evaluation Thin-Film-Composite Membrane for Municipal Sewage Concentration. Chem. Eng. J. 2016, 306, 531–537. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Tang, J.; Wang, X.; Wu, Z. A Pilot-Scale Forward Osmosis Membrane System for Concentrating Low-Strength Municipal Wastewater: Performance and Implications. Sci. Rep. 2016, 6, 21653. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yamamoto, K.; Tobino, T. Membrane Fouling and Long-Term Performance of Seawater-Driven Forward Osmosis for Enrichment of Nutrients in Treated Municipal Wastewater. J. Membr. Sci. 2016, 499, 555–562. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, J.; Zhao, Z.; Shi, W.; Liu, D.; Cui, F. Membrane Fouling of Forward Osmosis (FO) Membrane for Municipal Wastewater Treatment: A Comparison between Direct FO and OMBR. Water Res. 2016, 104, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Hey, T.; Bajraktari, N.; Davidsson, Å.; Vogel, J.; Madsen, H.T.; Hélix-Nielsen, C.; Jansen, J.L.C.; Jönsson, K. Evaluation of Direct Membrane Filtration and Direct forward Osmosis as Concepts for Compact and Energy-Positive Municipal Wastewater Treatment. Environ. Technol. 2018, 39, 264–276. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Liang, P.; Huang, X. Direct Concentration of Municipal Sewage by Forward Osmosis and Membrane Fouling Behavior. Bioresour. Technol. 2018, 247, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tian, J.; Song, L.; Gao, S.; Shi, W.; Cui, F. Dynamic Changes of the Fouling Layer in Forward Osmosis Based Membrane Processes for Municipal Wastewater Treatment. J. Membr. Sci. 2018, 549, 523–532. [Google Scholar] [CrossRef]
- Ferrari, F.; Pijuan, M.; Rodriguez-Roda, I.; Blandin, G. Exploring Submerged Forward Osmosis for Water Recovery and Pre-Concentration of Wastewater before Anaerobic Digestion: A Pilot Scale Study. Membranes 2019, 9, 97. [Google Scholar] [CrossRef]
- Zahedi, S.; Ferrari, F.; Blandin, G.; Balcazar, J.L.; Pijuan, M. Enhancing Biogas Production from the Anaerobic Treatment of Municipal Wastewater by Forward Osmosis Pretreatment. J. Clean. Prod. 2021, 315, 128140. [Google Scholar] [CrossRef]
- Almoalimi, K.; Liu, Y.Q. Fouling and Cleaning of Thin Film Composite Forward Osmosis Membrane Treating Municipal Wastewater for Resource Recovery. Chemosphere 2022, 288, 132507. [Google Scholar] [CrossRef]
- Ortega-Bravo, J.C.; Pavez, J.; Hidalgo, V.; Reyes-Caniupán, I.; Torres-Aravena, Á.; Jeison, D. Biogas Production from Concentrated Municipal Sewage by Forward Osmosis, Micro and Ultrafiltration. Sustainability 2022, 14, 2629. [Google Scholar] [CrossRef]
- Boix, C.; Ibáñez, M.; Fabregat-Safont, D.; Morales, E.; Pastor, L.; Sancho, J.V.; Sánchez-Ramírez, J.E.; Hernández, F. Behaviour of Emerging Contaminants in Sewage Sludge after Anaerobic Digestion. Chemosphere 2016, 163, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Mohsen, M.; Ismail, S.; Alhajeri, N.S.; Osman, A.I.; Rooney, D.W. Methods to alleviate the inhibition of sludge anaerobic digestion by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 3811–3836. [Google Scholar] [CrossRef]







| Emerging Contaminant Group | Examples |
|---|---|
| Antibiotics | Ciprofloxacin, ofloxacin, sulfamethoxazole, sulfadiazine, metronidazole, erythromycin, clarithromycin, amoxicilin |
| Analgesics/ antiinflammatories | Diclofenac, naproxen, ibuprofen, salicylic acid, acetaminophen |
| Lipid regulators | Clofibric acid, gemfibrozil |
| Psychiatric drug/anticonvulsants | Carbamazepine |
| Antimicrobials | Triclosan |
| Hormones | 17-α-Etinilestradiol (EE2), 17-β-estradiol (E2), estrone (E1), progesterone |
| X-ray contrast | Iohexol, iopromide |
| Stimulants | Caffeine |
| Anti-itching | Crotamiton |
| Insect repellant | DEET (N,N-diethyl-meta-toluamide) |
| Herbicides | Atrazine |
| Artificial sweeteners | Acesulsame, sucralose, aspartame |
| Preservatives | Methylparaben, ethylparaben |
| Cardiovascular drug | Propranolol |
| Plastics additives | Bisphenol A |
| Surfactants | 4-tert-Octylphenol, 4-nonylphenol |
| UV filters | Benzophenone |
| Year | Membrane Configuration | Membrane Material | Supplier | FS Location | DS | Contaminants | Reference |
|---|---|---|---|---|---|---|---|
| 2011 | Spiral wound | CTA with embedded polyester mesh | HTI | WWTP Amsterdam West (The Netherlands) | NaCl and MgCl2·6H2O | - | [106] |
| 2013 | Flat sheet | CTA with embedded woven mesh | HTI | WWTP Wollongong (New South Wales, Australia) | NaCl | √ | [176] |
| 2014 | Spiral wound | CTA with embedded polyester mesh | HTI | WWTP Queensland (Australia) | NaCl | - | [177] |
| 2015 | Three flat-sheet membranes (TFC, CTA-1, and CTA-2) | TFC with polyamide on polysulfone with embedded support CTA-1 with embedded polyester mesh CTA-2 with embedded nonwoven support | HTI | WWTP (Japan) | Synthetic seawater | - | [178] |
| 2016 | Three flat sheet membranes | CTA with embedded polyester mesh CTA with embedded nonwoven mesh TFC with embedded polyester screen support | HTI | WWTP Temuco (Chile) | NaCl | - | [179] |
| 2016 | Spiral-wound | CTA | HTI | WWTP Shanghai (China) | NaCl | - | [180] |
| 2016 | Flat sheet | CTA with embedded polyester mesh | HTI | WWTP (Japan) | Synthetic seawater | - | [181] |
| 2016 | Flat sheet | CTA with embedded polyester mesh | HTI | WWTP (China) | Synthetic seawater | - | [182] |
| 2018 | Flat sheet | TFC with polysulfone with embedded support | Porifera | WWTP New South Wales (Australia) | NaCl and NaOAc | - | [25] |
| 2018 | Flat sheet (proprietary, PFO-100) | ABS (wetted), carbon fiber (structural) with aquaporins | Porifera | WWTP (Sweden) | NaCl | - | [183] |
| 2018 | Flat sheet | CTA with embedded polyester mesh | HTI | WWTP, Beijing (China) | NaCl | √ | [184] |
| 2018 | Flat sheet | CTA | HTI | WWTP (China) | Synthetic seawater | - | [185] |
| 2019 | Spiral wound | TFC | Toray | WWTP Valencia (Spain) | NaCl and MgCl3 | - | [186] |
| 2019 | Flat sheet | CTA with embedded polyester mesh | HTI | WWTP Beijing (China) | NaCl | - | [89] |
| 2019 | Flaat sheet | TFC | Homemade | WWTP Jinan (China) | Synthetic seawater | - | [107] |
| 2021 | Spiral wound | TFC | Toray | WWTP Girona (Spain) | Sea salt | √ | [187] |
| 2022 | Hollow fiber and flat sheet | TFC | Singapore Membrane Technology Centre | WWTP Southampton (UK) | NaCl | - | [188] |
| 2022 | Hollow fiber | TFC with aquaporins | Aquaporin A/S | WWTP Valladolid (Spain) | NaCl, MgSO4·7H2O, C6H12O6, CH3COONa, and MgCl2·6H2O | √ | [8] |
| 2022 | Flat sheet | CTA with embedded polyester mesh | HTI | WWTP Temuco (Chile) | NaCl | - | [189] |
| 2023 | Tubular (TFO-D90) | PVC with aquporins | Berghof Membrane Technology GmbH | WWTP Valladolid (Spain) | NaCl | - | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca, M.; Peña, M.; Hernandez, A.; Prádanos, P.; Palacio, L. Forward Osmosis Application for the Removal of Emerging Contaminants from Municipal Wastewater: A Review. Membranes 2023, 13, 655. https://doi.org/10.3390/membranes13070655
Salamanca M, Peña M, Hernandez A, Prádanos P, Palacio L. Forward Osmosis Application for the Removal of Emerging Contaminants from Municipal Wastewater: A Review. Membranes. 2023; 13(7):655. https://doi.org/10.3390/membranes13070655
Chicago/Turabian StyleSalamanca, Mónica, Mar Peña, Antonio Hernandez, Pedro Prádanos, and Laura Palacio. 2023. "Forward Osmosis Application for the Removal of Emerging Contaminants from Municipal Wastewater: A Review" Membranes 13, no. 7: 655. https://doi.org/10.3390/membranes13070655
APA StyleSalamanca, M., Peña, M., Hernandez, A., Prádanos, P., & Palacio, L. (2023). Forward Osmosis Application for the Removal of Emerging Contaminants from Municipal Wastewater: A Review. Membranes, 13(7), 655. https://doi.org/10.3390/membranes13070655