Effect of Lu-Doping on Electrical Properties of Strontium Zirconate
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
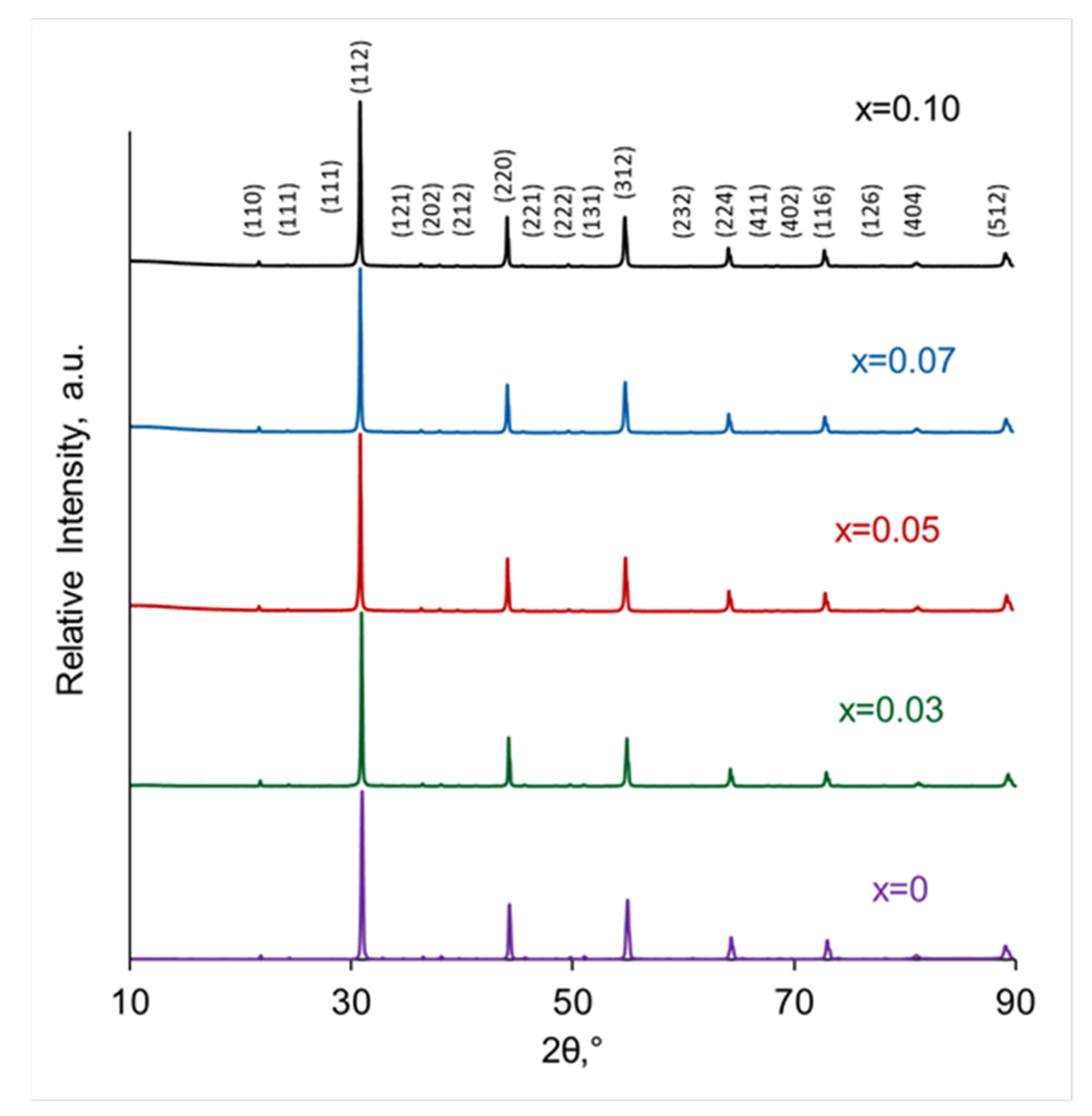
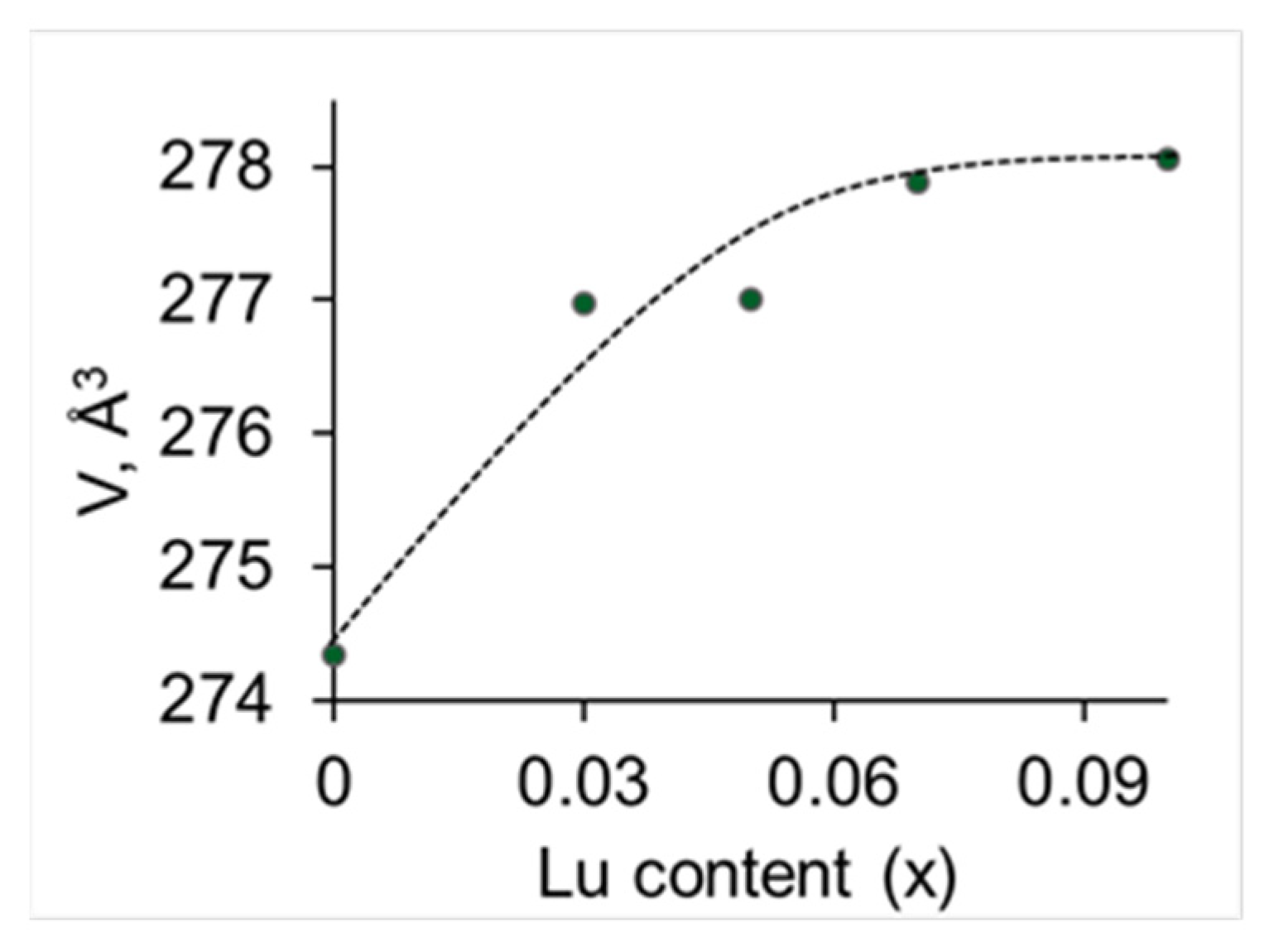
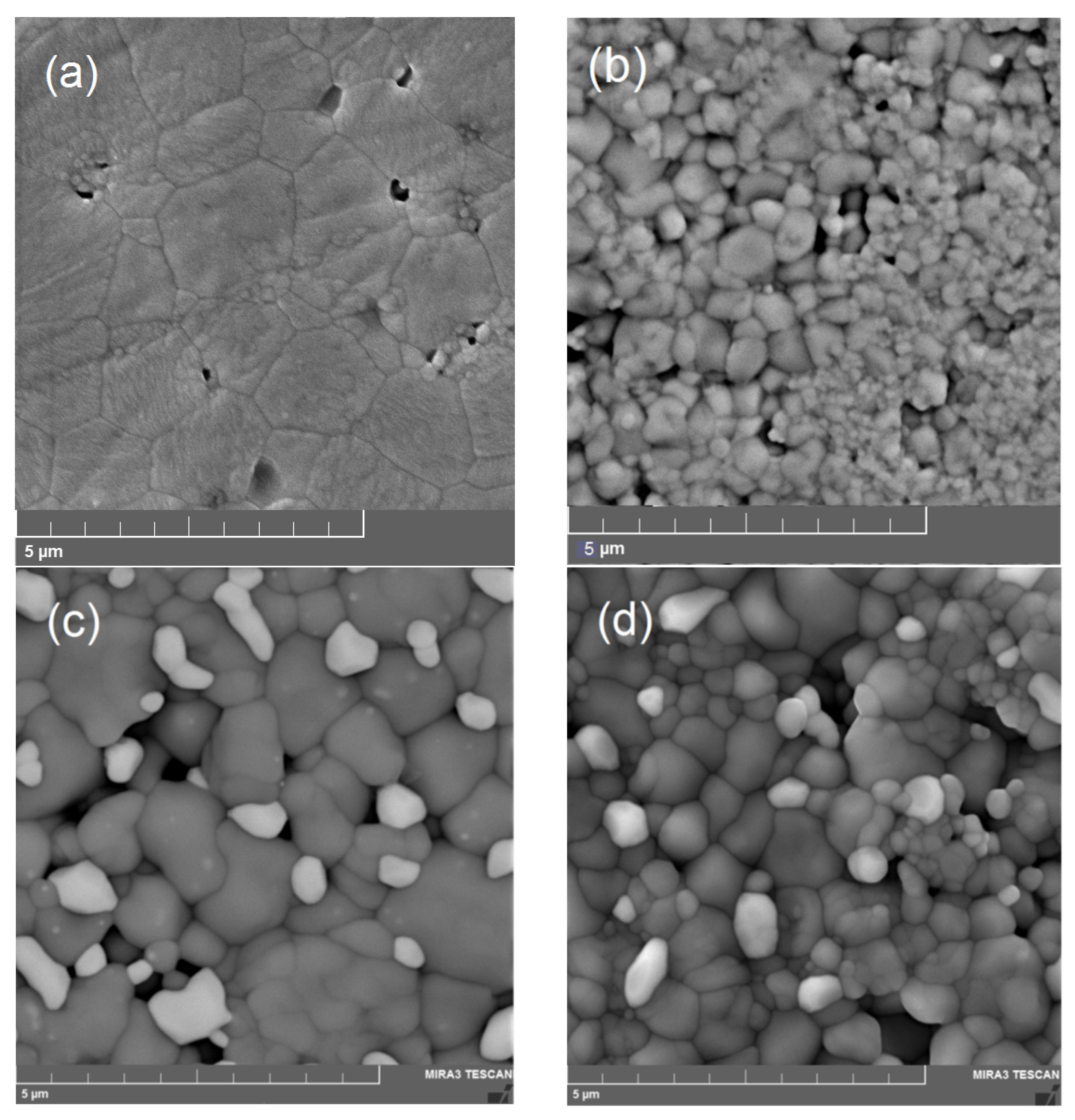
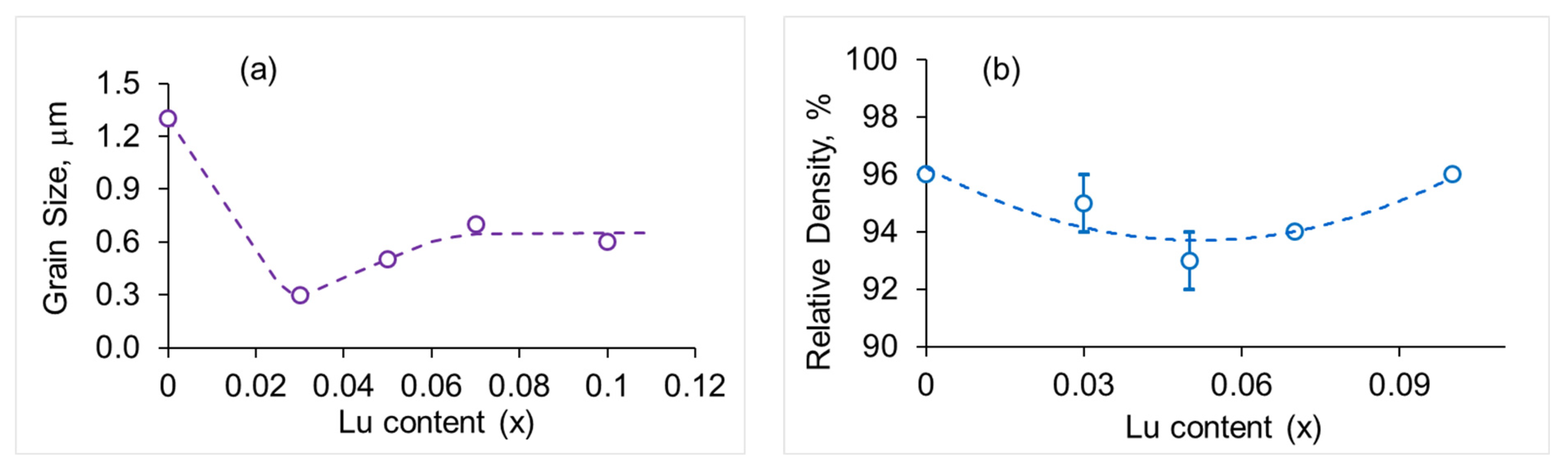
3.1. Characterization of Phase Purity, Structure and Morphology
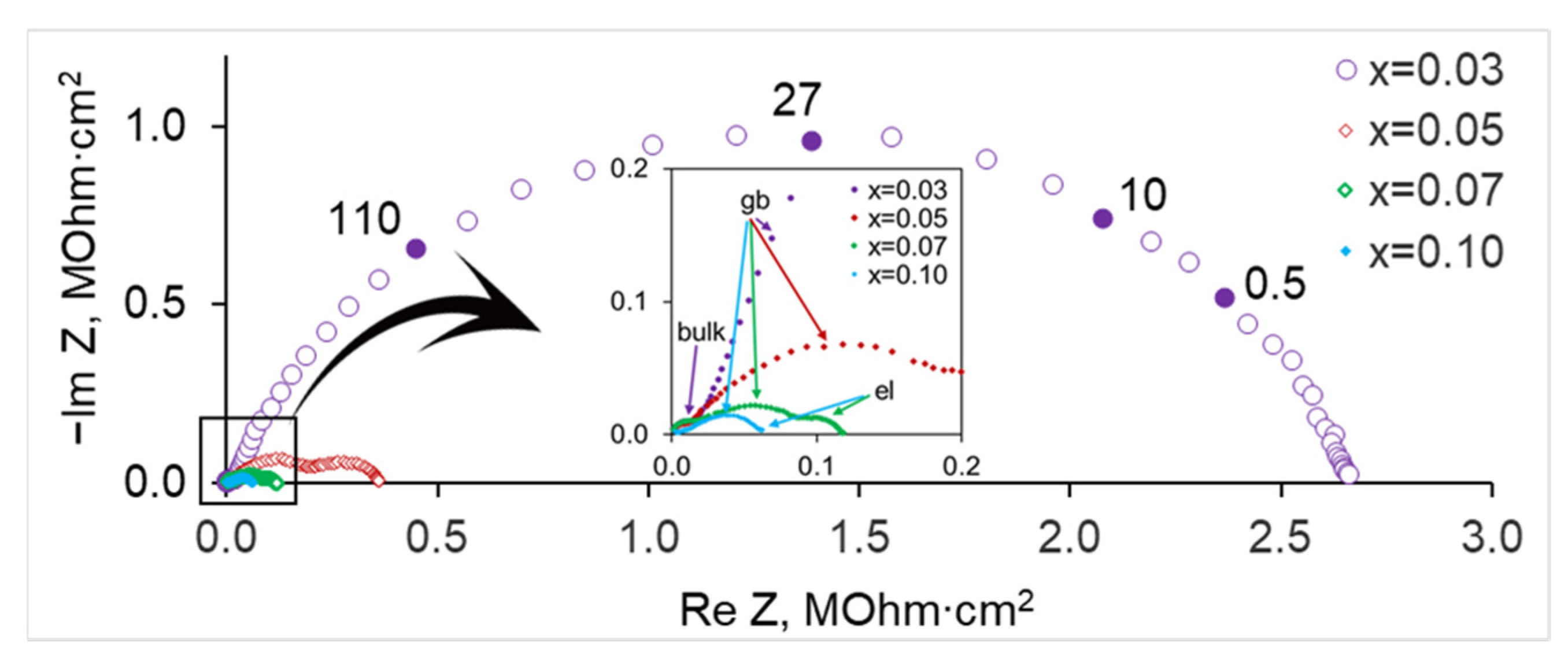
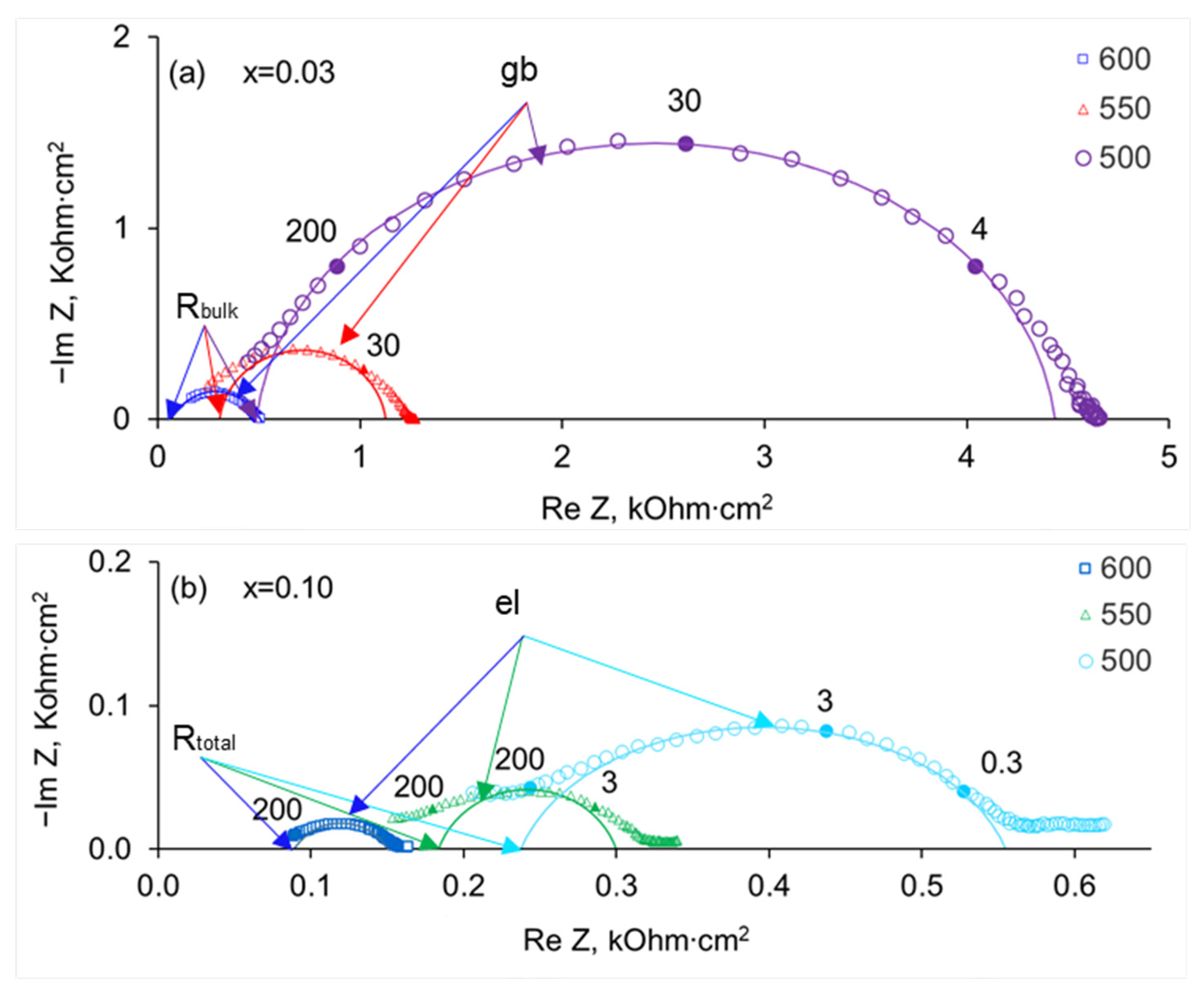
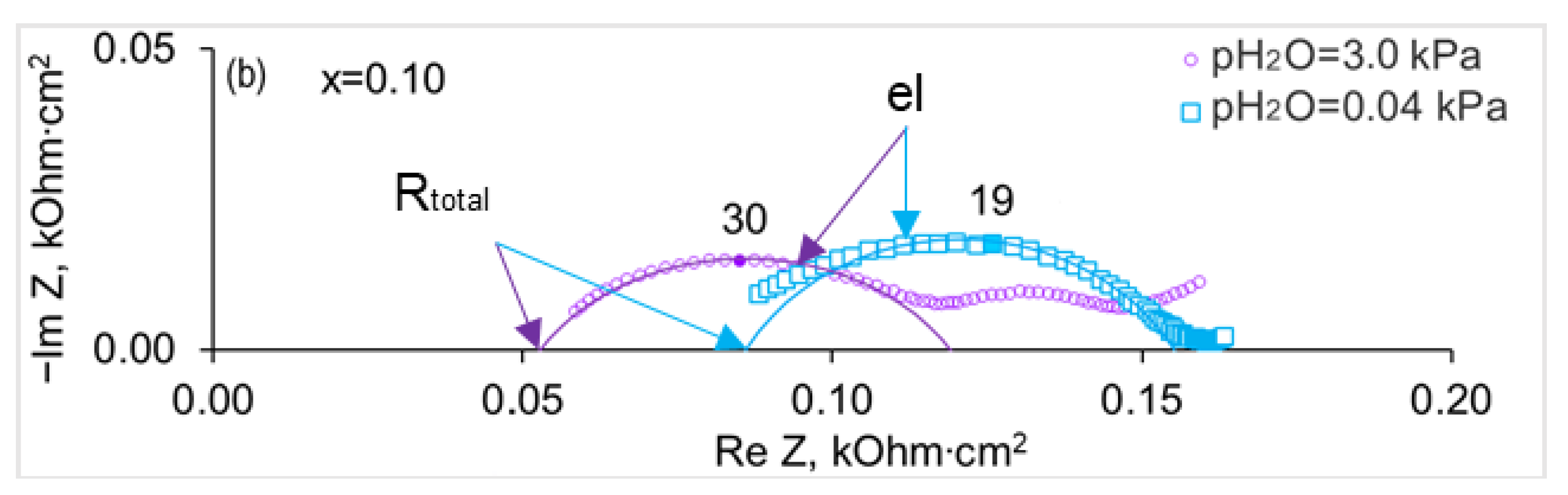
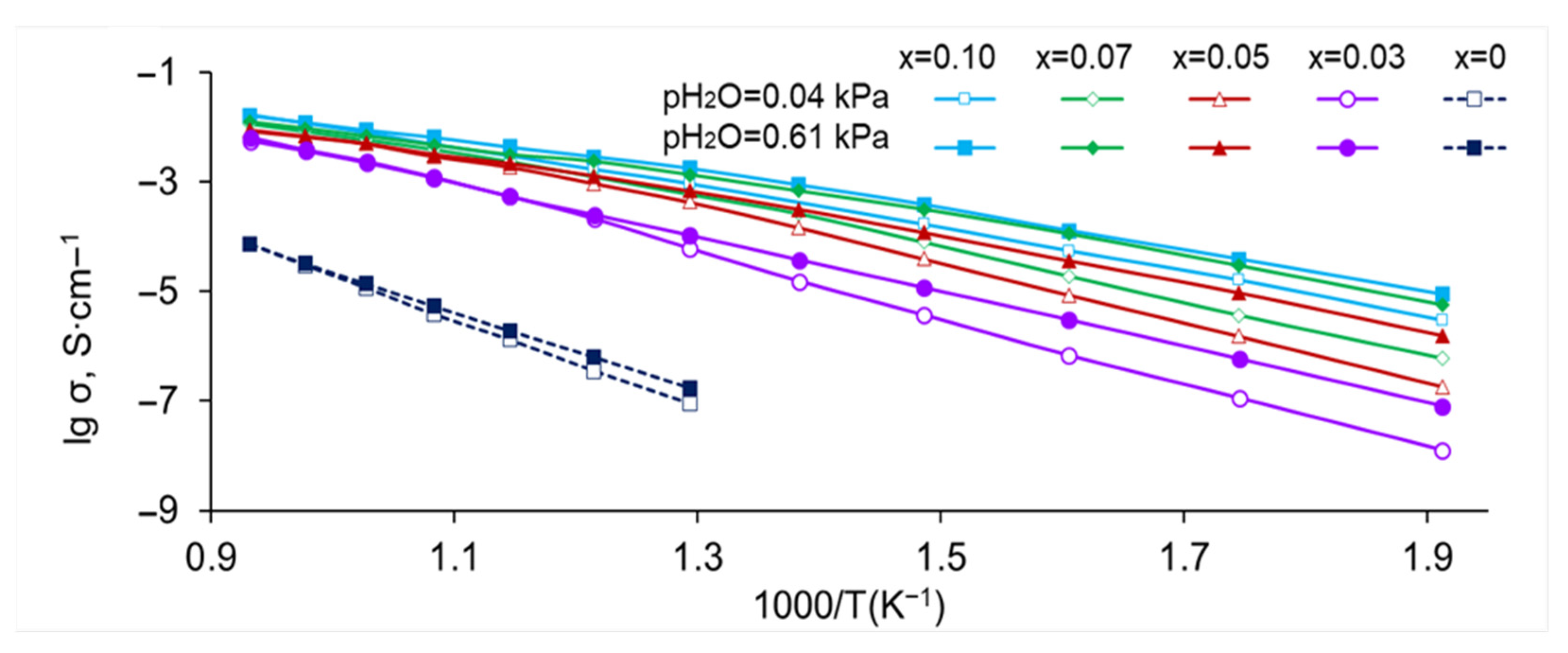
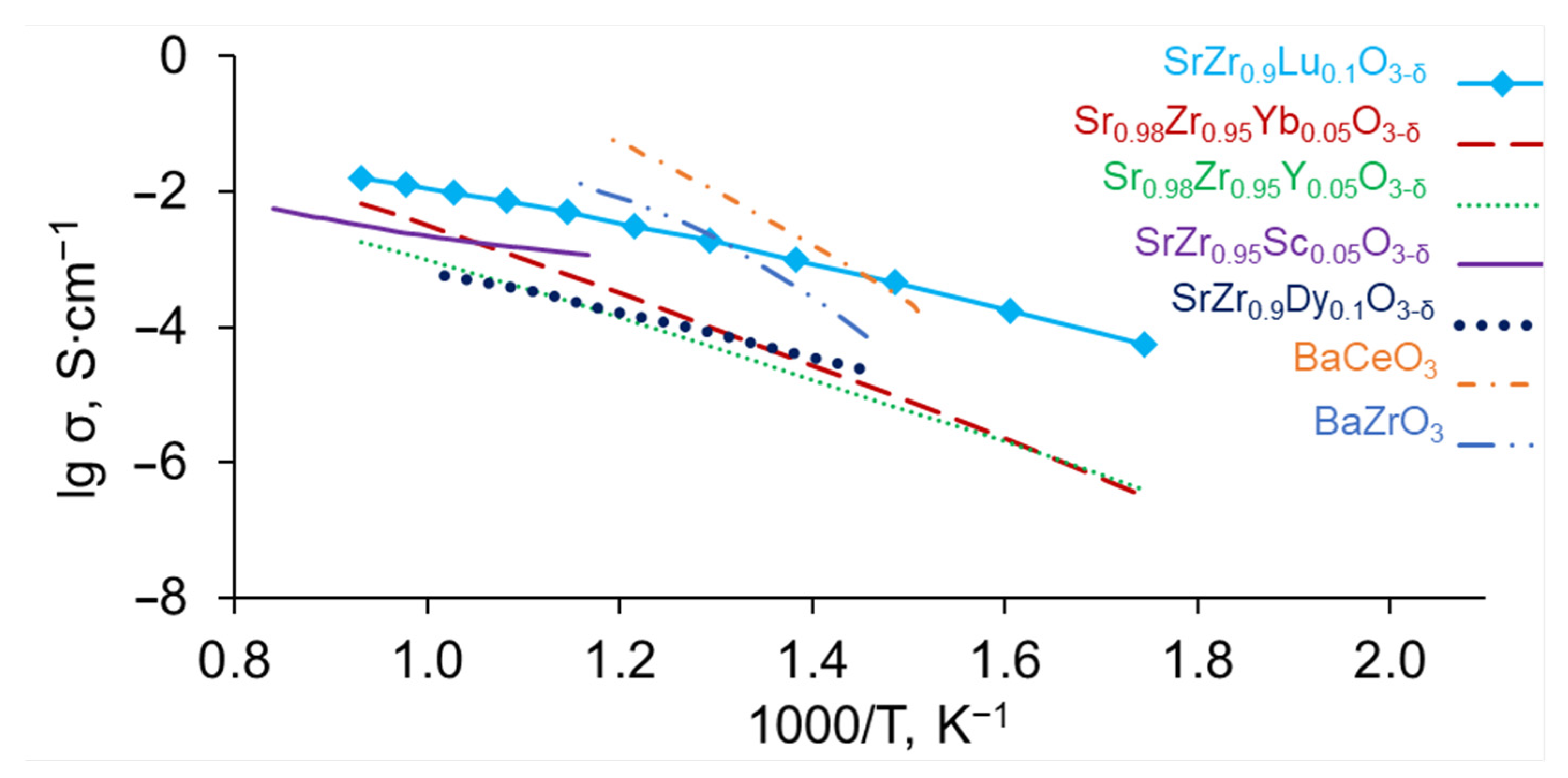
3.2. Electrical Conductivity of SrZr1−xLuxO3−δ
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreuer, K.D. Proton-Conducting Oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Azad, A.K.; Abdalla, A.M.; Afif, A.; Azad, A.; Afroze, S.; Idris, A.C.; Park, J.Y.; Saqib, M.; Radenahmad, N.; Hossain, S.; et al. Improved mechanical strength, proton conductivity and power density in an ‘all-protonic’ ceramic fuel cell at intermediate temperature. Sci. Rep. 2021, 11, 19382. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, J.; Yuan, Z.; Liu, J.; Ni, M.; Chen, F. Progress Report on Proton Conducting Solid Oxide Electrolysis Cells. Adv. Funct. Mater. 2019, 29, 1903805. [Google Scholar] [CrossRef]
- Herradon, C.; Le, L.; Meisel, C.; Huang, J.; Chmura, C.; Kim, Y.D.; Cadigan, C.; O’Hayre, R.; Sullivan, N.P. Proton-conducting ceramics for water electrolysis and hydrogen production at elevated pressure. Front. Energy Res. 2022, 10, 1020960. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hasan, S.M.K.; Hossain, M.I.; Das, R.C.; Bencherif, H.; Rubel, M.H.K.; Rahman, M.F.; Emrose, T.; Hashizume, K. A Review of Applications, Prospects, and Challenges of Proton-Conducting Zirconates in Electrochemical Hydrogen Devices. Nanomaterials 2022, 12, 3581. [Google Scholar] [CrossRef]
- Hung, I.M.; Chiang, Y.J.; Jang, J.S.C.; Lin, J.C.; Lee, S.W.; Chang, J.K.; His, C.S. The proton conduction and hydrogen permeation characteristic of Sr(Ce0.6Zr0.4)0.85Y0.15O3−δ ceramic separation membrane. J. Eur. Ceram. Soc. 2015, 35, 163–170. [Google Scholar] [CrossRef]
- Kalyakin, A.; Volkov, A.; Lyagaeva, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. Combined amperometric and potentiometric hydrogen sensors based on BaCe0.7Zr0.1Y0.2O3−δ proton-conducting ceramic. Sens. Actuators B Chem. 2016, 231, 175–182. [Google Scholar] [CrossRef]
- Tanaka, M.; Katahira, K.; Asakura, Y.; Ohshima, T. Hydrogen pump using a high-temperature proton conductor for nuclear fusion engineering applications. Solid State Ion. 2010, 181, 215–218. [Google Scholar] [CrossRef]
- Weston, L.; Janotti, A.; Cui, X.Y.; Stampfl, C.; Van deWalle, C.G. Acceptor doping in the proton conductor SrZrO3. Phys. Chem. Chem. Phys. 2017, 19, 11485–11491. [Google Scholar] [CrossRef]
- Dunyushkina, L.; Khaliullina, A.; Meshcherskikh, A.; Pankratov, A.; Osinkin, D. Effect of A-Site Nonstoichiometry on Defect Chemistry and Electrical Conductivity of Undoped and Y-Doped SrZrO3. Materials 2019, 12, 1258. [Google Scholar] [CrossRef] [Green Version]
- Yajima, T.; Suzuki, H.; Yogo, T.; Iwahara, H. Protonic conduction in SrZrO3-based oxides. Solid State Ion. 1992, 51, 101–107. [Google Scholar] [CrossRef]
- Huang, P.; Petric, A. Electrical conduction of Y-doped strontium zirconate. J. Mater. Chem. 1995, 5, 53–56. [Google Scholar] [CrossRef]
- Huang, H.H.; Ishigame, M.; Shin, S. Protonic conduction in the single-crystals of Y-doped SrZrO3. Solid State Ion. 1991, 47, 251–255. [Google Scholar] [CrossRef]
- Muller, J.; Kreuer, K.D.; Maier, J.; Matsuo, S.; Ishigame, M. A conductivity and thermal gravimetric analysis of a Y-doped SrZrO3 single crystal. Solid State Ion. 1997, 97, 421–427. [Google Scholar] [CrossRef]
- Zając, W.; Rusinek, D.; Zheng, K.; Molenda, J. Applicability of Gd-doped BaZrO3, SrZrO3, BaCeO3 and SrCeO3 proton conducting perovskites as electrolytes for solid oxide fuel cells. Cent. Eur. J. Chem. 2013, 11, 471–484. [Google Scholar] [CrossRef]
- Khaliullina, A.; Meshcherskikh, A.; Pankratov, A.; Dunyushkina, L. Effect of Sr Deficiency on Electrical Conductivity of Yb-Doped Strontium Zirconate. Materials 2022, 15, 4126. [Google Scholar] [CrossRef] [PubMed]
- Gharbage, B.; Marques, F.M.B.; Frade, J.R. Protonic conduction in Sr1-y(Zr1−xDyx)O3−δ Ceramics. J. Eur. Ceram. Soc. 1996, 16, 1149–1156. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Structural, thermal and electrical study of copper-doped strontium zirconate. Ionics 2019, 26, 6233–6244. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. Cation non-stoichiometry in yttrium-doped barium zirconate: Phase behavior, microstructure, and proton conductivity. J. Mater. Chem. 2010, 20, 8158–8166. [Google Scholar] [CrossRef] [Green Version]
- Sazinas, R.; Sunding, M.F.; Thøgersen, A.; Sakaguchi, I.; Norby, T.; Grande, T.; Polfus, J.M. Surface reactivity and cation non-stoichiometry in BaZr1−xYxO3−d (x = 0–0.2) exposed to CO2 at elevated temperature. J. Mater. Chem. A 2019, 7, 3848–3856. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Shinoda, K.; Uda, T. Dopant site occupancy and chemical expansion in rare earth-doped barium zirconate. J. Am. Ceram. Soc. 2013, 97, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Shkerin, S.N.; Rudakova, A.V.; Bulanin, K.M.; Khaliullina, A.S.; Meshcherskikh, A.N.; Vovkotrub, E.G.; Dunyushkina, L.A. Raman spectroscopy of SrZrO3 based proton conducting electrolyte: Effect of Y-doping and Sr nonstoichiometry. Int. J. Hydrog. En. 2019, 46, 17007–17018. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Huang, W.; Ding, Y. Preparation and conductive properties of CaHf1−xInxO3−δ. Ceram. Int. 2022, 48, 33773–33780. [Google Scholar] [CrossRef]
- Balakireva, V.B.; Gorelov, V.P.; Dunyushkina, L.A.; Kuzmin, A.V. Impact of Humidity on Charge Transport in Proton-Conducting Perovskites AZr0.95Sc0.05O3−α (A = Ca, Sr, Ba) Exposed to an Oxidative Atmosphere. Phys. Solid State 2019, 61, 515–522. [Google Scholar] [CrossRef]
- Rashid, N.L.R.M.; Samat, A.A.; Jais, A.A.; Somalu, M.R.; Muchtar, A.; Baharuddin, N.A.; Isahak, W.N.R.W. Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell. Ceram. Int. 2019, 45, 6605–6615. [Google Scholar] [CrossRef]




| Nominal Composition | Composition of Main Phase | Minor Phase |
|---|---|---|
| SrZrO3 | Sr0.99Zr1.00O3−δ | − |
| SrZr0.97Lu0.03O3−δ | Sr1.00Zr0.96Lu0.03O3−δ | − |
| SrZr0.95Lu0.05O3−δ | Sr1.00Zr0.96Lu0.04O3−δ | (Sr, Lu)Oy |
| SrZr0.93Lu0.07O3−δ | Sr1.00Zr0.93Lu0.04O3−δ | (Sr, Lu)Oy |
| SrZr0.90Lu0.10O3−δ | Sr1.00Zr0.94Lu0.04O3−δ | (Sr, Lu)Oy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlovich, A.; Pankratov, A.; Dunyushkina, L. Effect of Lu-Doping on Electrical Properties of Strontium Zirconate. Membranes 2023, 13, 663. https://doi.org/10.3390/membranes13070663
Pavlovich A, Pankratov A, Dunyushkina L. Effect of Lu-Doping on Electrical Properties of Strontium Zirconate. Membranes. 2023; 13(7):663. https://doi.org/10.3390/membranes13070663
Chicago/Turabian StylePavlovich, Anastasiya, Alexander Pankratov, and Liliya Dunyushkina. 2023. "Effect of Lu-Doping on Electrical Properties of Strontium Zirconate" Membranes 13, no. 7: 663. https://doi.org/10.3390/membranes13070663





