Anoxic Treatment of Agricultural Drainage Water in a Venturi-Integrated Membrane Bioreactor
Abstract
:1. Introduction
1.1. Environmental Effects of Agricultural Drainage Waters
1.2. Treatment Methods for Agricultural Drainage Waters
1.3. Membrane Bioreactors
1.4. Hydrogenotrophic Denitrification
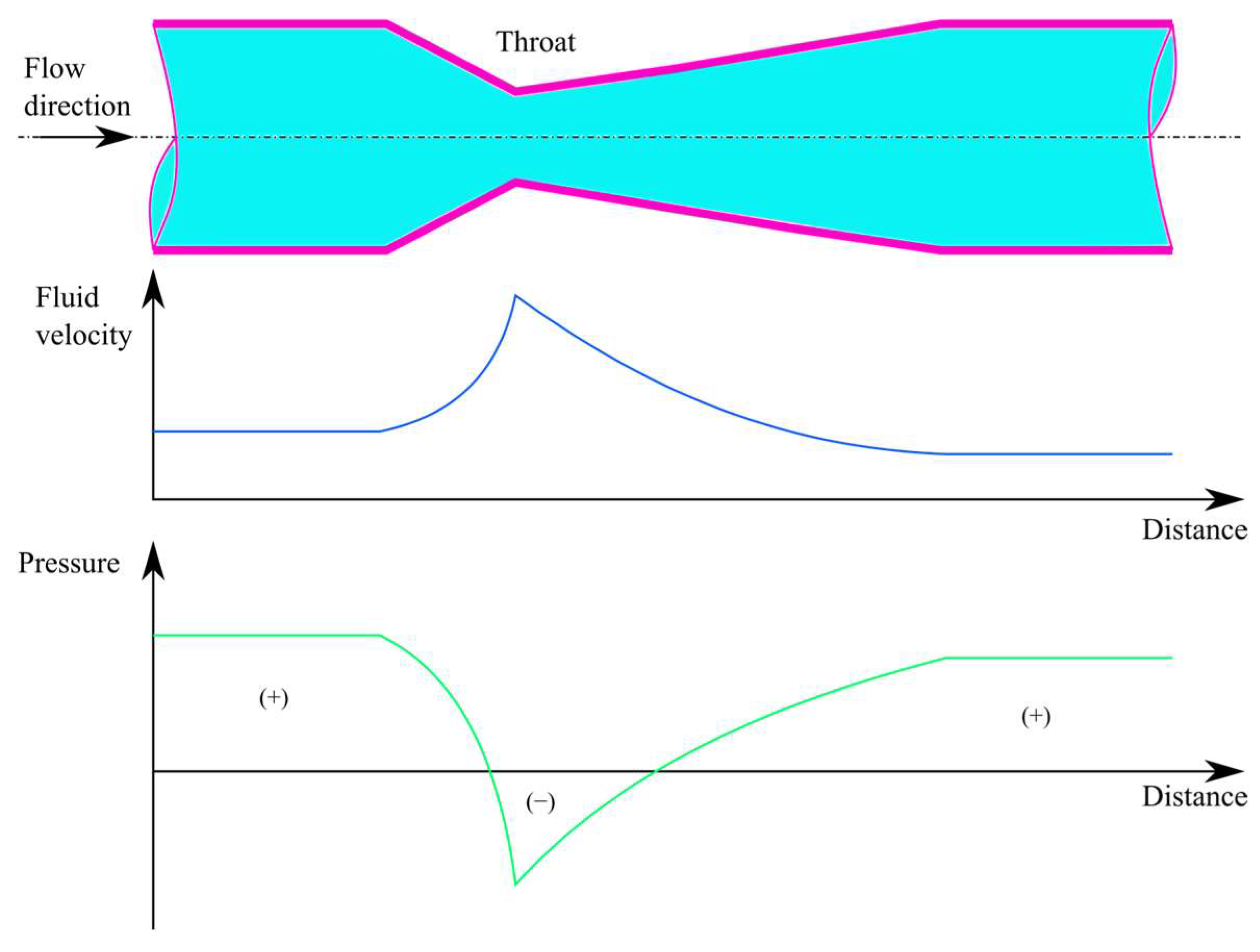
1.5. Venturi Injectors
2. Materials and Methods
2.1. Feedwater Characteristics
2.2. Analyses
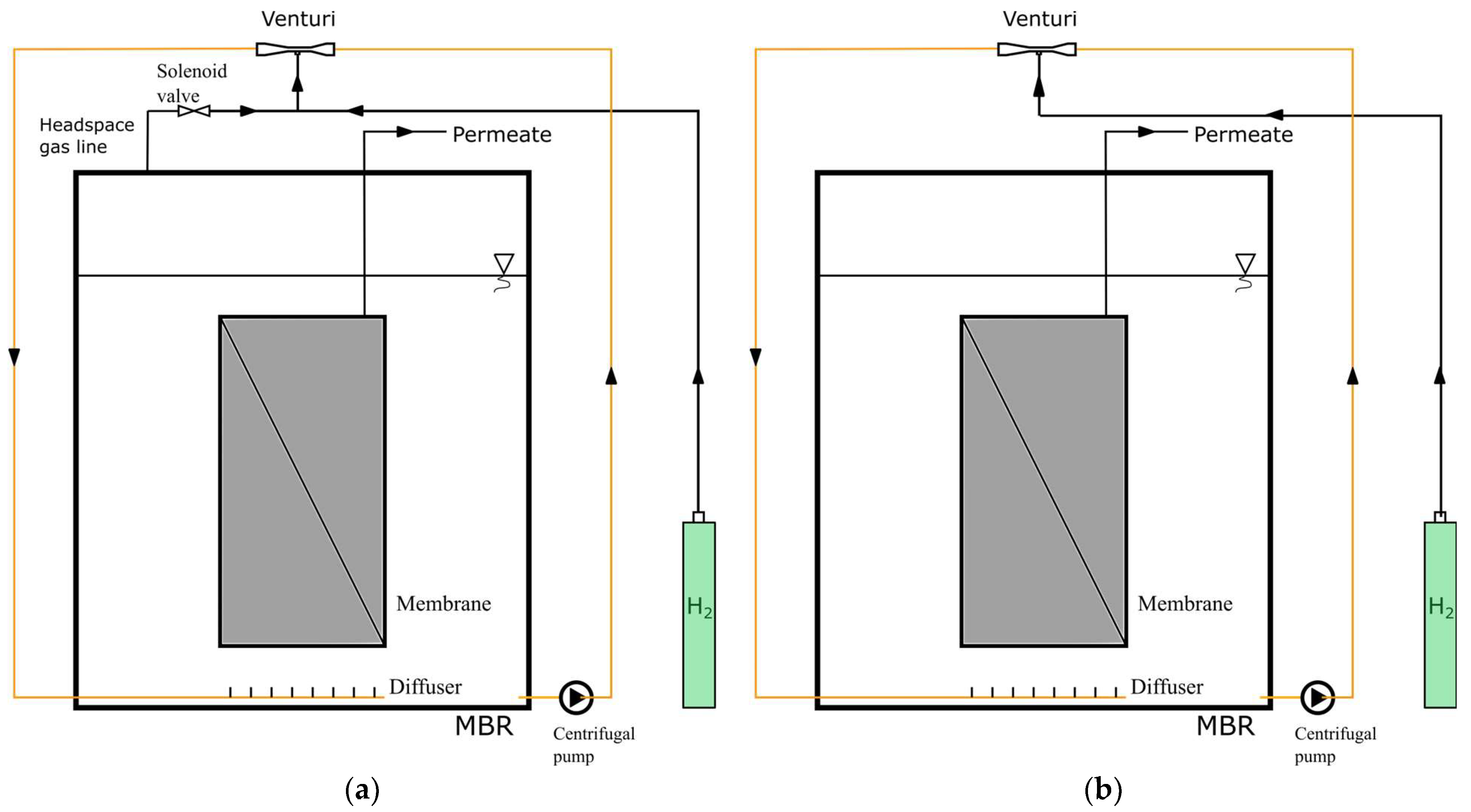
2.3. Experimental Setup
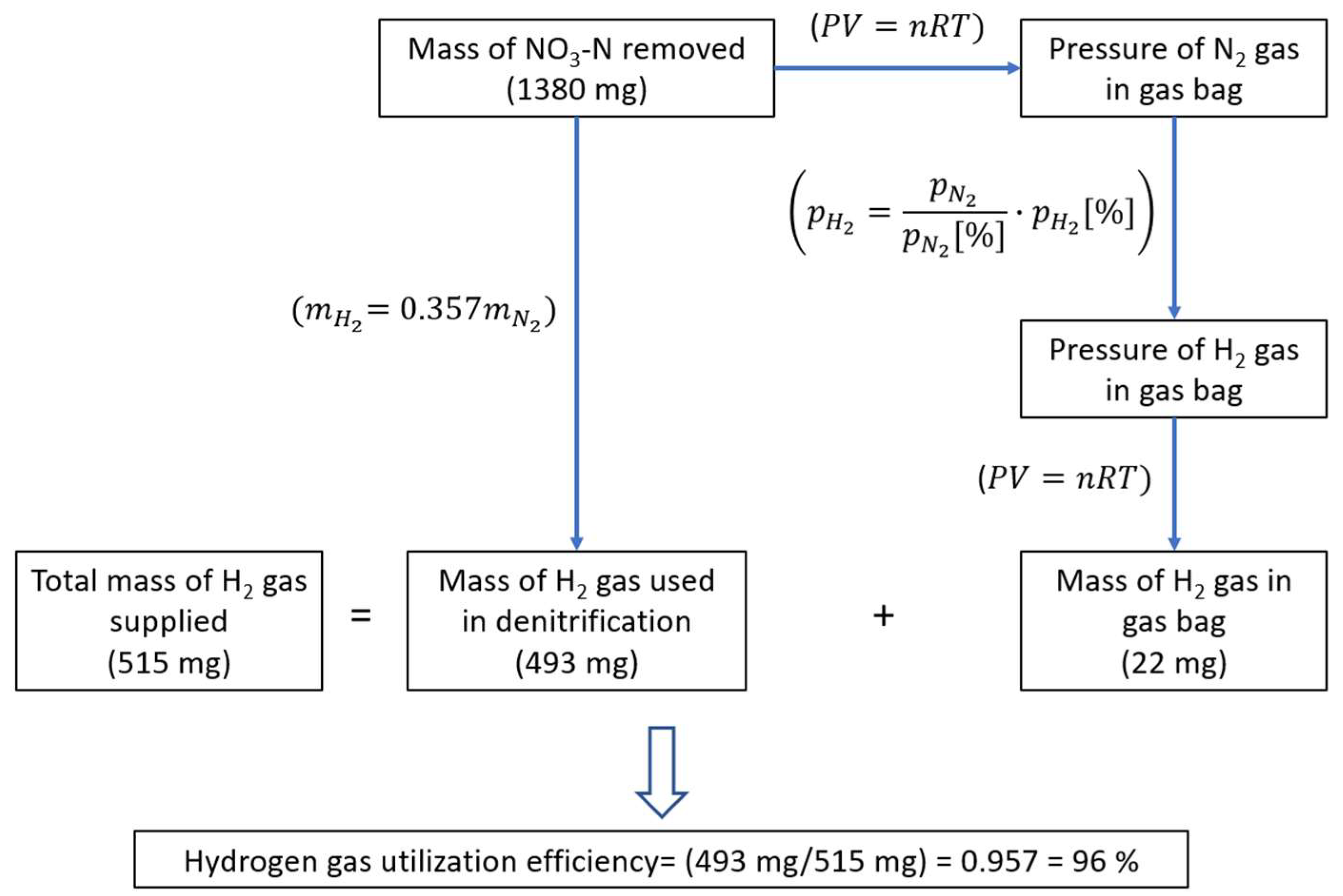
2.4. Calculation of H2 Gas Utilization Efficiency
3. Results and Discussion
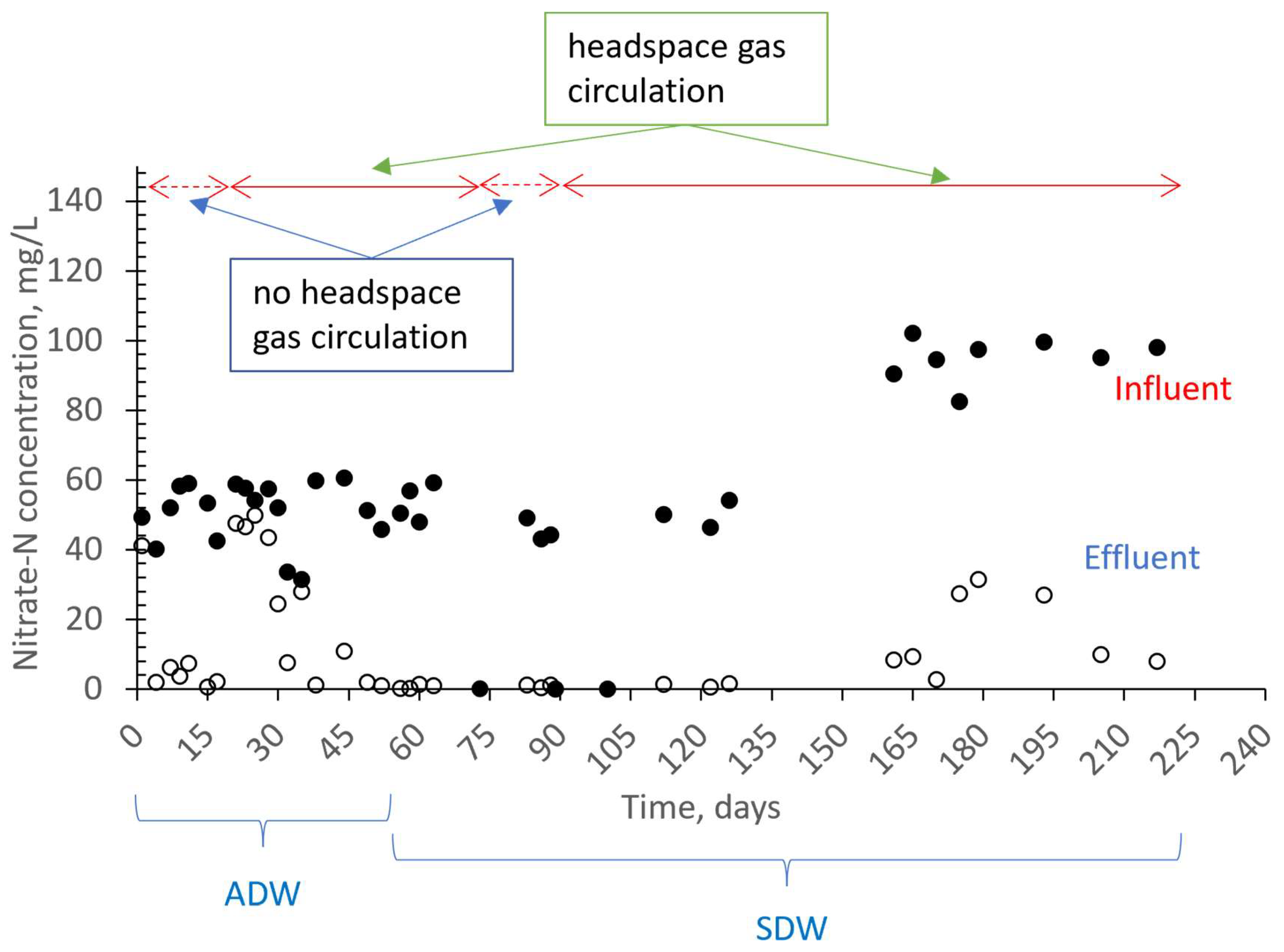
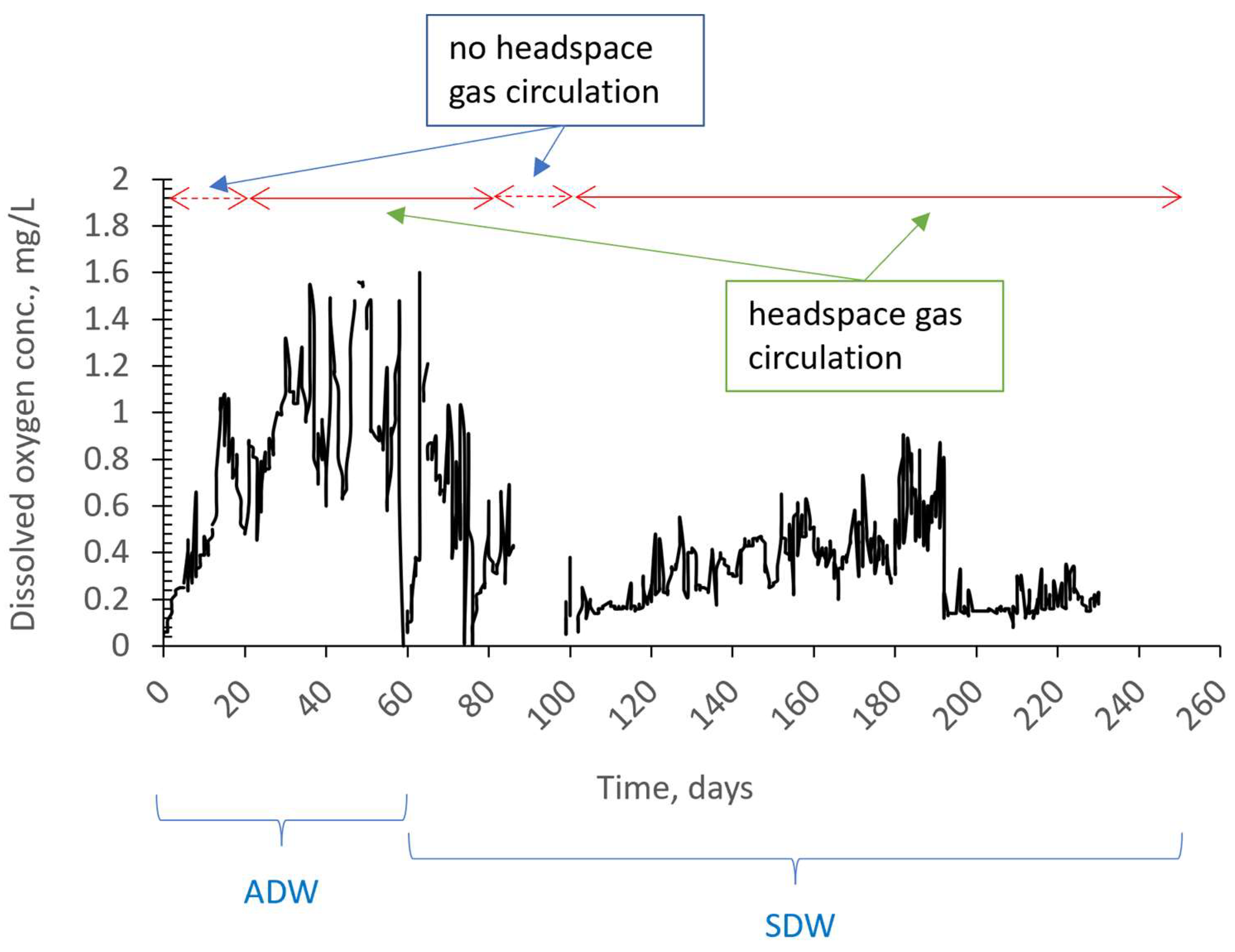
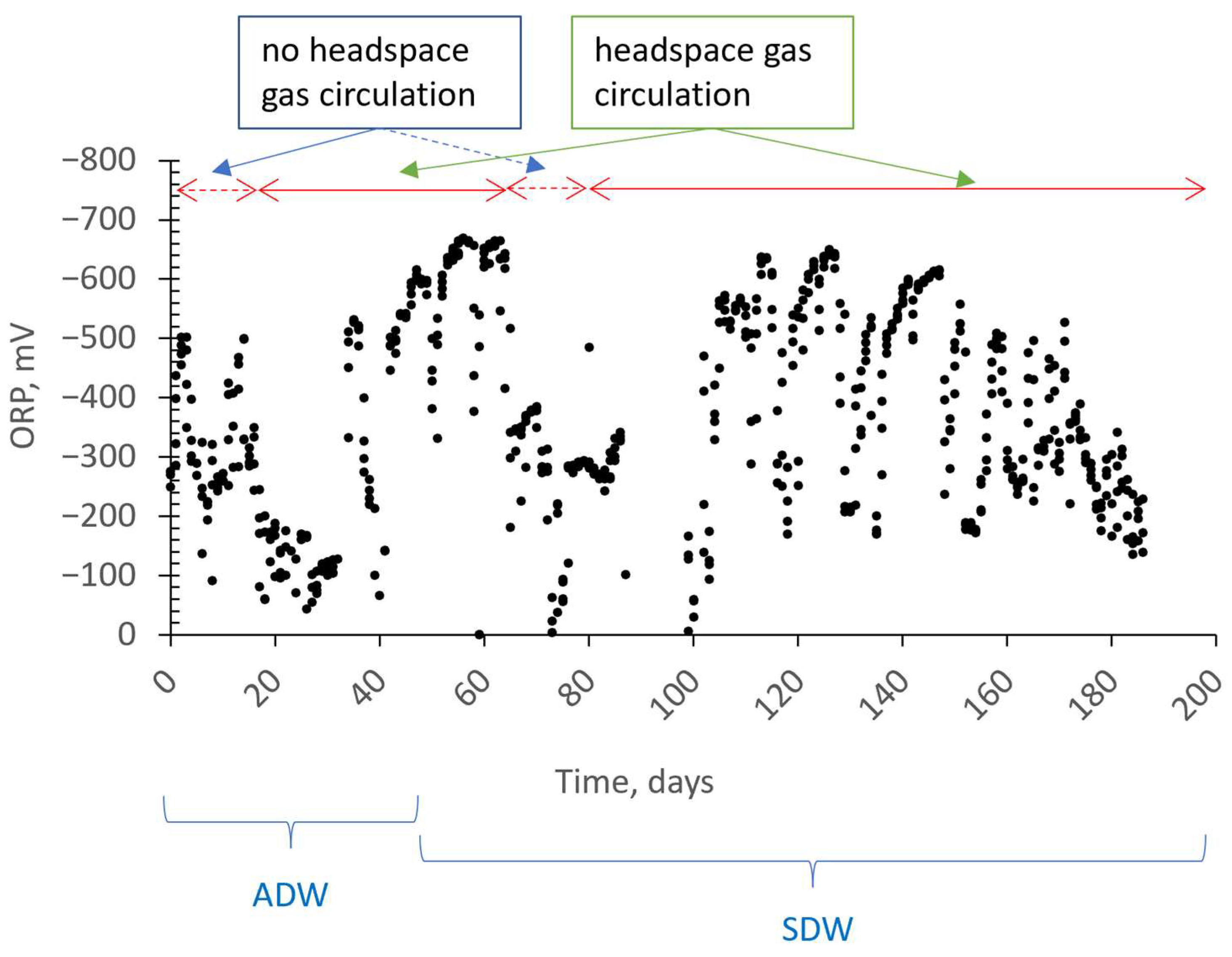
3.1. Removal Performance
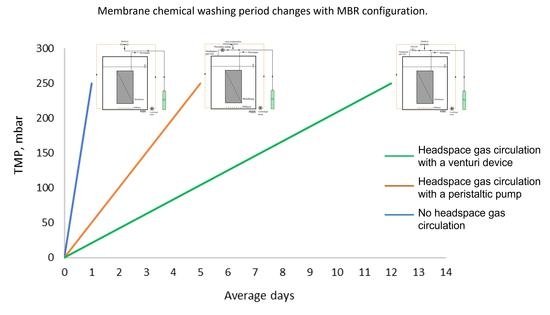
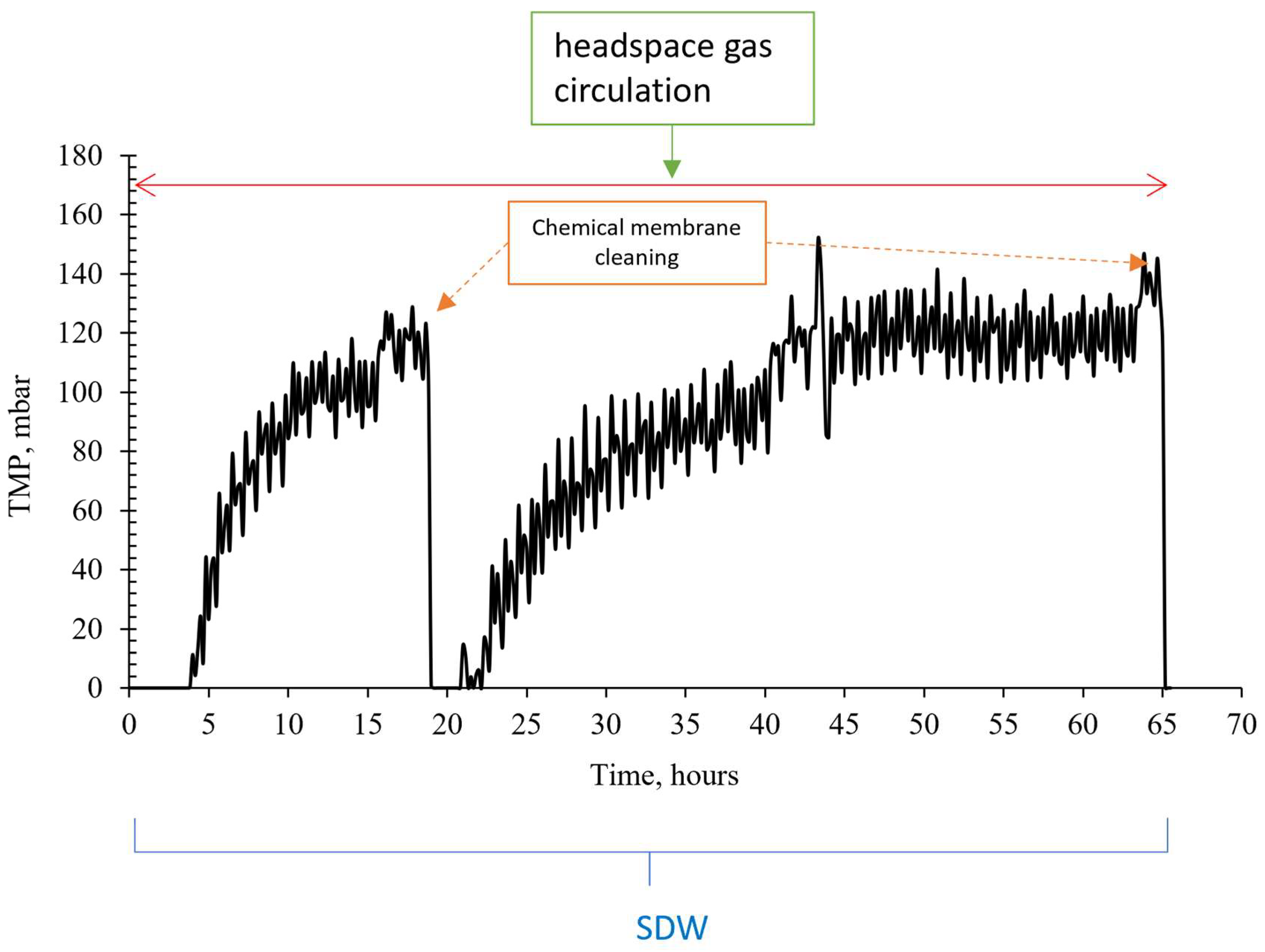
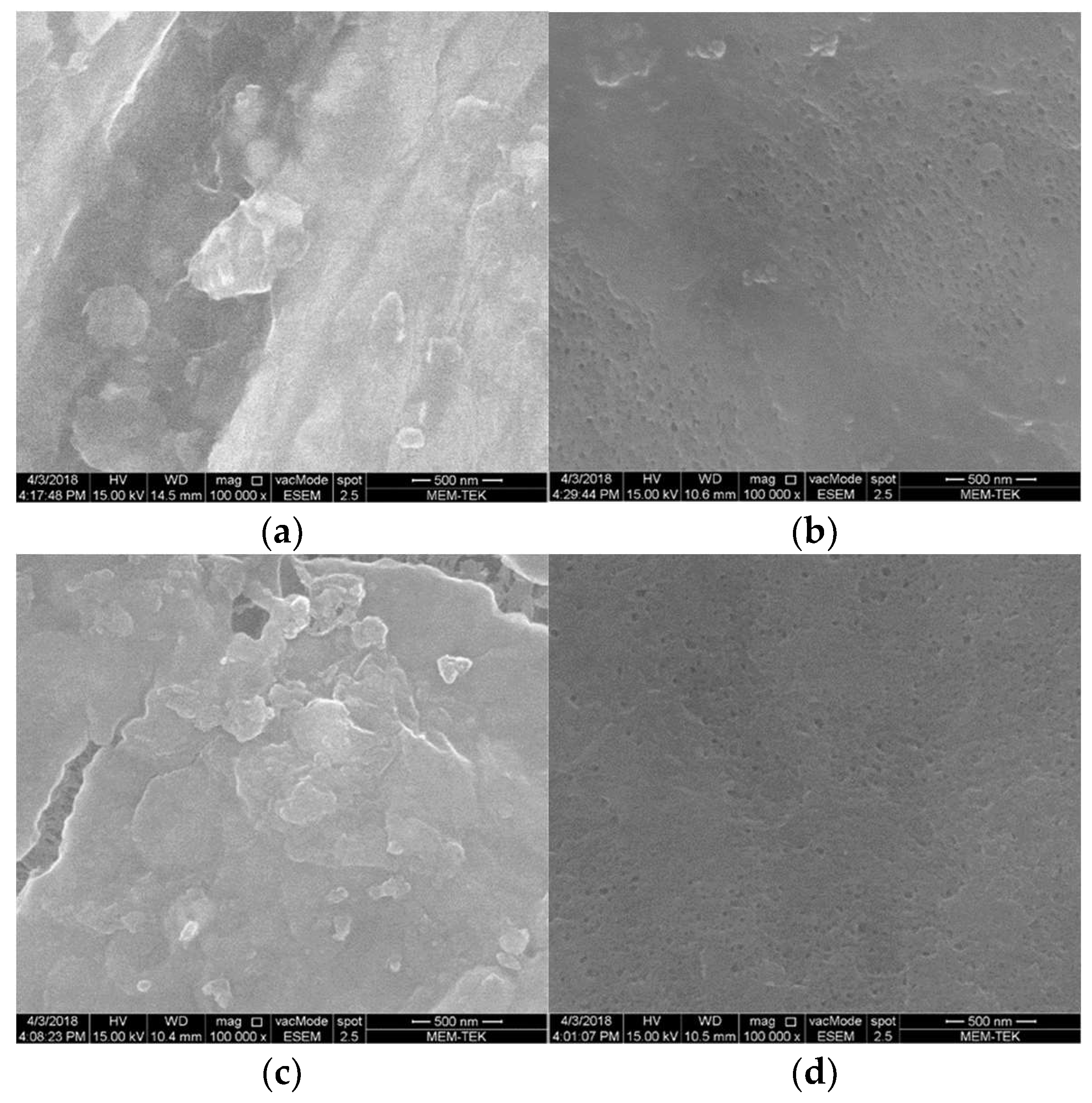
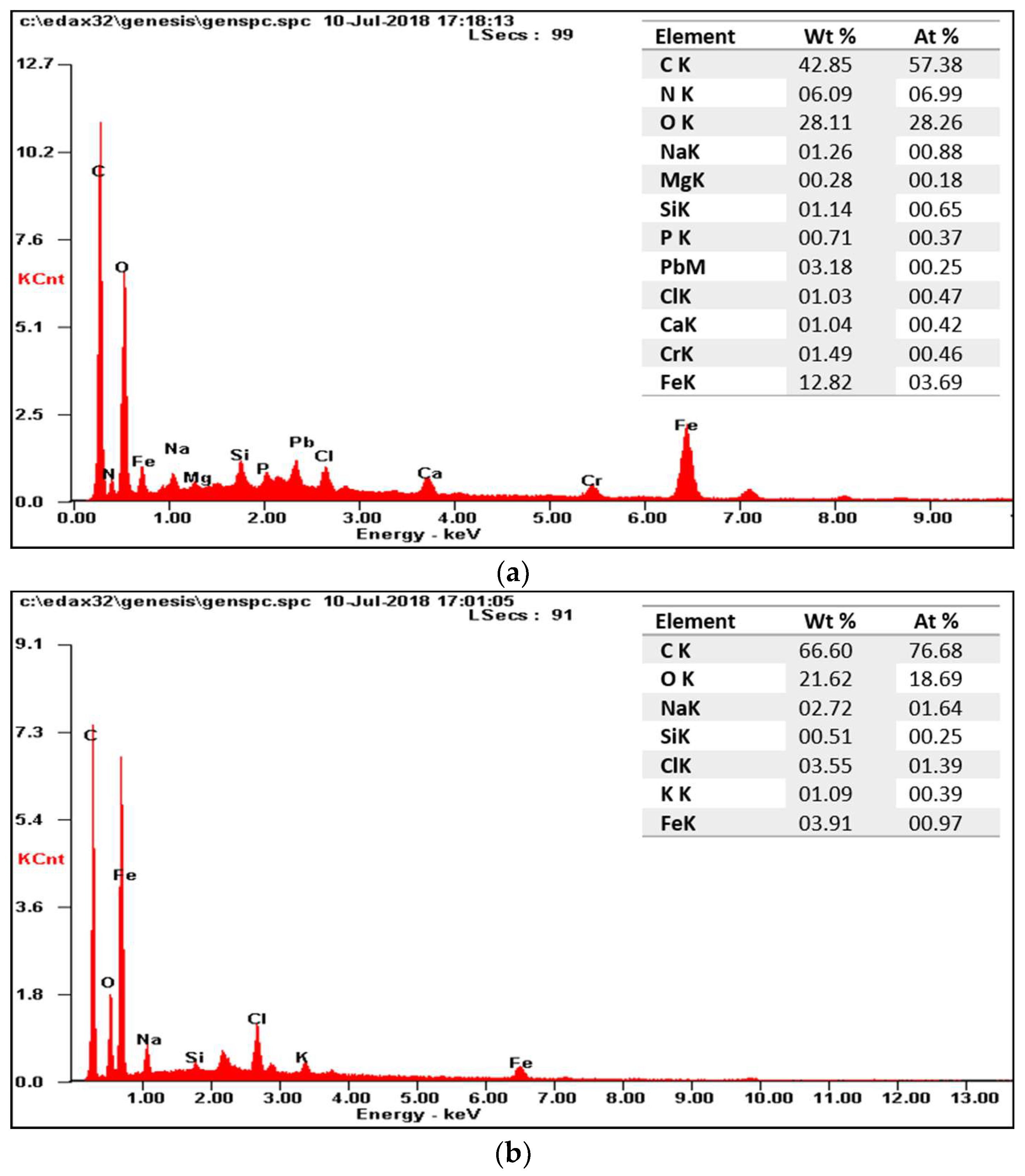
3.2. The Effect of Headspace Gas Circulation on Membrane Fouling
3.3. The Effect of Venturi Usage on Membrane Fouling
3.4. The Effect of Venturi Usage on Hydrogen Gas Utilization Efficiency
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Causape, J.; Quilez, D.; Aragües, R. Salt and nitrate concentrations in the surface waters of the CR-V irrigation district (Bardenas I, Spain): Diagnosis and prescriptions for reducing off-site contamination. J. Hydrol. 2004, 295, 87–100. [Google Scholar] [CrossRef]
- Mastrocicco, M.; Colombani, N.; Giuseppe, D.D.; Faccini, B.; Coltorti, M. Contribution of the subsurface drainage system in changing the nitrogen speciation of an agricultural soil located in a complex marsh environment (Ferrara, Italy). Agric. Water Manag. 2013, 119, 144–153. [Google Scholar] [CrossRef]
- Pasten-Zapata, E.; Ledesma-Ruiz, R.; Harter, T.; Ramirez, A.I.; Mahlknecht, J. Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi-tracer approach. Sci. Total Environ. 2014, 470–471, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Water Uses. Available online: https://www.fao.org/aquastat/en/overview/methodology/water-use (accessed on 7 December 2022).
- DSI (State Water Works) Soil Water Resources. Available online: https://www.dsi.gov.tr/Sayfa/Detay/754 (accessed on 7 December 2022).
- Yurtseven, E.; Cakmak, B.; Kesmez, D.; Polat, H.E. Reuse of Agricultural Drainage Waters in Irrigation. In Proceedings of the Turkish Agricultural Engineering 7th Congress, Ankara, Turkey, 11 January 2010. [Google Scholar]
- Tanji, K.K.; Kielen, N.C. Agricultural Drainage Water Management in Arid and Semi-Arid Areas; Food And Agriculture Organization of The United Nations: Rome, Italy, 2002; Volume Paper 61, ISBN 9251048398. [Google Scholar]
- Zhang, Y.; Okeke, B.C.; Frankenberger, W.T., Jr. Bacterial reduction of selenate to elemental selenium utilizing molasses as a carbon source. Bioresour. Technol. 2008, 99, 1267–1273. [Google Scholar] [CrossRef]
- Quinn, N.W.T.; Leighton, T.; Lundquist, T.J.; Green, F.B.; Zárate, M.A.; Oswald, W.J. Algal-Bacterial Treatment Facility Removes Selenium from Drainage Water. Calif. Agric. 2000, 54, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Hunt, P.G.; Matheny, T.A.; Ro, K.S.; Stone, K.C.; Vanotti, M.B. Denitrification of Agricultural Drainage Line Water via Immobilized Denitrification Sludge. J. Environ. Sci. Health Part A 2008, 43, 1077–1084. [Google Scholar] [CrossRef] [Green Version]
- Allred, B.J. Laboratory Evaluation of Zero Valent Iron and Sulfur-Modified Iron for Agricultural Drainage Water Treatment. Groundwater Monit. Remediat. 2012, 32, 81–95. [Google Scholar] [CrossRef]
- Messer, T.L.; Burchell, M.R.; Birgand, F.; Broome, S.W.; Chescheir, G. Nitrate Removal Potential of Restored Wetlands Loaded with Agricultural Drainage Water: A Mesocosm Scale Experimental Approach. Ecol. Eng. 2017, 106, 541–554. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Pollice, A.; Laera, G.; Saturno, D.; Giordano, C. Effects of Sludge Retention Time on the Performance of a Membrane Bioreactor Treating Municipal Sewage. J. Memb. Sci. 2008, 317, 65–70. [Google Scholar] [CrossRef]
- Casey, E. Membrane Bioreactors for Wastewater Treatment. In Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; Pabby, A.K., Rizvi, S.S.H., Sastre, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1007–1008. [Google Scholar]
- Vinardell, S.; Sanchez, L.; Astals, S.; Mata-Alvarez, J.; Dosta, J.; Heran, M.; Lesage, G. Impact of Permeate Flux and Gas Sparging Rate on Membrane Performance and Process Economics of Granular Anaerobic Membrane Bioreactors. Sci. Total Environ. 2022, 825, 153907. [Google Scholar] [CrossRef]
- Wang, K.M.; Cingolani, D.; Eusebi, A.L.; Soares, A.; Jefferson, B.; McAdam, E.J. Identification of Gas Sparging Regimes for Granular Anaerobic Membrane Bioreactor to Enable Energy Neutral Municipal Wastewater Treatment. J. Memb. Sci. 2018, 555, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Yang, F.; Shi, B.; Zhang, H. A Comprehensive Study on Membrane Fouling in Submerged Membrane Bioreactors Operated under Different Aeration Intensities. Sep. Purif. Technol. 2008, 59, 91–100. [Google Scholar] [CrossRef]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic Membrane Bioreactors for Wastewater Treatment: Novel Configurations, Fouling Control and Energy Considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Frankenberger, W.T. Removal of Selenium from River Water by a Microbial Community Enhanced with Enterobacter Taylorae in Organic Carbon Coated Sand Columns. Sci. Total Environ. 2005, 346, 280–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Frankenberger, W.T. Factors Affecting Removal of Selenate in Agricultural Drainage Water Utilizing Rice Straw. Sci. Total Environ. 2003, 305, 207–216. [Google Scholar] [CrossRef]
- Celmer-Repin, D.; Hwang, J.H.; Cicek, N.; Oleszkiewicz, J.A. Autotrophic Nitrogen-Removing Biofilms on Porous and Non-Porous Membranes. Environ. Technol. 2010, 31, 1391–1401. [Google Scholar] [CrossRef]
- Karanasios, K.A.; Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. Hydrogenotrophic Denitrification of Potable Water: A Review. J. Hazard Mater. 2010, 180, 20–37. [Google Scholar] [CrossRef]
- Hasar, H. Simultaneous Removal of Organic Matter and Nitrogen Compounds by Combining a Membrane Bioreactor and a Membrane Biofilm Reactor. Bioresour. Technol. 2009, 100, 2699–2705. [Google Scholar] [CrossRef]
- Lee, K.C.; Rittmann, B.E. A Novel Hollow-Fibre Membrane Biofilm Reactor for Autohydrogenotrophic Denitrification of Drinking Water. Water Sci. Technol. 2000, 41, 219–226. [Google Scholar] [CrossRef]
- Visvanathan, C.; Phong, D.D.; Jegatheesan, V. Hydrogenotrophic Denitrification of Highly Saline Aquaculture Wastewater Using Hollow Fiber Membrane Bioreactor. Environ. Technol. 2008, 29, 701–707. [Google Scholar] [CrossRef]
- Gül, E.; Kayaalp, N. Modelling of Hydrogenotrophic Denitrification Process in a Venturi-Integrated Membrane Bioreactor. Env. Technol. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Englehardt, J.D.; Wu, T.; Tchobanoglous, G. Urban Net-Zero Water Treatment and Mineralization: Experiments, Modeling and Design. Water Res. 2013, 47, 4680–4691. [Google Scholar] [CrossRef] [PubMed]
- Mazzei Injector Company Wastewater Airjection Systems. Available online: http://mazzei.net/files/MAZ_WW_AirjectionProductSheet.pdf (accessed on 7 December 2022).
- Thongsai, A.; Phuttaro, C.; Saritpongteeraka, K.; Charnnok, B.; Bae, J.; Noophan, P.L.; Chaiprapat, S. Efficacy of Anaerobic Membrane Bioreactor under Intermittent Liquid Circulation and Its Potential Energy Saving against a Conventional Activated Sludge for Industrial Wastewater Treatment. Energy 2022, 244, 122556. [Google Scholar] [CrossRef]


| Parameter/Statistic | pH | Conductivity (μS/cm) | COD (mg/L) | (mg/L) | (mg/L) | SO42− (mg/L) |
|---|---|---|---|---|---|---|
| Average | 7.7 | 2872 | 10.9 | 13.1 | 0.3 | 652 |
| Minimum | 7.1 | 654 | 2.5 | 2.6 | 0.0 | 80 |
| Maximum | 8.4 | 7130 | 47.8 | 44.7 | 1.4 | 1200 |
| Standard deviation | 0.3 | 1750 | 7.6 | 9.7 | 0.4 | 301 |
| Property | Value |
|---|---|
| Membrane Element | SINAP–10–PVDF |
| Effective surface area, m2 | 0.1 |
| Dimensions, W × L × D, mm | 220 × 320 × 6 |
| Weight, kg | 0.4 |
| Pore size, µm | 0.1 |
| Capacity, L/min/pc | 40–60 |
| Aeration volume, L/min/pc | 6 |
| pH | 3–12 |
| Effluent turbidity, NTU | 1.0 |
| Effluent TDS, mg/L | 1 |
| H2 Gas Supply Valve Open/Closed | Gaz Component (%) | |||||
|---|---|---|---|---|---|---|
| H2 | O2 | N2 | CO2 | Others † | Total | |
| 4 s/5 min | 95.9 | 0.97 | 3.13 | - | - | 100 |
| 2 s/25 min | 17.0 | 3.4 | 76.6 | 1.7 | 1.3 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayaalp, N. Anoxic Treatment of Agricultural Drainage Water in a Venturi-Integrated Membrane Bioreactor. Membranes 2023, 13, 666. https://doi.org/10.3390/membranes13070666
Kayaalp N. Anoxic Treatment of Agricultural Drainage Water in a Venturi-Integrated Membrane Bioreactor. Membranes. 2023; 13(7):666. https://doi.org/10.3390/membranes13070666
Chicago/Turabian StyleKayaalp, Necati. 2023. "Anoxic Treatment of Agricultural Drainage Water in a Venturi-Integrated Membrane Bioreactor" Membranes 13, no. 7: 666. https://doi.org/10.3390/membranes13070666
APA StyleKayaalp, N. (2023). Anoxic Treatment of Agricultural Drainage Water in a Venturi-Integrated Membrane Bioreactor. Membranes, 13(7), 666. https://doi.org/10.3390/membranes13070666