e-Beam and γ-rays Induced Synthesis and Catalytic Properties of Copper Nanoclusters-Deposited Composite Track-Etched Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
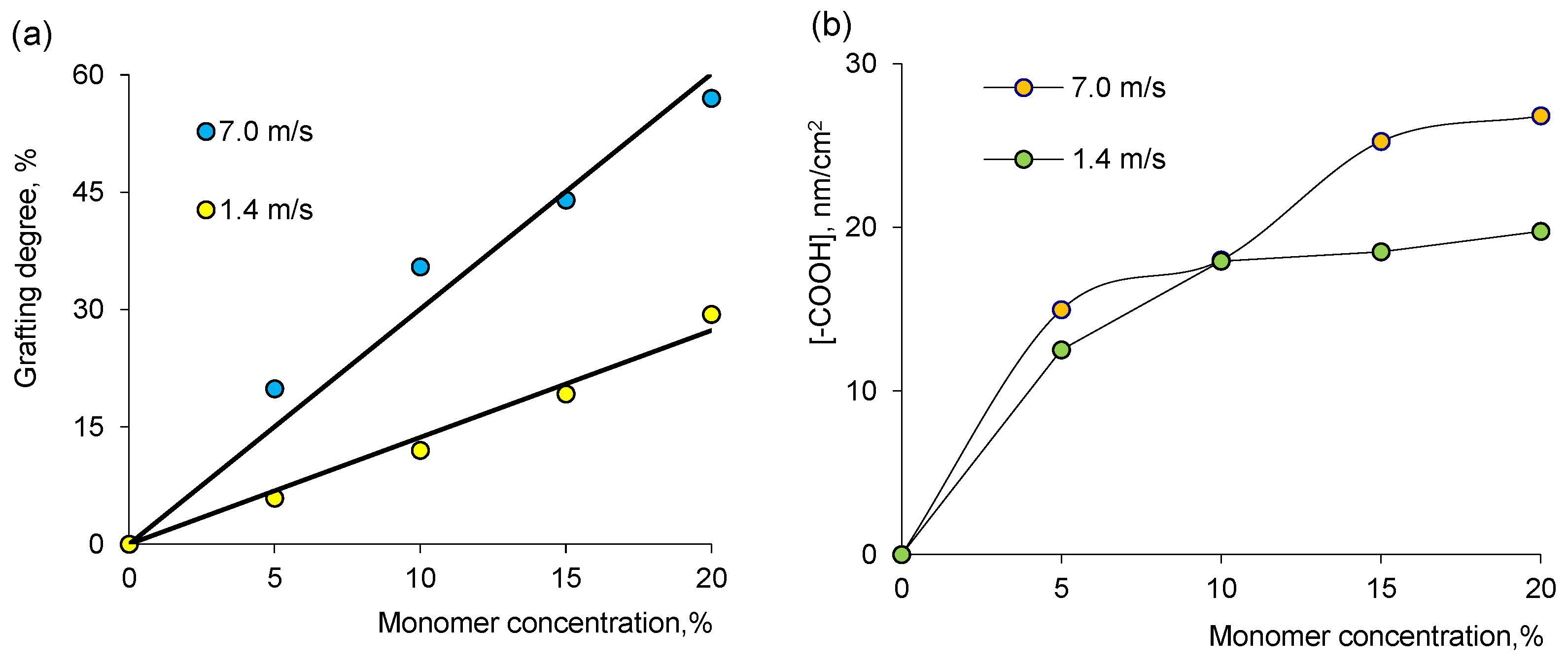
2.2. Electron Beam-Induced Grafting of Acrylic Acid
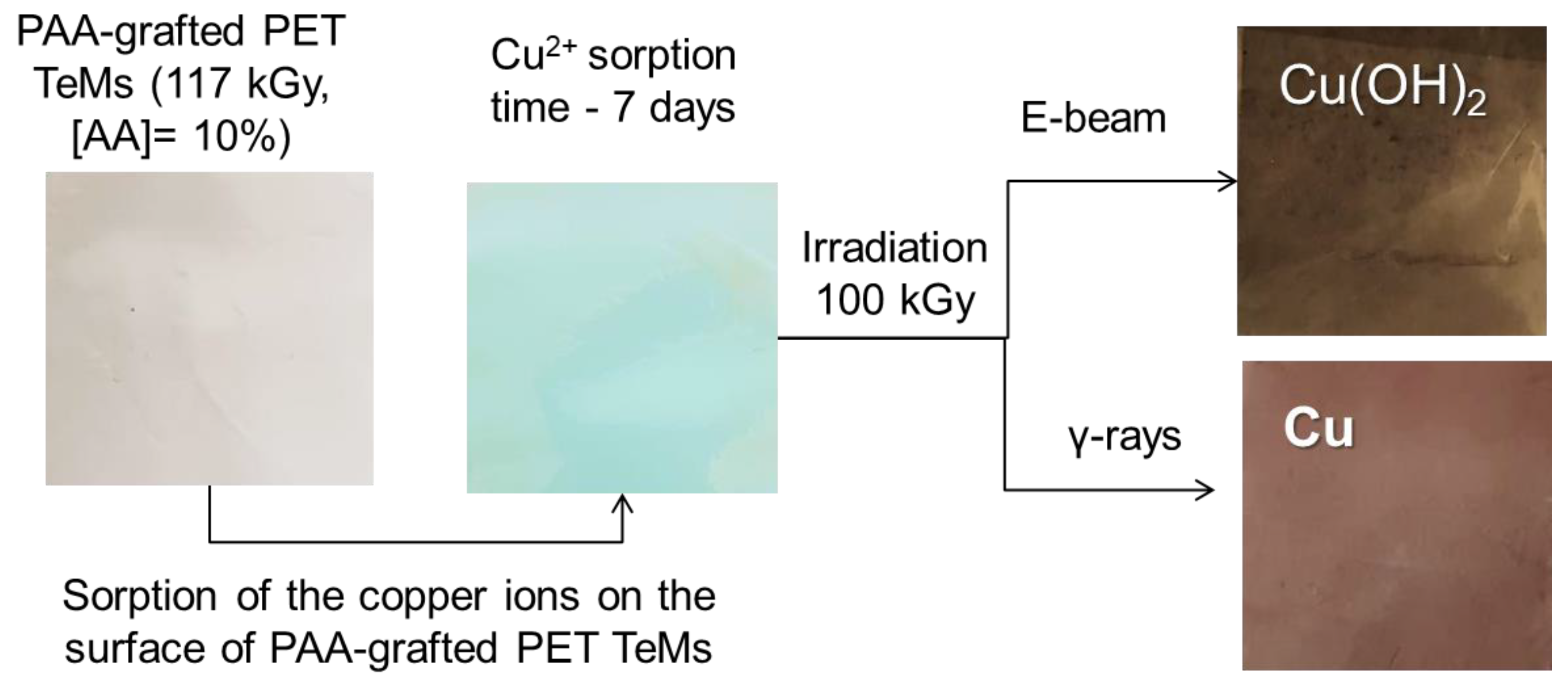
2.3. Radiation-Induced Synthesis of Copper Nanoclusters (NCs)
e-Beam and γ-rays Treatment of PET-g-PAA TeMs
2.4. Characterization
2.5. Photocatalytic Activity
3. Results and Discussion
3.1. Synthesis of PET-g-PAA TeMs by e-Beam-Mediated Grafting and Loading Them with NCs by e-Beam and γ-rays Treatments
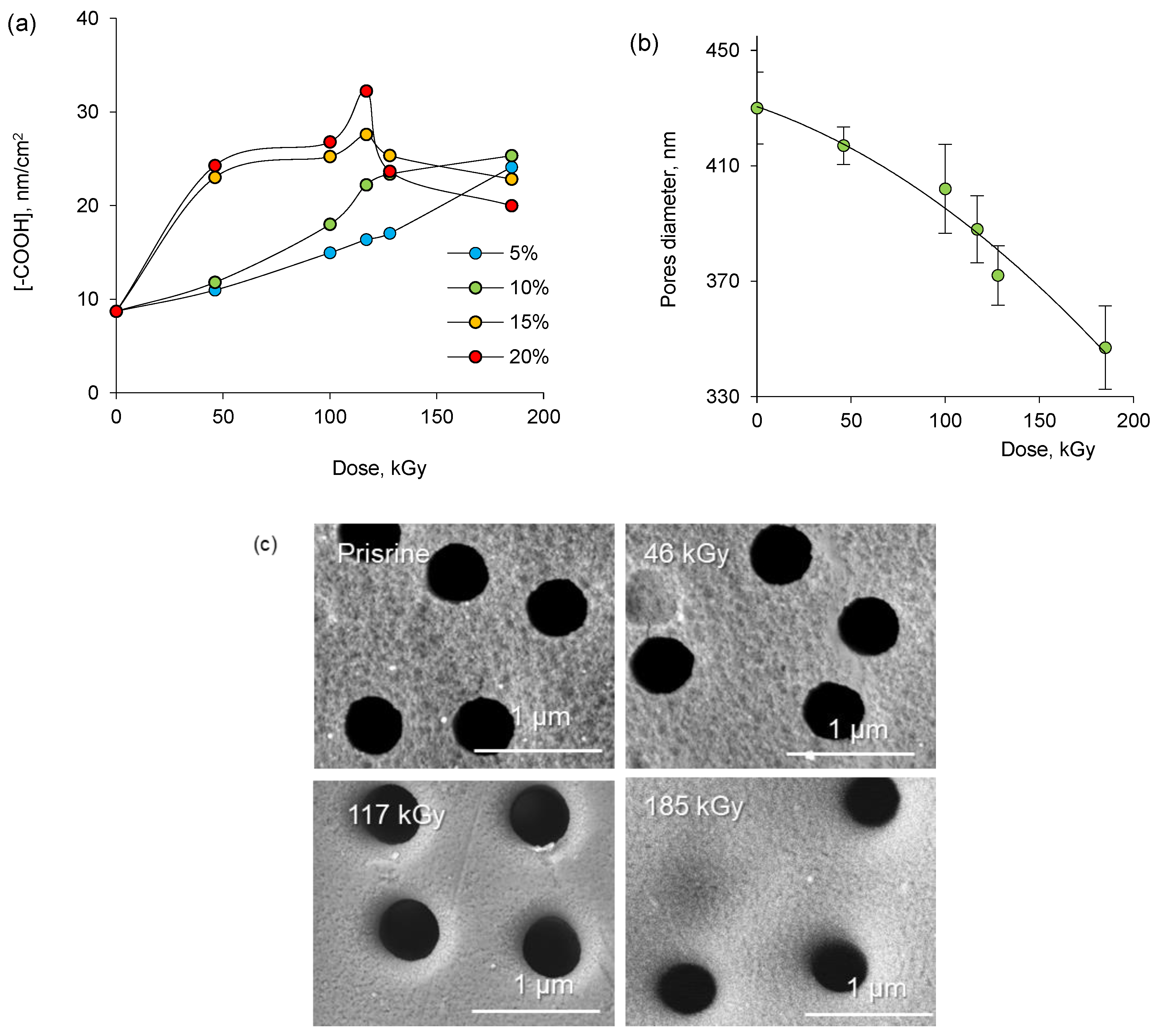
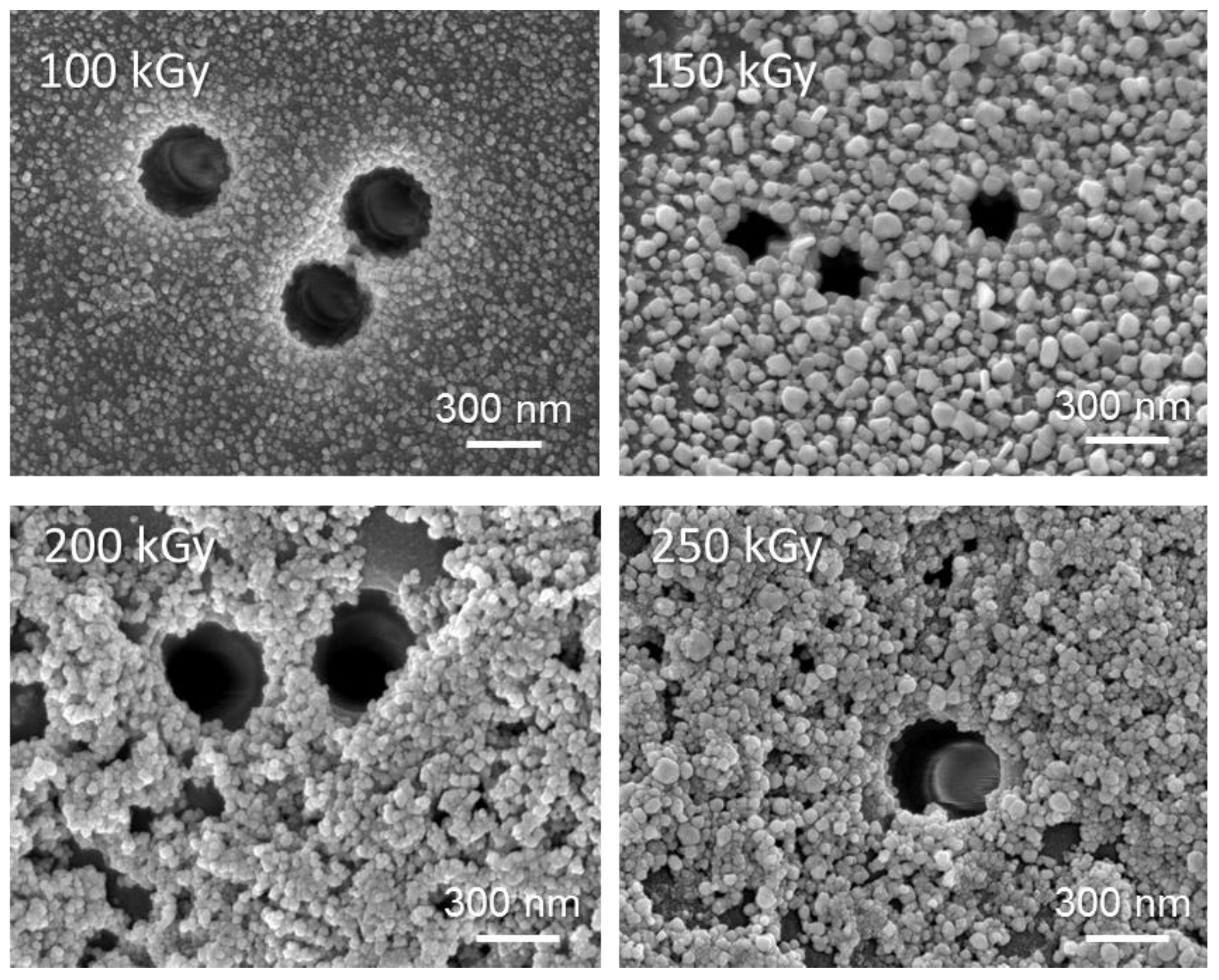
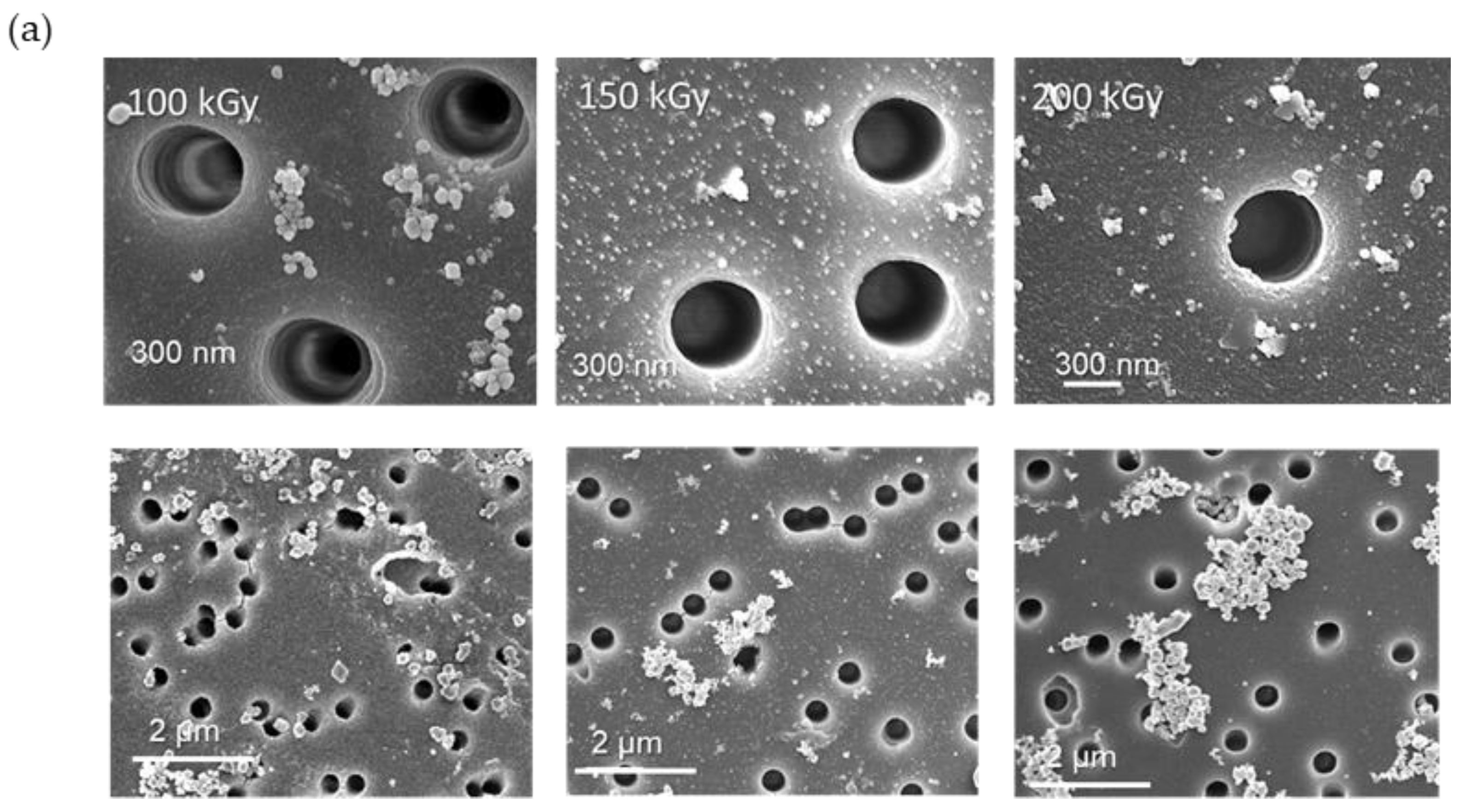
3.2. Deposition and Characterization of Copper NCs
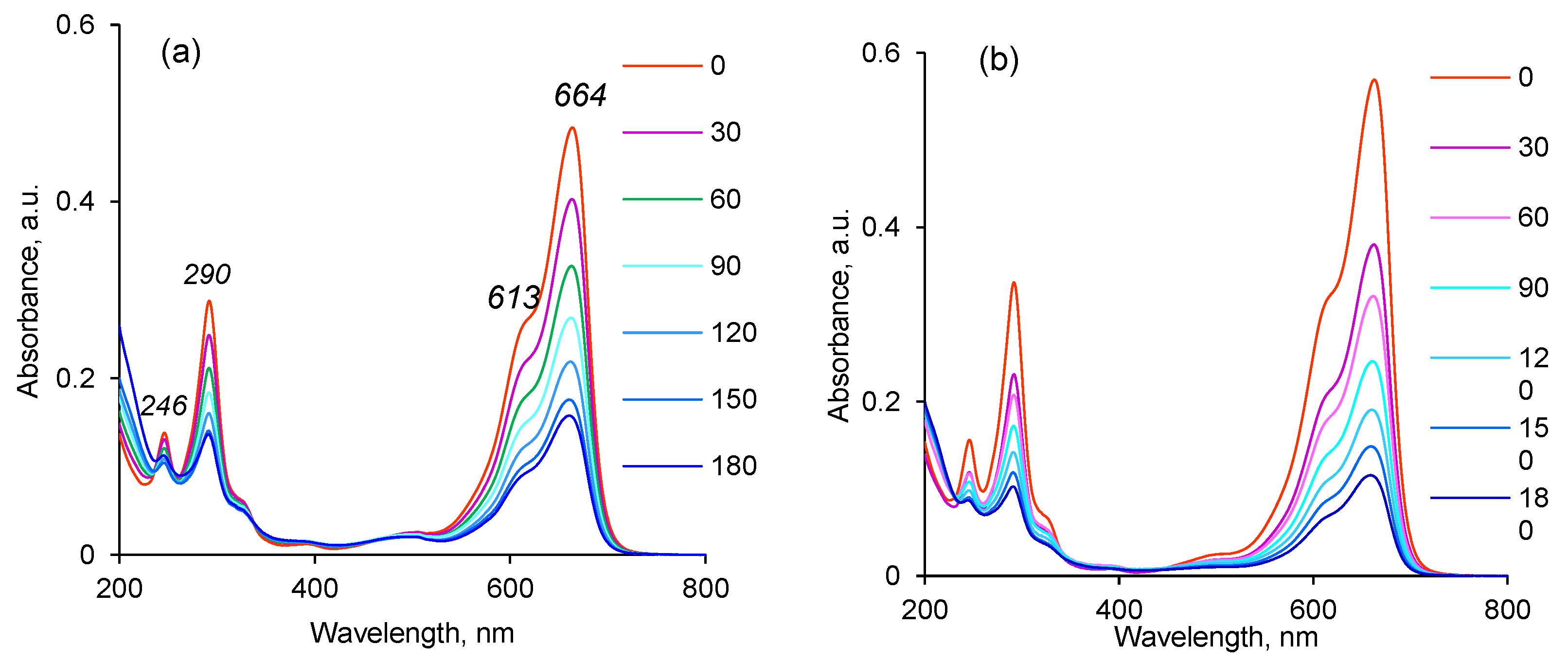
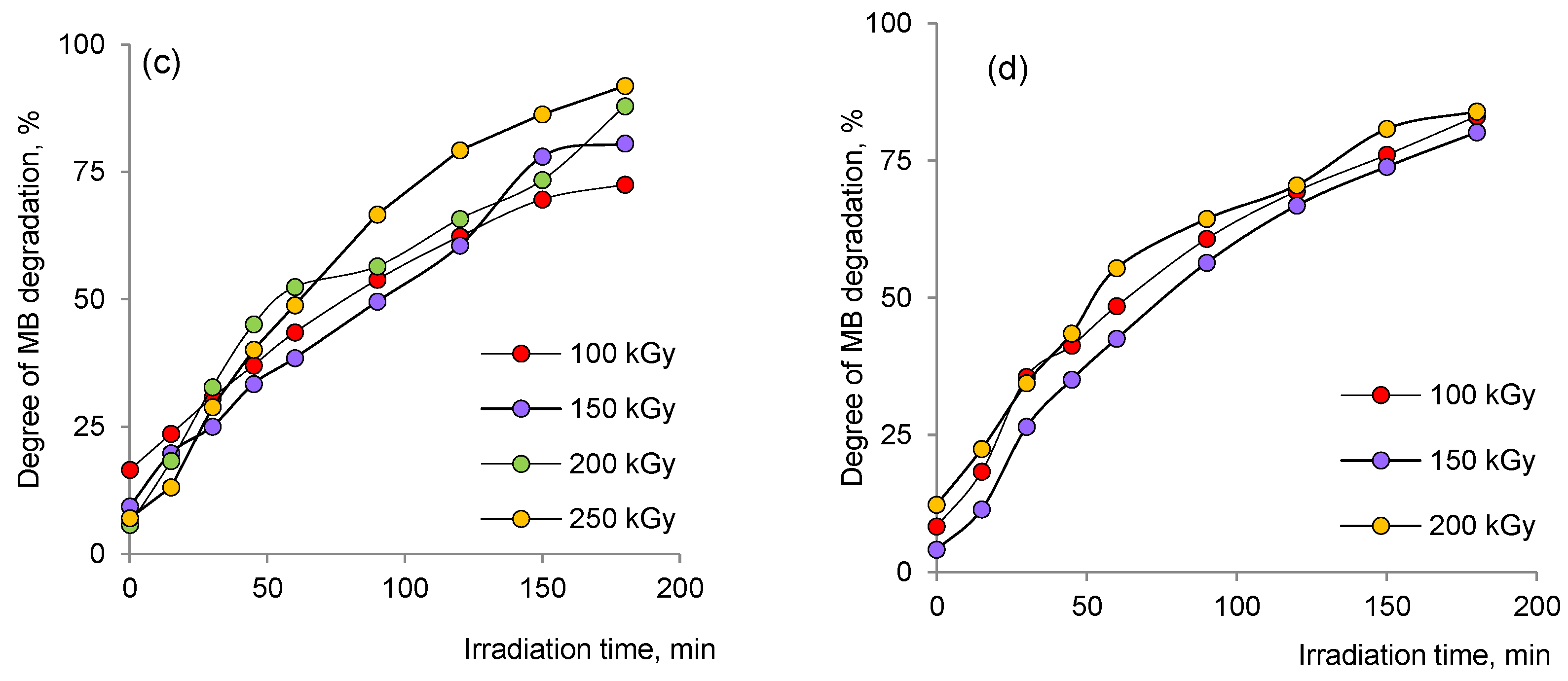
3.3. Application of the Hybrid Composites for Methylene Blue Degradation
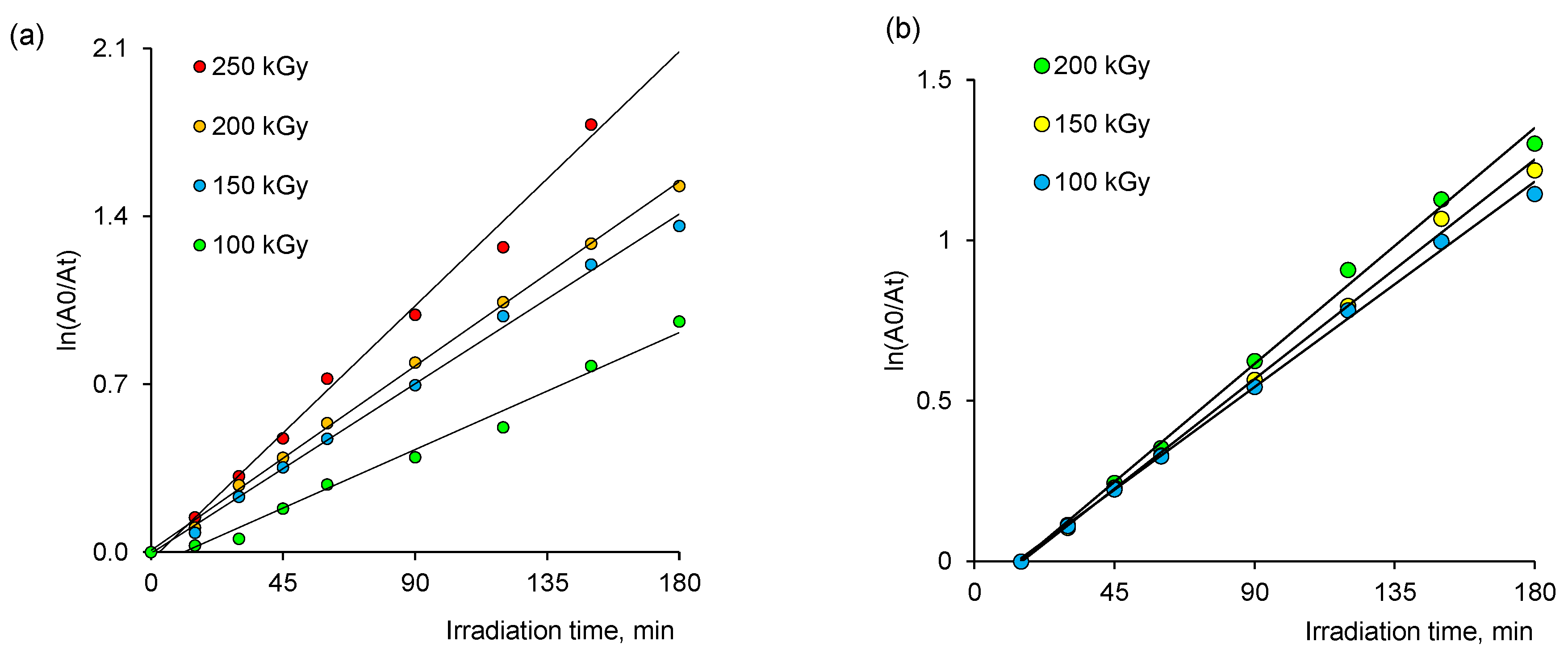
3.3.1. Thermodynamic Parameters of Photocatalytic Degradation
3.3.2. Mechanism of MB Decomposition in the Presence of Composite TeMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TeM | Track-etched membrane |
| AA | Acrylic acid |
| PAA | Poly(acrylic acid) |
| PET | Poly(ethylene terephthalate) |
| XRD | X-ray diffraction |
| PET-g-PAA | PAA-grafted PET TeM |
| Cu(OH)2@PET-g-PAA | PAA-grafted composite membrane with loaded Cu(OH)2 nanoclusters synthesized using e-beam |
| Cu@PET-g-PAA | PAA-grafted composite membrane with loaded Cu nanoclusters synthesized using γ-rays |
| NC | Nanocluster |
| SEM | Scanning electron microscopy |
| DC | Degree of crystallinity (%) |
| L | Average crystallite size (nm) |
| ka | Aate constant (min−1) |
| D | Degradation rate (%) |
| EA | Activation energy (kJ/mol) |
| ∆H | Activation enthalpy (kJ/mol) |
| ∆S | Activation entropy (kJ/mol·K) |
| ∆G | Gibbs free energy (kJ/mol) |
References
- Abadulla, E.; Tzanov, T.; Costa, S.; Cavaco, A.; Gübitz, G. Decolorization and Detoxification of Textile Dyes with a Laccase from Trametes Hirsuta Decolorization and Detoxification of Textile Dyes with a Laccase from Trametes hirsuta. Appl. Environ. Microbiol. 2000, 66, 3357–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Kant, R. Textile Dyeing Industry an Environmental Hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef] [Green Version]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Selvam, K.; Awasthi, M.K.; Gobinath, R.; Karri, R.R.; Ragunathan, M.G.; Jayanthi, J.; Mani, V.; Poudineh, M.A.; et al. Plant Microbe Based Remediation Approaches in Dye Removal: A Review. Bioengineered 2022, 13, 7798–7828. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Atalay, S.; Ersöz, G. Advanced Oxidation Processes for Removal of Dyes from Aqueous Media. In Green Chemistry for Dyes Removal from Wastewater; Wiley: Hoboken, NJ, USA, 2015; pp. 83–117. [Google Scholar]
- Ledakowicz, S.; Paździor, K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.; Sahu, S.N.; Tripathy, R.R.; Sahoo, T.; Sahu, J.R.; Pattanayak, S.K.; Sahu, R. Metal–Organic Frameworks for Heterogeneous Photocatalysis of Organic Dyes. In Photocatalytic Degradation of Dyes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 489–508. [Google Scholar]
- Beydaghdari, M.; Hooriabad Saboor, F.; Babapoor, A.; Karve, V.; Asgari, M. Recent Advances in MOF-Based Adsorbents for Dye Removal from the Aquatic Environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Grzegorzek, M.; Wartalska, K.; Kaźmierczak, B. Review of Water Treatment Methods with a Focus on Energy Consumption. Int. Commun. Heat Mass Transf. 2023, 143, 106674. [Google Scholar] [CrossRef]
- Gupta, V.K. Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Guo, C.; Wang, Y. Molybdenum Carbide-Based Photocatalysts: Synthesis, Functionalization, and Applications. Langmuir 2022, 38, 12739–12756. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent Advances in Photocatalysis for Environmental Applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Barsbay, M.; Aimanova, N.A.; Zdorovets, M.V. Application of Silver-Loaded Composite Track-Etched Membranes for Photocatalytic Decomposition of Methylene Blue under Visible Light. Membranes 2021, 11, 60. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Zhang, S.; Malik, S.; Ali, N.; Khan, A.; Bilal, M.; Rasool, K. Covalent and Non-Covalent Functionalized Nanomaterials for Environmental Restoration. Top. Curr. Chem. 2022, 380, 44. [Google Scholar] [CrossRef] [PubMed]
- Korolkov, I.V.; Güven, O.; Mashentseva, A.A.; Atıcı, A.B.; Gorin, Y.G.; Zdorovets, M.V.; Taltenov, A.A. Radiation Induced Deposition of Copper Nanoparticles inside the Nanochannels of Poly(Acrylic Acid)-Grafted Poly(Ethylene Terephthalate) Track-Etched Membranes. Radiat. Phys. Chem. 2017, 130, 480–487. [Google Scholar] [CrossRef]
- Khdary, N.H.; Almuarqab, B.T.; El Enany, G. Nanoparticle-Embedded Polymers and Their Applications: A Review. Membranes 2023, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Zdorovets, M. V Catalytic Activity of Composite Track-Etched Membranes Based on Copper Nanotubes in Flow and Static Modes. Pet. Chem. 2019, 59, 552–557. [Google Scholar] [CrossRef]
- Borgekov, D.; Mashentseva, A.; Kislitsin, S.; Kozlovskiy, A.; Russakova, A.; Zdorovets, M. Temperature Dependent Catalytic Activity of Ag/PET Ion-Track Membranes Composites. Acta Phys. Pol. A 2015, 128, 871–875. [Google Scholar] [CrossRef]
- Bräuer, P.; Muench, F.; Stojkovikj, S.; Gupta, S.; Mayer, M.T.; Ensinger, W.; Roth, C.; El-Nagar, G.A. Shape-Controlled Electroless Plating of Hetero-Nanostructures: AgCu- and AgNi-Decorated Ag Nanoplates on Carbon Fibers as Catalysts for the Oxygen Evolution Reaction. ACS Appl. Nano Mater. 2022, 5, 10348–10357. [Google Scholar] [CrossRef]
- Muench, F. Electroless Plating of Metal Nanomaterials. ChemElectroChem 2021, 8, 2993–3012. [Google Scholar] [CrossRef]
- Boettcher, T.; Schaefer, S.; Antoni, M.; Stohr, T.; Kunz, U.; Dürrschnabel, M.; Molina-Luna, L.; Ensinger, W.; Muench, F. Shape-Selective Electroless Plating within Expanding Template Pores: Etching-Assisted Deposition of Spiky Nickel Nanotube Networks. Langmuir 2019, 35, 4246–4253. [Google Scholar] [CrossRef]
- Pérez-Mitta, G.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.A.; Azzaroni, O. Molecular Design of Solid-State Nanopores: Fundamental Concepts and Applications. Adv. Mater. 2019, 31, 1–46. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Cygańczuk, K.; Pastuszka, Ł.; Jurecki, L. Nanometals-Containing Polymeric Membranes for Purification Processes. Materials 2021, 14, 513. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A Comprehensive Review on Polymeric Nano-Composite Membranes for Water Treatment. J. Membr. Sci. Technol. 2018, 8, 1000179. [Google Scholar] [CrossRef]
- Parmanbek, N.; Sütekin, D.S.; Barsbay, M.; Mashentseva, A.A.; Zheltov, D.A.; Aimanova, N.A.; Jakupova, Z.Y.; Zdorovets, M.V. Hybrid PET Track-Etched Membranes Grafted by Well-Defined Poly(2-(dimethylamino)ethyl methacrylate) Brushes and Loaded with Silver Nanoparticles for the Removal of As(III). Polymers 2022, 14, 4026. [Google Scholar] [CrossRef] [PubMed]
- Güven, O. Radiation-Assisted Synthesis of Polymer-Based Nanomaterials. Appl. Sci. 2021, 11, 7913. [Google Scholar] [CrossRef]
- Feldman, V.I.; Zezin, A.A.; Abramchuk, S.S.; Zezina, E.A. X-ray Induced Formation of Metal Nanoparticles from Interpolyelectrolyte Complexes with Copper and Silver Ions: The Radiation-Chemical Contrast. J. Phys. Chem. C 2013, 117, 7286–7293. [Google Scholar] [CrossRef]
- Zezin, A.A. Synthesis of Hybrid Materials in Polyelectrolyte Matrixes: Control over Sizes and Spatial Organization of Metallic Nanostructures. Polym. Sci. Ser. C 2016, 58, 118–130. [Google Scholar] [CrossRef]
- Bakar, A.; Güven, O.; Zezin, A.A.; Feldman, V.I. Controlling the Size and Distribution of Copper Nanoparticles in Double and Triple Polymer Metal Complexes by X-ray Irradiation. Radiat. Phys. Chem. 2014, 94, 62–65. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Gorin, Y.G.; Kozlovskiy, A.L.; Zdorovets, M.V.; Zhidkov, I.S.; Cholach, S.O. Electron/Gamma Radiation-Induced Synthesis and Catalytic Activity of Gold Nanoparticles Supported on Track-Etched Poly(Ethylene Terephthalate) Membranes. Mater. Chem. Phys. 2018, 217, 31–39. [Google Scholar] [CrossRef]
- Abedini, A.; Daud, A.R.; Abdul Hamid, M.A.; Kamil Othman, N.; Saion, E. A Review on Radiation-Induced Nucleation and Growth of Colloidal Metallic Nanoparticles. Nanoscale Res. Lett. 2013, 8, 474. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, K.; Saion, E.; Rezaee, K.; Yunus, W.M.M. Influence of Dose on Particle Size of Colloidal Silver Nanoparticles Synthesized by Gamma Radiation. Radiat. Phys. Chem. 2010, 79, 1203–1208. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Aliev, G.; Naumova, A.; Melnikov, P.; Gervald, A. Recent Developments and Perspectives of Recycled Poly(Ethylene Terephthalate)-Based Membranes: A Review. Membranes 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Z.; Wei, W.T.; Chen, W. Copper Nanoclusters: Synthesis, Characterization and Properties. Chin. Sci. Bull. 2012, 57, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Russakova, A.V.; Altynbaeva, L.S.; Barsbay, M.; Zheltov, D.A.; Zdorovets, M.V.; Mashentseva, A.A. Kinetic and Isotherm Study of As(III) Removal from Aqueous Solution by PET Track-Etched Membranes Loaded with Copper Microtubes. Membranes 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Mashentseva, A.A.; Barsbay, M.; Zdorovets, M.V.; Zheltov, D.A.; Güven, O. Cu/CuO Composite Track-Etched Membranes for Catalytic Decomposition of Nitrophenols and Removal of As(III). Nanomaterials 2020, 10, 1552. [Google Scholar] [CrossRef] [PubMed]
- Barsbay, M.; Çaylan Özgür, T.; Sütekin, S.D.; Güven, O. Effect of Brush Length of Stabilizing Grafted Matrix on Size and Catalytic Activity of Metal Nanoparticles. Eur. Polym. J. 2020, 134, 109811. [Google Scholar] [CrossRef]
- Mantilaka, M.M.M.G.P.G.; Rajapakse, R.M.G.; Karunaratne, D.G.G.P.; Pitawala, H.M.T.G.A. Preparation of Amorphous Calcium Carbonate Nanoparticles from Impure Dolomitic Marble with the Aid of Poly(Acrylic Acid) as a Stabilizer. Adv. Powder Technol. 2014, 25, 591–598. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Niyazova, D.T.; Barsbay, M.; Zdorovets, M.V. The Effect of Oxidizing Agents/Systems on the Properties of Track-Etched PET Membranes. Polym. Degrad. Stab. 2014, 107, 150–157. [Google Scholar] [CrossRef]
- Kavaklı, C.; Barsbay, M.; Tilki, S.; Güven, O.; Kavaklı, P.A. Activation of Polyethylene/Polypropylene Nonwoven Fabric by Radiation-Induced Grafting for the Removal of Cr(VI) from Aqueous Solutions. Water Air Soil Pollut. 2016, 227, 473. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Nanostructuring of Polymers by Controlling of Ionizing Radiation-Induced Free Radical Polymerization, Copolymerization, Grafting and Crosslinking by RAFT Mechanism. Radiat. Phys. Chem. 2020, 169, 107816. [Google Scholar] [CrossRef]
- Wittenberg, N.F.G.; Preusser, C.; Kattner, H.; Stach, M.; Lacík, I.; Hutchinson, R.A.; Buback, M. Modeling Acrylic Acid Radical Polymerization in Aqueous Solution. Macromol. React. Eng. 2016, 10, 95–107. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Taltenov, A.A. UV-Induced Graft Polymerization of Acrylic Acid in the Sub-Micronchannels of Oxidized PET Track-Etched Membrane. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2015, 365, 419–423. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Borgekov, D.B.; Mashentseva, A.A.; Güven, O.; Atlcl, A.B.; Kozlovskiy, A.L.; Zdorovets, M.V. The Effect of Oxidation Pretreatment of Polymer Template on the Formation and Catalytic Activity of Au/PET Membrane Composites. Chem. Pap. 2017, 71, 2353–2358. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene Blue Dye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of Methylene Blue Dye from Artificially Contaminated Water Using Citrus Limetta Peel Waste as a Very Low Cost Adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, N.; Jiang, Y.; Han, J.; Feng, G.; Shi, Z.; He, C. Rapid Photodegradation of Methylene Blue by Laser-Induced Plasma. RSC Adv. 2022, 12, 21056–21065. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yuan, S.; Jiang, W.; Yue, H.; Cui, Z.; Liang, B. Adsorption and Photocatalytic Degradation Behaviors of Rhodamine Dyes on Surface-Fluorinated TiO2 under Visible Irradiation. RSC Adv. 2016, 6, 4090–4100. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chern, J.-M. Kinetics of Photocatalytic Decomposition of Methylene Blue. Ind. Eng. Chem. Res. 2006, 45, 6450–6457. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, W.; Dong, Z.; Fan, H.-J.S. Kinetics of Catalytic Oxidation of Methylene Blue with La/Cu Co-Doped in Attapulgite. Materials 2023, 16, 2087. [Google Scholar] [CrossRef]
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts 2021, 11, 591. [Google Scholar] [CrossRef]
- Parmanbek, N.; Sütekin, S.D.; Barsbay, M.; Aimanova, N.A.; Mashentseva, A.A.; Alimkhanova, A.N.; Zhumabayev, A.M.; Yanevich, A.; Almanov, A.A.; Zdorovets, M.V. Environmentally Friendly Loading of Palladium Nanoparticles on Nanoporous PET Track-Etched Membranes Grafted by Poly(1-Vinyl-2-Pyrrolidone) via RAFT Polymerization for the Photocatalytic Degradation of Metronidazole. RSC Adv. 2023, 13, 18700–18714. [Google Scholar] [CrossRef]
- Al-Mahamad, L.L.G. Analytical Study to Determine the Optical Properties of Gold Nanoparticles in the Visible Solar Spectrum. Heliyon 2022, 8, e09966. [Google Scholar] [CrossRef] [PubMed]
- Boltaev, G.S.; Ganeev, R.A.; Krishnendu, P.S.; Zhang, K.; Guo, C. Nonlinear Optical Characterization of Copper Oxide Nanoellipsoids. Sci. Rep. 2019, 9, 11414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhusari, R.; Thomann, J.-S.; Guillot, J.; Leturcq, R. Morphology Control of Copper Hydroxide Based Nanostructures in Liquid Phase Synthesis. J. Cryst. Growth 2021, 570, 126225. [Google Scholar] [CrossRef]
- Gurav, K.V.; Patil, U.M.; Shin, S.W.; Agawane, G.L.; Suryawanshi, M.P.; Pawar, S.M.; Patil, P.S.; Lokhande, C.D.; Kim, J.H. Room Temperature Chemical Synthesis of Cu(OH)2 Thin Films for Supercapacitor Application. J. Alloys Compd. 2013, 573, 27–31. [Google Scholar] [CrossRef]
- Chandan, M.R.; Kumar, K.R.; Shaik, A.H. Two-Dimensional Cu Nanostructures for Efficient Photo-Catalytic Degradation of Methylene Blue. Environ. Sci. Adv. 2022, 1, 814–826. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. [Google Scholar] [CrossRef]
- Molinari, R.; Pirillo, F.; Falco, M.; Loddo, V.; Palmisano, L. Photocatalytic Degradation of Dyes by Using a Membrane Reactor. Chem. Eng. Process. Process Intensif. 2004, 43, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Pava-Gómez, B.; Vargas-Ramírez, X.; Díaz-Uribe, C. Physicochemical Study of Adsorption and Photodegradation Processes of Methylene Blue on Copper-Doped TiO2 Films. J. Photochem. Photobiol. A Chem. 2018, 360, 13–25. [Google Scholar] [CrossRef]
- Rouabah, N.; Nazir, R.; Djaballah, Y.; Mir, A.Q.; Ameur, I.; Beldjebli, O. Synthesis of a Thin Film of CuO/MgO/PVC Nanocomposites for Photocatalytic Applications. Iran. J. Catal. 2023, 13, 23–34. [Google Scholar] [CrossRef]
- Razi, R.; Sheibani, S. Photocatalytic Activity Enhancement by Composition Control of Mechano-Thermally Synthesized BiVO4-Cu2O Nanocomposite. Ceram. Int. 2021, 47, 29795–29806. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Aimanova, N.A.; Temirgaziev, B.S.; Zhumazhanova, A.T.; Tuleuov, B.I. Photocatalytic Activity of Copper(II) Oxide Nanoparticles Synthesized Using Serratula coronata L. Extract. Pet. Chem. 2020, 60, 1141–1147. [Google Scholar] [CrossRef]
- Karazmoudeh, N.J.; Soltanieh, M.; Hasheminiasari, M. Structural and Photocatalytic Properties of Undoped and Zn-Doped CuO Thin Films Deposited by Reactive Magnetron Sputtering. J. Alloys Compd. 2023, 947, 169564. [Google Scholar] [CrossRef]
- Akter, J.; Sapkota, K.P.; Hanif, M.A.; Islam, M.A.; Abbas, H.G.; Hahn, J.R. Kinetically Controlled Selective Synthesis of Cu2O and CuO Nanoparticles toward Enhanced Degradation of Methylene Blue Using Ultraviolet and Sun Light. Mater. Sci. Semicond. Process. 2021, 123, 105570. [Google Scholar] [CrossRef]
- Raees, A.; Jamal, M.A.; Ahmed, I.; Silanpaa, M.; Algarni, T.S. Synthesis and Characterization of Ceo2/Cuo Nanocomposites for Photocatalytic Degradation of Methylene Blue in Visible Light. Coatings 2021, 11, 305. [Google Scholar] [CrossRef]
- Poorsajadi, F.; Sayadi, M.H.; Hajiani, M.; Rezaei, M.R. Synthesis of CuO/Bi2O3 Nanocomposite for Efficient and Recycling Photodegradation of Methylene Blue Dye. Int. J. Environ. Anal. Chem. 2022, 102, 7165–7178. [Google Scholar] [CrossRef]
- Yasin, S.A.; Zeebaree, S.Y.S.; Zeebaree, A.Y.S.; Zebari, O.I.H.; Saeed, I.A. The Efficient Removal of Methylene Blue Dye Using CuO/PET Nanocomposite in Aqueous Solutions. Catalysts 2021, 11, 241. [Google Scholar] [CrossRef]
- Rafique, M.; Khalid, N.R.; Irshad, M.; Shafiq, F.; Usman, M.; Fouad, Y.; Imran, M.; Assiri, M.A.; Ashraf, W.M. Visible Light-Active Pure and Lanthanum-Doped Copper Oxide Nanostructures for Photocatalytic Degradation of Methylene Blue Dye and Hydrogen Production. Energy Sci. Eng. 2023, 1, 1–13. [Google Scholar] [CrossRef]

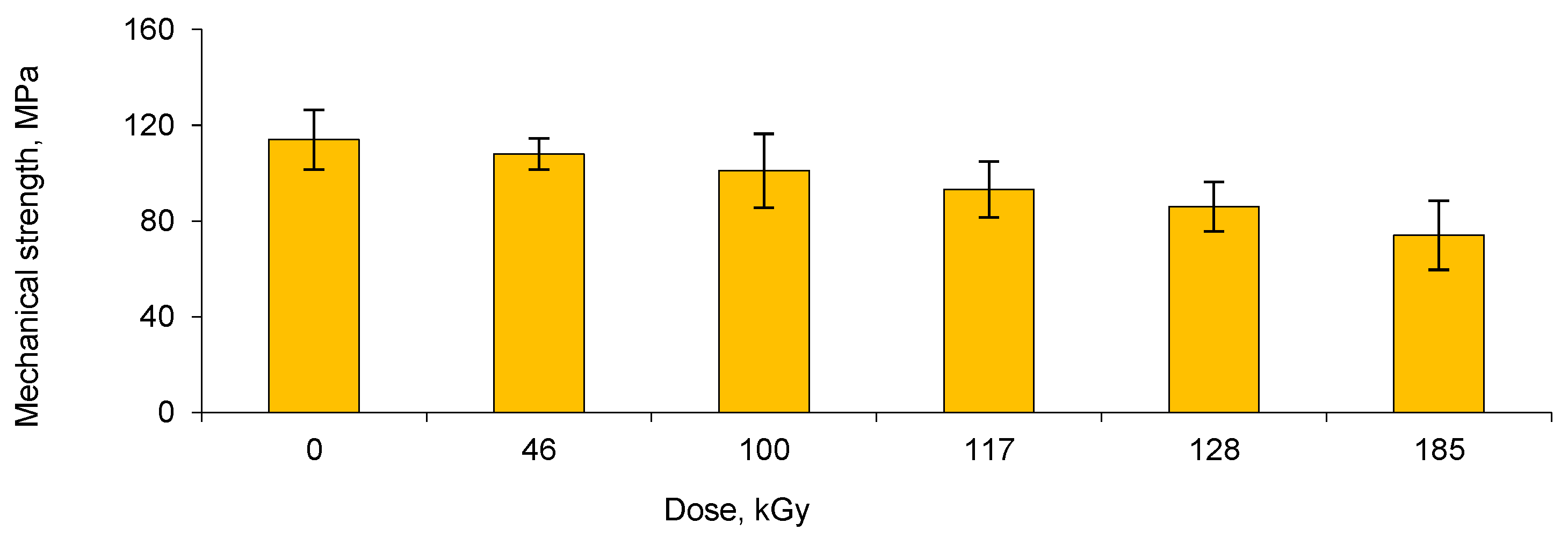
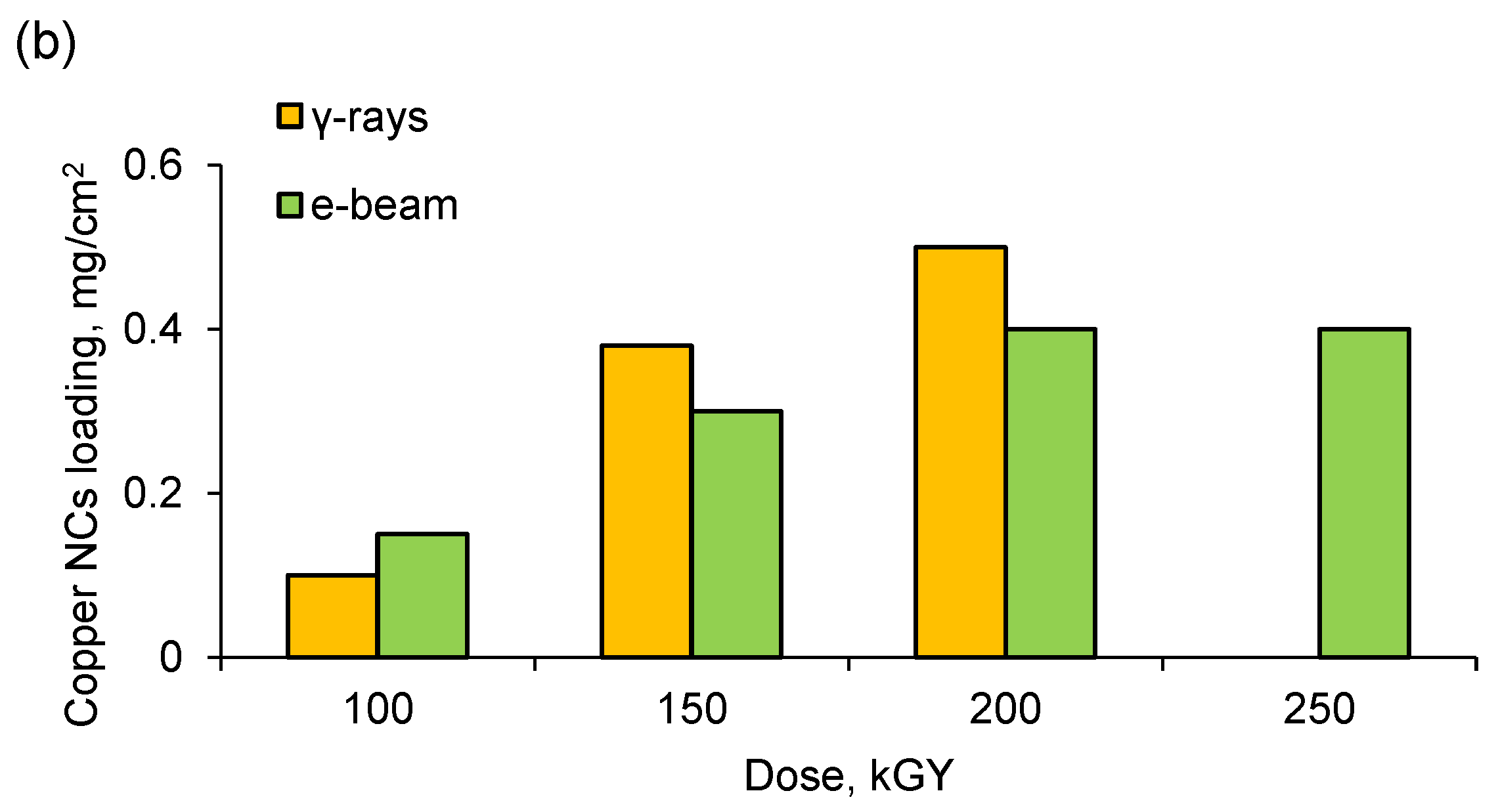
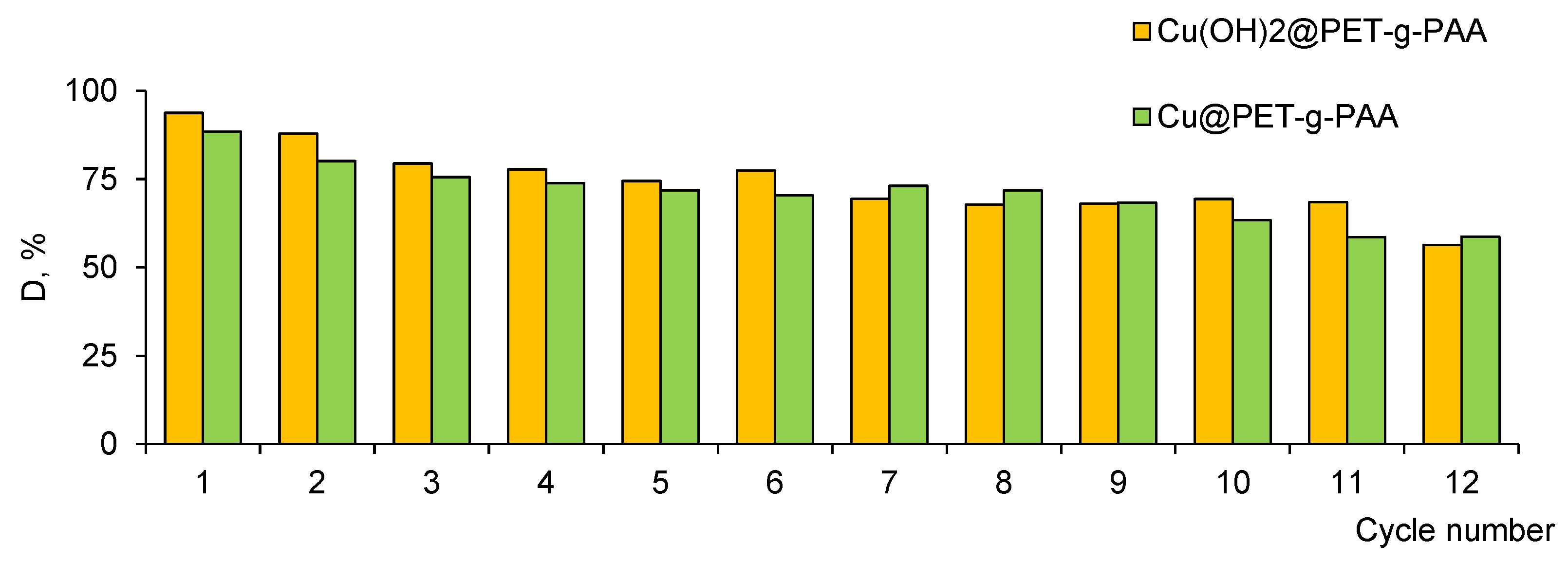

| Dose, kGy | Phase/Phase Content, % | (hkl) | 2θ° a | d, Å b | L, nm c | FWHM d | а, Å e | Crystallinity Degree, % |
|---|---|---|---|---|---|---|---|---|
| 100 | Cu(OH)2 100% | 022 | 38.20 | 2.354 | 51.48 | 0.181 | a = 2.950, b = 10.439, c = 5.283 | 67.4 |
| 131 | 44.38 | 2.040 | 30.88 | 0.309 | ||||
| 152 | 64.57 | 1.442 | 35.59 | 0.293 | ||||
| 153 | 77.50 | 1.230 | 46.44 | 0.244 | ||||
| 044 | 81.55 | 1.179 | 80.10 | 0.146 | ||||
| 150 | Cu(OH)2 100% | 022 | 38.20 | 2.354 | 42.84 | 0.218 | a = 2.947, b = 10.582, c = 5.252 | 59.3 |
| 131 | 44.38 | 2.040 | 22.80 | 0.418 | ||||
| 152 | 64.64 | 1.441 | 35.88 | 0.291 | ||||
| 222 | 75.23 | 1.262 | 71.62 | 0.155 | ||||
| 153 | 77.64 | 1.229 | 31.02 | 0.365 | ||||
| 200 | Cu(OH)2 100% | 022 | 38.23 | 2.350 | 25.13 | 0.372 | a = 2.924, b = 10.486, c = 5.233 | 43.2 |
| 131 | 43.39 | 2.084 | 44.91 | 0.212 | ||||
| 250 | Cu(OH)2 100% | 111 | 36.42 | 2.451 | 25.37 | 0.336 | a = 2.917, b = 10.530, c = 5.211 | 44.6 |
| 022 | 38.20 | 2.354 | 27.70 | 0.337 | ||||
| 131 | 43.46 | 2.081 | 45.90 | 0.207 |
| Dose, kGy | Phase/ Phase Content, % | (hkl) a | 2θ° | d, Å b | L, nm c | FWHM d | а, Å e | Crystallinity Degree, % |
|---|---|---|---|---|---|---|---|---|
| 100 | Cu 100% | 111 | 43.38 | 2.084 | 40.46 | 0.235 | a = 3.613 | 65.6 |
| 200 | 50.56 | 1.804 | 27.46 | 0.356 | ||||
| 220 | 74.16 | 1.278 | 30.51 | 0.363 | ||||
| 311 | 90.01 | 1.089 | 26.28 | 0.475 | ||||
| 150 | Cu 100% | 111 | 43.38 | 2.084 | 95.38 | 0.100 | a = 3.603 | 81.3 |
| 200 | 50.54 | 1.804 | 44.54 | 0.219 | ||||
| 200 | Cu 100% | 111 | 43.46 | 2.081 | 64.45 | 0.147 | a = 3.598 | 75.3 |
| 200 | 50.56 | 1.804 | 26.90 | 0.363 | ||||
| 220 | 74.16 | 1.278 | 57.90 | 0.191 | ||||
| 311 | 89.88 | 1.091 | 49.46 | 0.252 |
| Dose, kGy | Testing Temperature, °C | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| γ-rays/ Cu@PET-g-PAA | e-Beam/ Cu(OH)2@PET-g-PAA | |||||||||||
| D,% | ka × 10−2 (min−1) | D,% | ka × 10−2 (min−1) | |||||||||
| 20 | 30 | 40 | 20 | 30 | 40 | 20 | 30 | 40 | 20 | 30 | 40 | |
| 100 | 74.4 | 81.4 | 82.2 | 0.71 | 0.87 | 0.95 | 72.9 | 72.5 | 77.2 | 0.54 | 0.65 | 0.74 |
| 150 | 75.1 | 80.7 | 98.3 | 0.76 | 0.90 | 1.00 | 74.7 | 80.6 | 89.5 | 0.79 | 0.88 | 1.04 |
| 200 | 81.2 | 83.9 | 88.4 | 0.83 | 0.96 | 1.08 | 79.2 | 87.9 | 85.5 | 0.85 | 1.00 | 1.07 |
| 250 | - | - | - | - | - | - | 89.3 | 91.9 | 93.8 | 1.18 | 1.38 | 1.47 |
| Dose, kGy | γ-rays/ Cu@PET-g-PAA | e-Beam/ Cu(OH)2@PET-g-PAA | |||||
|---|---|---|---|---|---|---|---|
| 100 | 150 | 200 | 100 | 150 | 200 | 250 | |
| EA, kJ/mol | 11.15 | 10.49 | 10.05 | 12.03 | 10.45 | 8.81 | 8.41 |
| ∆H, kJ/mol | 8.63 | 7.97 | 7.53 | 9.51 | 7.94 | 6.29 | 5.90 |
| ∆S, kJ/mol·K | −0.26 | −0.26 | −0.26 | −0.26 | −0.26 | −0.26 | −0.26 |
| ∆G, kJ/mol (293K) | 83.73 | 83.58 | 83.38 | 84.42 | 83.54 | 83.30 | 82.50 |
| Catalyst | Catalyst Test Conditions | Catalyst Efficiency | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| MB, mg/L | Contact Time, min | Catalyst Dosage | Light Source | Lifetime, Cycle | D,% | k × 10−4, min−1 | ||
| Copper-doped TiO2 powder | 10.0 | 300 | 300 mg | Visible light | 3 | 25.1 | 14.0 | [70] |
| Copper-impregnated TiO2 powder | 42.4 | 3.7 | ||||||
| CuO/MgO/PVC | 0.05 | 150 | 5 × 2.5 cm2 | UV light | - | 44.0 | 52.0 | [71] |
| Cu2O-BiVO4 | 5.0 | 240 | 4.5 mg | Visible light | 3 | 97.0 | 9.0 | [72] |
| CuO NPs (biogenic) | 1.0 | 100 | 100.0 mg | Visible light | 5 | 69.3 | 140.0 | [73] |
| CuO/Zn film | 5.0 | 240 | 30 × 25 mm2 | Visible light | 5 | 82.0 | 67.5 | [74] |
| Cu2O | 1.0 | 120 | 0.5 mg | Sunlight | 4 | 70.0 | 108.0 | [75] |
| CuO | 4 | 63.0 | 83.0 | |||||
| CuO/CeO | 1.0 | 150 | 1.0 g/L | Visible light | - | 85.66 | - | [76] |
| Cu nanosheets | 42.0 | 100 | 500.0 mg | UV light | - | 40.0 | - | [67] |
| CuO/Bi2O3 | 20.0 | 120 | 0.2 g/L | UV light | 4 | 88.32 | 173.0 | [77] |
| CuO/PET nanocomposite | 10.0 | 30 | 40.0 mg | UV light | - | 99.0 | 2380.0 | [78] |
| CuO-3La | 0.625 | 90 | 13 mg/L | Visible light | 5 | 99.0 | 182.0 | [79] |
| Cu(OH)2@PET-g-AA (e-beam, 250 kGy) | 3.0 | 180 | 0.5 mg | UV light | 12 | 91.85 | 138.0 | This study |
| Cu@PET-g-AA (γ-rays, 200 kGy) | 0.4 mg | 12 | 83.96 | 96.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmanbek, N.; Aimanova, N.A.; Mashentseva, A.A.; Barsbay, M.; Abuova, F.U.; Nurpeisova, D.T.; Jakupova, Z.Y.; Zdorovets, M.V. e-Beam and γ-rays Induced Synthesis and Catalytic Properties of Copper Nanoclusters-Deposited Composite Track-Etched Membranes. Membranes 2023, 13, 659. https://doi.org/10.3390/membranes13070659
Parmanbek N, Aimanova NA, Mashentseva AA, Barsbay M, Abuova FU, Nurpeisova DT, Jakupova ZY, Zdorovets MV. e-Beam and γ-rays Induced Synthesis and Catalytic Properties of Copper Nanoclusters-Deposited Composite Track-Etched Membranes. Membranes. 2023; 13(7):659. https://doi.org/10.3390/membranes13070659
Chicago/Turabian StyleParmanbek, Nursanat, Nurgulim A. Aimanova, Anastassiya A. Mashentseva, Murat Barsbay, Fatima U. Abuova, Dinara T. Nurpeisova, Zhanar Ye. Jakupova, and Maxim V. Zdorovets. 2023. "e-Beam and γ-rays Induced Synthesis and Catalytic Properties of Copper Nanoclusters-Deposited Composite Track-Etched Membranes" Membranes 13, no. 7: 659. https://doi.org/10.3390/membranes13070659


_Dionysiou.jpg)






