Experimental Evaluation of the Process Performance of MF and UF Membranes for the Removal of Nanoplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Membranes
2.2. Filtration Experiments
2.3. NPs and Membranes Characterization
2.3.1. Dynamic Light Scattering (DLS) and ζ-Potential
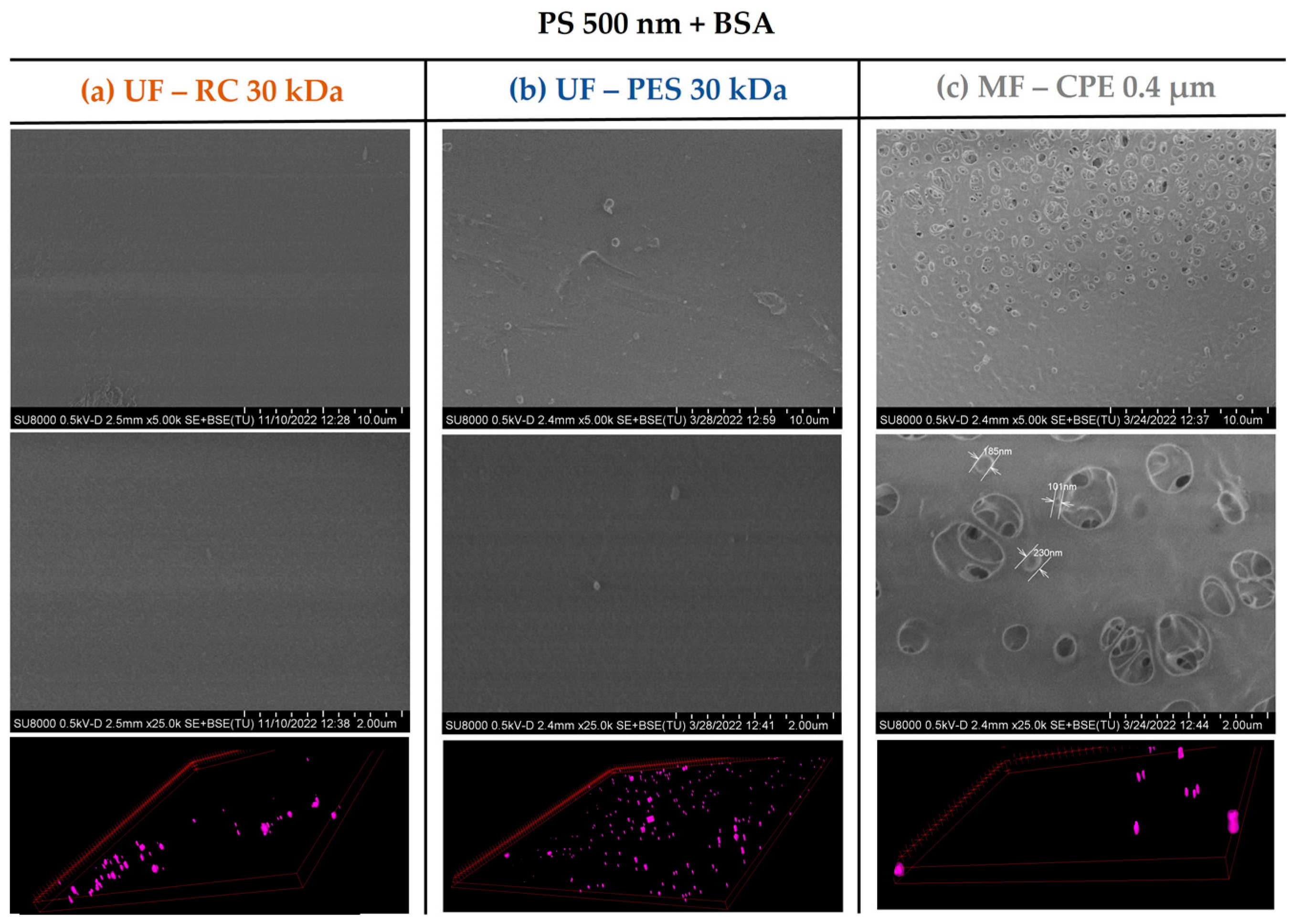
2.3.2. Scanning Electron Microscopy (SEM) and Confocal Laser Scanning Microscopy (CLSM)
3. Results and Discussion
3.1. Rejection Coefficients
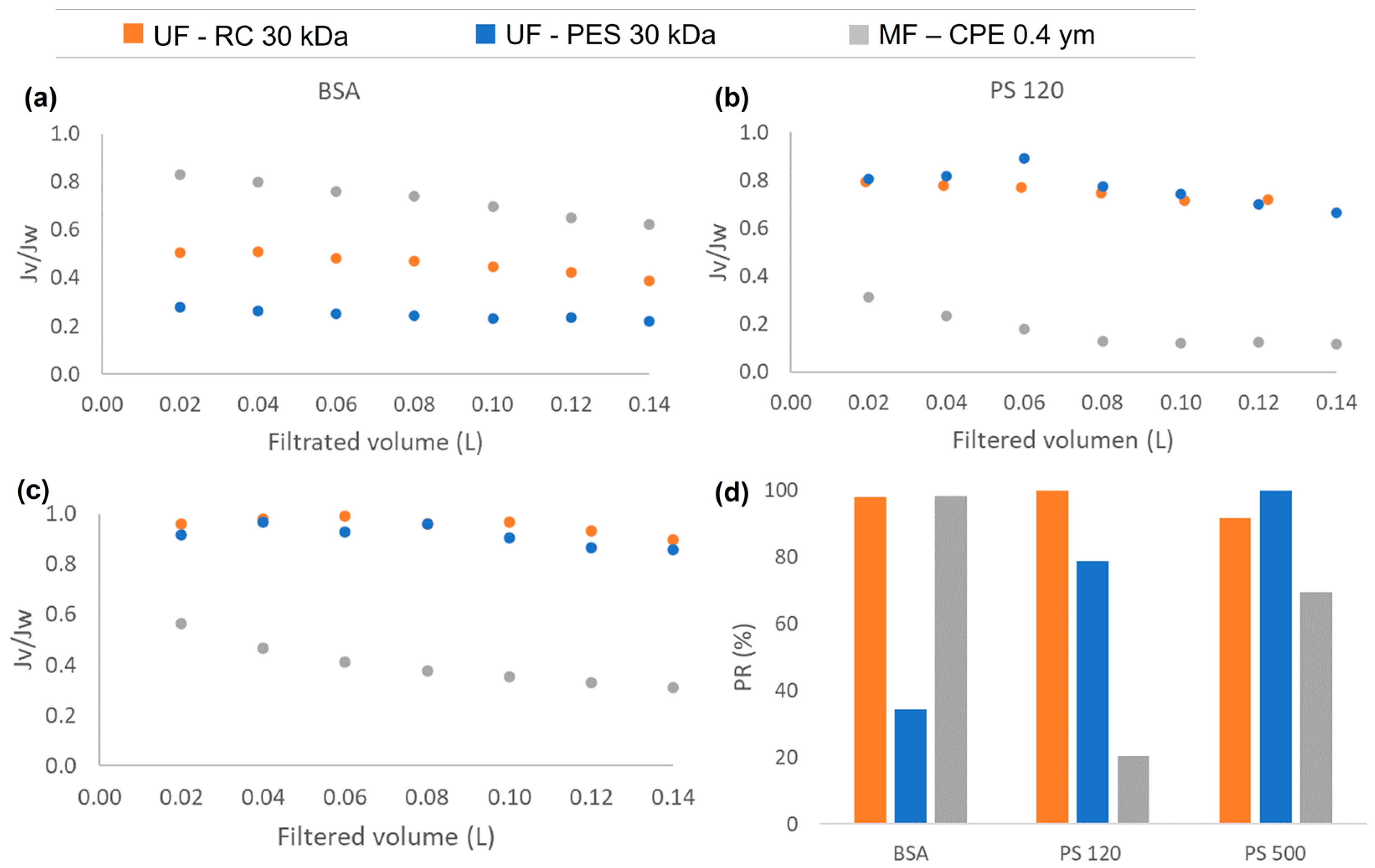
3.2. Permeate Flux and Fouling Phenomena of Single Compounds
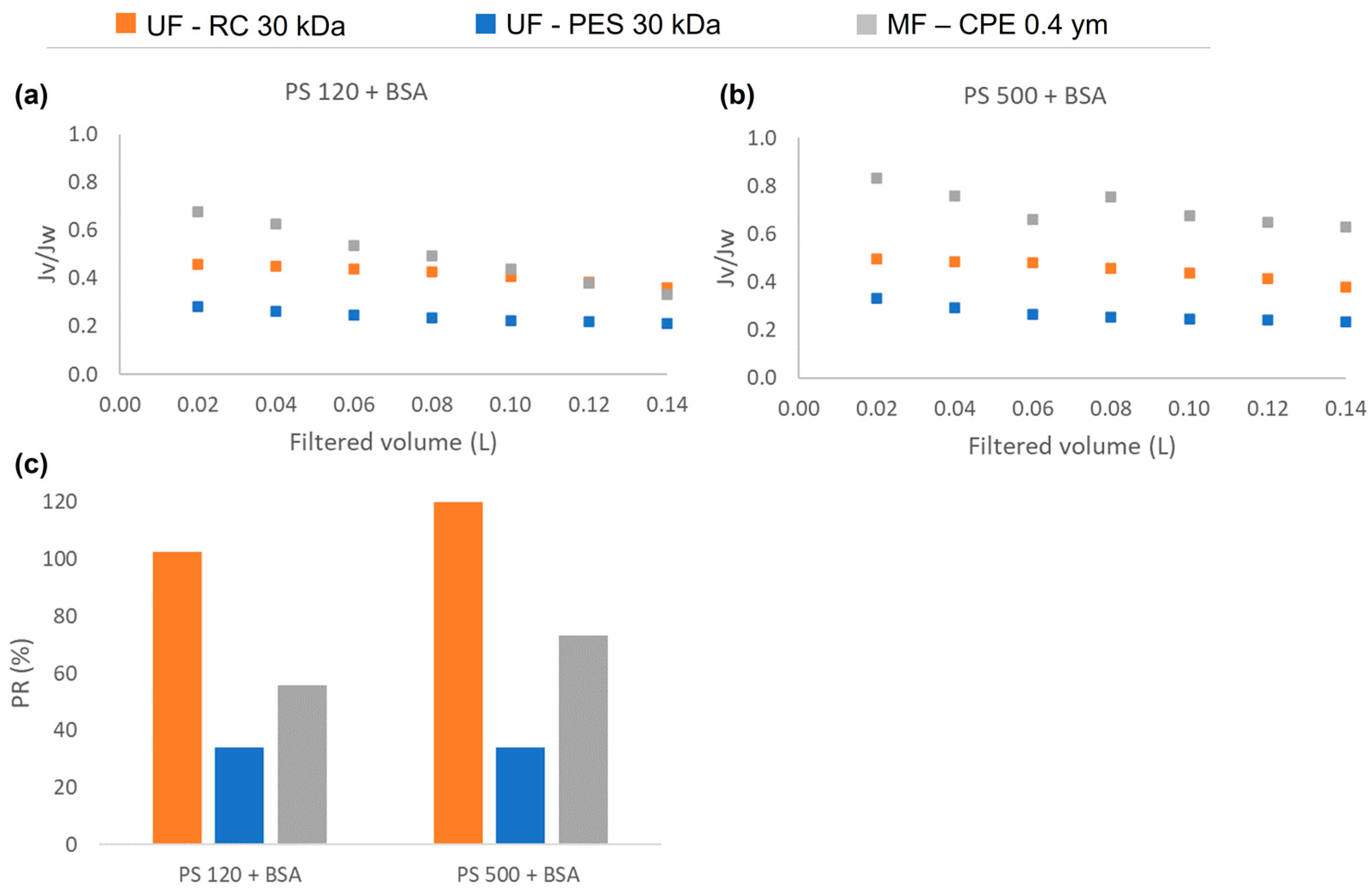
3.3. Permeate Flux and Synergetic Fouling Phenomena of Mixtures
4. Conclusions
- All the membranes studied showed successful removal towards the single solutions of the 120 nm and 500 nm PS nanosphere, except for the MF membrane that showed a very low rejection coefficient of PS 120 nm that passed the membrane readily.
- NPs that were successfully rejected by the membranes were deposited on the membrane surface generating pore blocking and/or cake layer formation. However, the permeability recovery values were very successful. It was concluded that even for the PES membrane, where a thick cake layer was observed, the fouling was reversible.
- For the removal of the 120 nm PS nanospheres, the MF membranes obtained very low permeability recoveries due to the irreversible internal pore blocking caused by the partial retention of the NPs inside the membrane pores.
- This study shows that the membrane material has a direct effect on the membrane fouling. The PES membranes have a higher hydrophobic character, which enhances the hydrophobic–hydrophobic interaction between the foulants and the membrane.
- The mixed solutions helped to understand the synergetic effect of PS NPs and BSA. It was concluded that the BSA acted in two different ways: (i) as a stabilizer that helped to have a better dispersion of NPs, which hindered the pore blocking and the cake layer formation of the PS NPs and (ii) as the main foulant that showed the highest contribution to the normalized flux reduction, decreasing the permeability recovery factor.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe Plastics—The Facts 2022. 2022. Available online: https://Plasticseurope.Org/Knowledge-Hub/Plastics-the-Facts-2022/ (accessed on 22 May 2023).
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [Green Version]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Hadibarata, T. Microplastics Removal through Water Treatment Plants: Its Feasibility, Efficiency, Future Prospects and Enhancement by Proper Waste Management. Environ. Chall. 2021, 5, 100264. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, Identification and Removal of Microplastic Particles and Fibers in Conventional Activated Sludge Process and Advanced MBR Technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to Microplastic Pollution—Removal of Microplastics from Wastewater Effluent with Advanced Wastewater Treatment Technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Landaburu-Aguirre, J.; Molina, S. Polymeric Membranes for Micronanoplastic Sampling and Removal from Water Effluents Crimson Publishers Polymer Science: Peer Review Journal. Polym. Sci. Peer Rev. J. 2022, 4, 10–12. [Google Scholar] [CrossRef]
- Vighi, M.; Bayo, J.; Fernández-Piñas, F.; Gago, J.; Gómez, M.; Hernández-Borges, J.; Herrera, A.; Landaburu, J.; Muniategui-Lorenzo, S.; Muñoz, A.R.; et al. Micro and Nano-Plastics in the Environment: Research Priorities for the Near Future. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 257, pp. 163–218. [Google Scholar]
- Wolff, S.; Kerpen, J.; Prediger, J.; Barkmann, L.; Müller, L. Determination of the Microplastics Emission in the Effluent of a Municipal Waste Water Treatment Plant Using Raman Microspectroscopy. Water Res. X 2019, 2, 100014. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A Review of the Removal of Microplastics in Global Wastewater Treatment Plants: Characteristics and Mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef]
- Wan, H.; Shi, K.; Yi, Z.; Ding, P.; Zhuang, L.; Mills, R.; Bhattacharyya, D.; Xu, Z. Removal of Polystyrene Nanoplastic Beads Using Gravity-Driven Membrane Filtration: Mechanisms and Effects of Water Matrices. Chem. Eng. J. 2022, 450, 138484. [Google Scholar] [CrossRef]
- Enfrin, M.; Lee, J.; Le-Clech, P.; Dumée, L.F. Kinetic and Mechanistic Aspects of Ultrafiltration Membrane Fouling by Nano- and Microplastics. J. Memb. Sci. 2020, 601, 117890. [Google Scholar] [CrossRef]
- Pizzichetti, A.R.P.; Pablos, C.; Álvarez-Fernández, C.; Reynolds, K.; Stanley, S.; Marugán, J. Kinetic and Mechanistic Analysis of Membrane Fouling in Microplastics Removal from Water by Dead-End Microfiltration. J. Environ. Chem. Eng. 2023, 11, 109338. [Google Scholar] [CrossRef]
- Ali, I.; Tan, X.; Li, J.; Peng, C.; Naz, I.; Duan, Z.; Ruan, Y. Interaction of Microplastics and Nanoplastics with Natural Organic Matter (NOM) and the Impact of NOM on the Sorption Behavior of Anthropogenic Contaminants—A Critical Review. J. Clean. Prod. 2022, 376, 134314. [Google Scholar] [CrossRef]
- Tian, J.; Ernst, M.; Cui, F.; Jekel, M. Effect of Particle Size and Concentration on the Synergistic UF Membrane Fouling by Particles and NOM Fractions. J. Membr. Sci. 2013, 446, 1–9. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Qi, B.; Cao, W.; Wan, Y. NOM Fouling Behavior during Ultrafiltration: Effect of Membrane Hydrophilicity. J. Water Process Eng. 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Ayoubian Markazi, S.; Karimi, M.; Yousefi, B.; Sadati, M.; Khoramshahi, H.; Khoee, S.; Karimi, M.R. Experimental and Modeling Study on the Simultaneous Fouling Behavior of Micro/Nanoplastics and Bovine Serum Albumin in Ultrafiltration Membrane Separation. J. Environ. Chem. Eng. 2023, 11, 109354. [Google Scholar] [CrossRef]
- Vakondios, N.; Koukouraki, E.E.; Diamadopoulos, E. Effluent Organic Matter (EfOM) Characterization by Simultaneous Measurement of Proteins and Humic Matter. Water Res. 2014, 63, 62–70. [Google Scholar] [CrossRef]
- Westgate, P.J.; Park, C. Evaluation of Proteins and Organic Nitrogen in Wastewater Treatment Effluents. Environ. Sci. Technol. 2010, 44, 5352–5357. [Google Scholar] [CrossRef] [PubMed]
- Polat, H.; Eren, M.C.; Polat, M. The Effect of Protein BSA on the Stability of Lipophilic Drug (Docetaxel)-Loaded Polymeric Micelles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127712. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, J.; Li, Y.; Chen, Q.; Xie, W.; Wang, J. Characterizing Membrane Fouling Formation during Ultrafiltration of High-Salinity Organic Wastewater. Chemosphere 2022, 287, 132057. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Liu, Z.; Liu, S.; You, G.; Qu, H.; Hou, J. Effects of Nanoplastics on Freshwater Biofilm Microbial Metabolic Functions as Determined by BIOLOG ECO Microplates. Int. J. Environ. Res. Public Health 2019, 16, 4639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Sáez, L.; Patsios, S.I.; Senán-Salinas, J.; Landaburu-Aguirre, J.; Molina, S.; García-Calvo, E. A Novel Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (AMBR): A Proof-of-Concept Study. Membranes 2022, 12, 218. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Jamil, S.M. Feasibility of Recycled Newspaper as Cellulose Source for Regenerated Cellulose Membrane Fabrication. J. Appl. Polym. Sci. 2015, 132, 42684. [Google Scholar] [CrossRef]
- Rohani, M.M.; Zydney, A.L. Effect of Surface Charge Distribution on Protein Transport through Semipermeable Ultrafiltration Membranes. J. Memb. Sci. 2009, 337, 324–331. [Google Scholar] [CrossRef]
- Burns, D.B.; Zydney, A.L. Buffer Effects on the Zeta Potential of Ultrafiltration Membranes. J. Memb. Sci. 2000, 172, 39–48. [Google Scholar] [CrossRef]
- da Costa, P.R.; Alkmin, A.R.; Amaral, M.C.S.; de França Neta, L.S.; Cerqueira, A.C.; Santiago, V.M.J. Ageing Effect on Chlorinated Polyethylene Membrane of an MBR Caused by Chemical Cleaning Procedures. Desalin. Water Treat. 2015, 53, 1460–1470. [Google Scholar] [CrossRef]
- Gao, F.; Li, L.; Shi, Y.; Xue, X.; Mao, J.; Xing, L.; Yao, X. Interaction Mechanisms of Fouling and Cleaning Protocol for A/O-MBR Treatment of Coal⁃to⁃hydrogen Wastewater. Process Saf. Environ. Prot. 2023, 169, 515–525. [Google Scholar] [CrossRef]
- Rohani, M.M.; Zydney, A.L. Role of Electrostatic Interactions during Protein Ultrafiltration. Adv. Colloid Interface Sci. 2010, 160, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Tanudjaja, H.J.; Anantharaman, A.; Ng, A.Q.Q.; Ma, Y.; Tanis-Kanbur, M.B.; Zydney, A.L.; Chew, J.W. A Review of Membrane Fouling by Proteins in Ultrafiltration and Microfiltration. J. Water Process Eng. 2022, 50, 103294. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, F. Membrane Fouling in Aerobic Granular Sludge (AGS)-Membrane Bioreactor (MBR): Effect of AGS Size. Water Res. 2019, 157, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Golgoli, M.; Khiadani, M.; Sen, T.K.; Razmjou, A.; Johns, M.L.; Zargar, M. Synergistic Effects of Microplastics and Organic Foulants on the Performance of Forward Osmosis Membranes. Chemosphere 2023, 311, 136906. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A. A Mechanistic Model for Ultrafiltration Membrane Fouling by Latex. J. Memb. Sci. 2013, 433, 88–99. [Google Scholar] [CrossRef]




| Sample | D (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| BSA | 3 [24] | - | −15.7 [25] |
| PS 120 nm | 122.6 ± 0.7 | 0.029 | −45.5 ± 0.3 |
| PS 500 nm | 517.3 ± 2.9 | 0.048 | −40.5 ± 0.1 |
| PS 120 nm + BSA | 128.1 ± 1.1 | 0.050 | −38.3 ± 0.2 |
| PS 500 nm + BSA | 517.8 ± 2.3 | 0.053 | −41.5 ± 0.8 |
| Membrane | Material | Nominal Pore Size | Ra (nm) | Rq (nm) | Contact Angle | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| UF—RC 30 kDa | Regenerated cellulose | <15 nm | 6.7 ± 1.9 | 8.4 ± 2.3 | 26 ± 3.0° [28] | −2.0 [29] |
| UF—PES 30 kDa | Polyethersulfone | <15 nm | 7.0 ± 1.5 | 8.8 ± 1.9 | 67.6 ± 3.0° [16] | −15.1 ± 0.8 [30] |
| MF—CPE 0.4 μm | Chlorinated polyethylene | 0.4 µm | 184 ± 21 [27] | 234 ± 26 [27] | 104° [31] | −60.5 ± 0.7 [32] |
| Membrane Identification | Solution Description | PS Rejection (%) | BSA Rejection (%) | |
|---|---|---|---|---|
| Material | Name | Size | ||
| UF—RC 30 kDa | PS 120 | 120 nm | 100 | - |
| PS 500 | 500 nm | 100 | - | |
| BSA | 66 kDa | - | 91.61 | |
| PS 120 + BSA | Mixture | 100 | 100 | |
| PS 500 + BSA | Mixture | 100 | 100 | |
| UF—PES 30 kDa | PS 120 | 120 nm | 100 | - |
| PS 500 | 500 nm | 100 | - | |
| BSA | 66 kDa | - | 96.79 | |
| PS 120 + BSA | Mixture | 100 | 100 | |
| PS 500 + BSA | Mixture | 100 | 100 | |
| MF—CPE 0.4 μm | PS 120 | 120 nm | 26.72 | - |
| PS 500 | 500 nm | 100 | - | |
| BSA | 66 kDa | - | 0.76 | |
| PS 120 + BSA | Mixture | 0 | 3 | |
| PS 500 + BSA | Mixture | 100 | 86.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, S.; Ocaña-Biedma, H.; Rodríguez-Sáez, L.; Landaburu-Aguirre, J. Experimental Evaluation of the Process Performance of MF and UF Membranes for the Removal of Nanoplastics. Membranes 2023, 13, 683. https://doi.org/10.3390/membranes13070683
Molina S, Ocaña-Biedma H, Rodríguez-Sáez L, Landaburu-Aguirre J. Experimental Evaluation of the Process Performance of MF and UF Membranes for the Removal of Nanoplastics. Membranes. 2023; 13(7):683. https://doi.org/10.3390/membranes13070683
Chicago/Turabian StyleMolina, Serena, Helena Ocaña-Biedma, Laura Rodríguez-Sáez, and Junkal Landaburu-Aguirre. 2023. "Experimental Evaluation of the Process Performance of MF and UF Membranes for the Removal of Nanoplastics" Membranes 13, no. 7: 683. https://doi.org/10.3390/membranes13070683
APA StyleMolina, S., Ocaña-Biedma, H., Rodríguez-Sáez, L., & Landaburu-Aguirre, J. (2023). Experimental Evaluation of the Process Performance of MF and UF Membranes for the Removal of Nanoplastics. Membranes, 13(7), 683. https://doi.org/10.3390/membranes13070683









