Safety and Effectiveness of Carbon Dioxide Removal CO2RESET Device in Critically Ill Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Intervention
2.3. Data Collection
2.4. Patients’ Selection and Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. ECCO2R Implementation
3.3. Primary Outcome
3.4. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marini, J.J. Mechanical ventilation: Past lessons and the near future. Crit. Care 2013, 17, S1. [Google Scholar] [CrossRef] [Green Version]
- Dreyfuss, D.; Soler, P.; Basset, G.; Saumon, G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am. Rev. Respir. Dis. 1988, 137, 1159–1164. [Google Scholar] [CrossRef]
- Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Physiological and Pathophysiological Consequences of Mechanical Ventilation. Semin. Respir. Crit. Care Med. 2022, 43, 321–334. [Google Scholar] [CrossRef]
- Jaber, S.; Jung, B.; Matecki, S.; Petrof, B.J. Clinical review: Ventilator-induced diaphragmatic dysfunction-human studies confirm animal model findings! Crit. Care 2011, 15, 206. [Google Scholar] [CrossRef] [Green Version]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Neto, A.S.; Simonis, F.D.; Barbas, C.S.; Biehl, M.; Determann, R.M.; Elmer, J.; Friedman, G.; Gajic, O.; Goldstein, J.N.; Linko, R.; et al. Lung-Protective Ventilation With Low Tidal Volumes and the Occurrence of Pulmonary Complications in Patients Without Acute Respiratory Distress Syndrome: A Systematic Review and Individual Patient Data Analysis. Crit. Care Med. 2015, 43, 2155–2163. [Google Scholar] [CrossRef]
- Yang, D.; Grant, M.C.; Stone, A.; Wu, C.L.; Wick, E.C. A Meta-analysis of Intraoperative Ventilation Strategies to Prevent Pulmonary Complications: Is Low Tidal Volume Alone Sufficient to Protect Healthy Lungs? Ann. Surg. 2016, 263, 881–887. [Google Scholar] [CrossRef]
- Contreras, M.; Masterson, C.; Laffey, J.G. Permissive hypercapnia: What to remember. Curr. Opin. Anaesthesiol. 2015, 28, 26–37. [Google Scholar] [CrossRef]
- Combes, A.; Auzinger, G.; Capellier, G.; Du Cheyron, D.; Clement, I.; Consales, G.; Dabrowski, W.; De Bels, D.; De Molina Ortiz, F.J.G.; Gottschalk, A.; et al. ECCO2R therapy in the ICU: Consensus of a European round table meeting. Crit. Care 2020, 24, 490. [Google Scholar] [CrossRef]
- Ficial, B.; Vasques, F.; Zhang, J.; Whebell, S.; Slattery, M.; Lamas, T.; Daly, K.; Agnew, N.; Camporota, L. Physiological Basis of Extracorporeal Membrane Oxygenation and Extracorporeal Carbon Dioxide Removal in Respiratory Failure. Membranes 2021, 11, 225. [Google Scholar] [CrossRef]
- Nentwich, J.; Wichmann, D.; Kluge, S.; Lindau, S.; Mutlak, H.; John, S. Low-flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: A pilot study. Ann. Intensive Care 2019, 9, 3. [Google Scholar] [CrossRef]
- Moerer, O.; Harnisch, L.O.; Barwing, J.; Heise, D.; Heuer, J.F.; Quintel, M. Minimal-flow ECCO2R in patients needing CRRT does not facilitate lung-protective ventilation. J. Artif. Organs 2019, 22, 68–76. [Google Scholar] [CrossRef]
- Combes, A.; Tonetti, T.; Fanelli, V.; Pham, T.; Pesenti, A.; Mancebo, J.; Brodie, D.; Ranieri, V.M. Efficacy and safety of lower versus higher CO2 extraction devices to allow ultraprotective ventilation: Secondary analysis of the SUPERNOVA study. Thorax 2019, 74, 1179–1181. [Google Scholar] [CrossRef]
- Dhamija, A.; Thibault, D.; Fugett, J.; Hayanga, H.K.; McCarthy, P.; Badhwar, V.; Awori Hayanga, J.W. Incremental effect of complications on mortality and hospital costs in adult ECMO patients. Perfusion 2022, 37, 461–469. [Google Scholar] [CrossRef]
- Di Nardo, M.; Annoni, F.; Su, F.; Belliato, M.; Lorusso, R.; Broman, L.M.; Malfertheiner, M.; Creteur, J.; Taccone, F.S. Evaluation of a New Extracorporeal CO2 Removal Device in an Experimental Setting. Membranes 2020, 11, 8. [Google Scholar] [CrossRef]
- Montalti, A.; Belliato, M.; Gelsomino, S.; Nalon, S.; Matteucci, F.; Parise, O.; De Jong, M.; Makhoul, M.; Johnson, D.M.; Lorusso, R. Continuous monitoring of membrane lung carbon dioxide removal during ECMO: Experimental testing of a new volumetric capnometer. Perfusion 2019, 34, 538–543. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Morelli, A.; Del Sorbo, L.; Pesenti, A.; Ranieri, V.M.; Fan, E. Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med. 2017, 43, 519–530. [Google Scholar] [CrossRef]
- Schmidt, M.; Jaber, S.; Zogheib, E.; Godet, T.; Capellier, G.; Combes, A. Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit. Care 2018, 22, 122. [Google Scholar] [CrossRef] [Green Version]
- Combes, A.; Fanelli, V.; Pham, T.; Ranieri, V.M. European Society of Intensive Care Medicine Trials Group and the “Strategy of Ultra-Protective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to severe ARDS” (SUPERNOVA) investigators. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: The SUPERNOVA study. Intensive Care Med. 2019, 45, 592–600. [Google Scholar]
- Zhu, Y.; Zhen, W.; Zhang, X.; Shi, Z.; Zhang, L.; Zhou, J.; Meng, X. Extracorporeal Carbon Dioxide Removal in Patients with Acute Respiratory Distress Syndrome or Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Blood Purif. 2023, 52, 103–113. [Google Scholar] [CrossRef]
- McNamee, J.J.; Gillies, M.A.; Barrett, N.A.; Perkins, G.D.; Tunnicliffe, W.; Young, D.; Bentley, A.; Harrison, D.A.; Brodie, D.; Boyle, A.J.; et al. Effect of Lower Tidal Volume Ventilation Facilitated by Extracorporeal Carbon Dioxide Removal vs Standard Care Ventilation on 90-Day Mortality in Patients With Acute Hypoxemic Respiratory Failure: The REST Randomized Clinical Trial. JAMA 2021, 326, 1013–1023. [Google Scholar] [CrossRef]
- Duscio, E.; Cipulli, F.; Vasques, F.; Collino, F.; Rapetti, F.; Romitti, F.; Behnemann, T.; Niewenhuys, J.; Tonetti, T.; Pasticci, I.; et al. Extracorporeal CO2 Removal: The Minimally Invasive Approach, Theory, and Practice. Crit. Care Med. 2019, 47, 33–40. [Google Scholar] [CrossRef]
- Hospach, I.; Goldstein, J.; Harenski, K.; Laffey, J.G.; Pouchoulin, D.; Raible, M.; Votteler, S.; Storr, M. In vitro characterization of PrismaLung+: A novel ECCO2R device. Intensive Care Med. Exp. 2020, 8, 14. [Google Scholar] [CrossRef]
- Ghasem, N. Modeling and Simulation of the Impact of Feed Gas Perturbation on CO2 Removal in a Polymeric Hollow Fiber Membrane. Polymers 2022, 14, 3783. [Google Scholar] [CrossRef]
- Sun, L.; Kaesler, A.; Fernando, P.; Thompson, A.J.; Toomasian, J.M.; Bartlett, R.H. CO2 clearance by membrane lungs. Perfusion 2018, 33, 249–253. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Kampe, K.A.; Sipmann, F.S.; Larsson, A.; Hedenstierna, G.; Windisch, W.; Mueller, T. Veno-venous extracorporeal CO2 removal for the treatment of severe respiratory acidosis: Pathophysiological and technical considerations. Crit. Care 2014, 18, R124. [Google Scholar] [CrossRef] [Green Version]
- Taccone, F.S.; Malfertheiner, M.V.; Ferrari, F.; Di Nardo, M.; Swol, J.; Broman, L.M.; Vercaemst, L.; Barrett, N.; Pappalardo, F.; Belohlavek, J.; et al. Extracorporeal CO2 removal in critically ill patients: A systematic review. Minerva Anestesiol. 2017, 83, 762–772. [Google Scholar] [CrossRef]
- Materne, L.A.; Hunsicker, O.; Menk, M.; Graw, J.A. Hemolysis in patients with Extracorporeal Membrane Oxygenation therapy for severe Acute Respiratory Distress Syndrome—A systematic review of the literature. Int. J. Med. Sci. 2021, 18, 1730–1738. [Google Scholar] [CrossRef]


| Characteristic | N = 11 |
|---|---|
| Age, years | 60 (43–72) |
| Male sex—n (%) | 9 (82) |
| Weight, Kgs | 88 (62–160) |
| APACHE II score on admission | 19 (16–24) |
| Medical History | |
| Chronic Heart Disease—n (%) | 0 |
| Coronary Artery Disease—n (%) | 0 |
| COPD, n (%) | 2 (18) |
| Diabetes—n (%) | 1 (9) |
| Immunosuppression—n (%) | 2 (18) |
| ECCO2R characteristics | |
| Time from admission to MV, days | 1 (1–3) |
| Time from admission to ECCO2R, days | 3 (2–39) |
| SOFA Score on the day of ECCO2R | 9 (7–10) |
| Duration of therapy, days | 6 (3–17) |
| Adverse Events | |
| Circuit Change, n (%) | 3 (27) |
| Device Malfunctioning, n (%) | 1 (9) |
| Catheter Kinking, n (%) | 0 |
| Catheter Dislodgement, n (%) | 0 |
| Cannulation Site Hematoma, n (%) | 3 (27) |
| Bleeding requiring RBCT, n (%) | 2 (18) |
| Air Embolism, n (%) | 0 |
| Cannula infection, n (%) | 0 |
| Arterial Hypotension, n (%) | 0 |
| New onset of vasopressors, n (%) | 0 |
| Outcomes | |
| ICU length of stay, days | 19 (8–71) |
| Tracheostomy, n (%) | 3 (27) |
| Hospital mortality, n (%) | 7 (64) |
| Variable | Baseline | 1-h | 12-h | 24-h | p Value |
|---|---|---|---|---|---|
| pH | 7.27 (7.12–7.33) | 7.40 (7.30–7.50) | 7.40 (7.35–7.48) | 7.41 (7.35–7.50) | <0.001 |
| PaCO2, mmHg | 65 (50–84) | 50 (31–63) | 47 (32–63) | 45 (39–49) | <0.001 |
| PaO2, mmHg | 94 (70–129) | 90 (58–207) | 90 (67–131) | 91 (72–118) | 0.83 |
| ECCO2R BF, mL/min | 0 (0–0) | 800 (500–800) | 800 (500–800) | 800 (550–800) | <0.001 |
| ECCO2R GF, L/min | 0 (0–0) | 4 (2–14) | 6 (1.5–14) | 6 (1–14) | <0.001 |
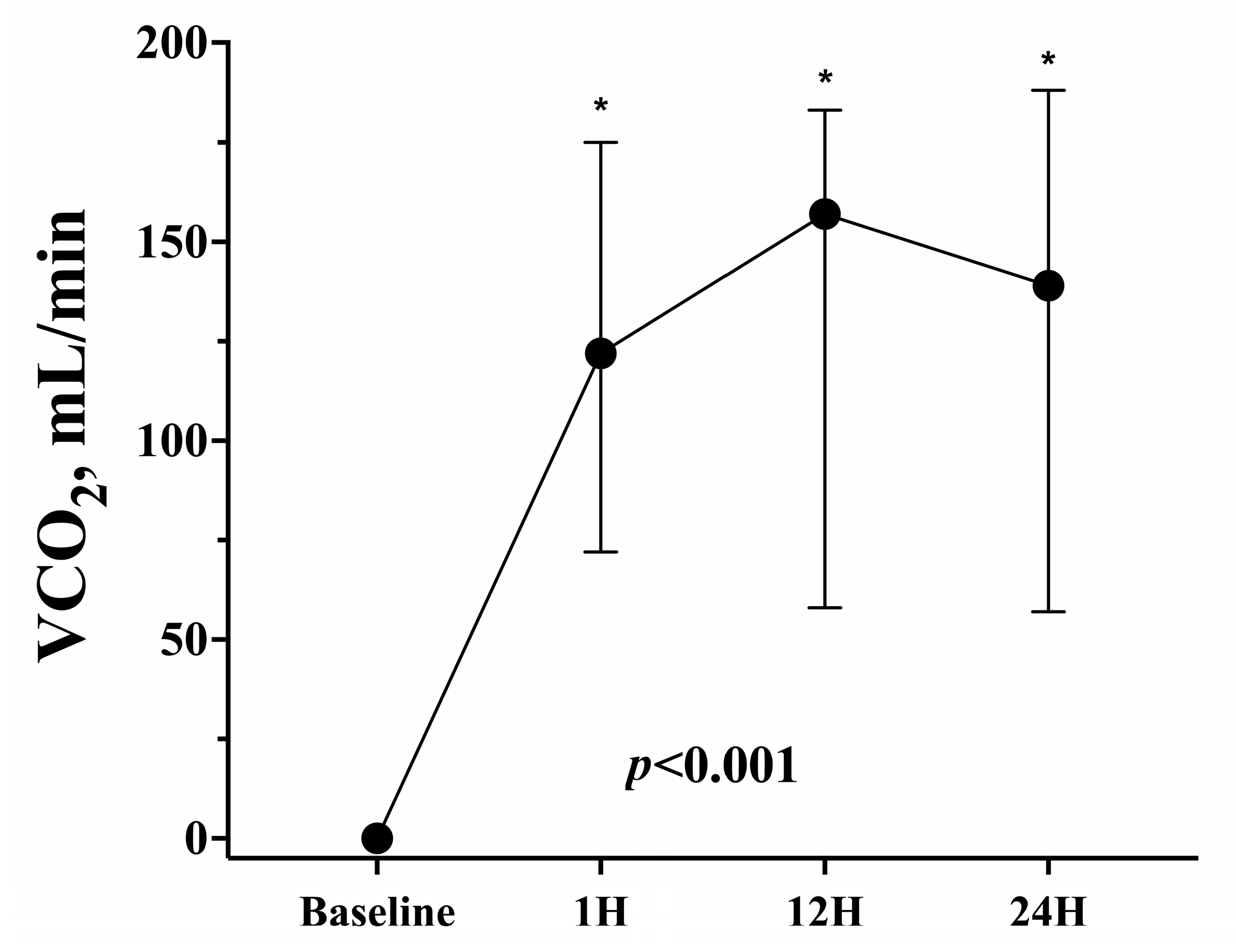
| VCO2, mL/min | 0 (0–0) | 122 (72–175) | 157 (58–183) | 139 (57–188) | <0.001 |
| FiO2, % | 60 (40–100) | 60 (40–90) | 50 (40–95) | 50 (40–100) | 0.85 |
| PEEP, cmH2O | 8 (4–12) | 8 (4–14) | 8 (4–12) | 8 (4–14) | 0.93 |
| TV, mL | 355 (310–446) | 300 (240–422) | 280 (240–409) | 280 (200–350) | 0.004 |
| TV/PBW, mL/kg | 4.9 (4.4–6.0) | 4.1 (3.7–5.3) | 3.9 (3.3–5.4) | 3.8 (2.5–4.6) | 0.001 |
| RR | 28 (20–32) | 22 (12–28) | 19 (12–25) | 18 (11–25) | 0.001 |
| Driving Pressure, cmH2O | 21 (14–24) | 15 (12–20) | 14 (11–18) | 14 (11–15) | 0.004 |
| NE dose, mcg/min | 8 (3–12), n = 9 | 10 (3–19), n = 9 | 8 (2–22), n = 9 | 8 (5–18), n = 7 | 0.67 |
| Sedatives, n (%) | 11 (100) | 11 (100) | 11 (100) | 11 (100) | 0.99 |
| Opioids, n (%) | 11 (100) | 11 (100) | 11 (100) | 11 (100) | 0.99 |
| NMBA, n (%) | 10 (91) | 10 (91) | 10 (91) | 10 (91) | 0.99 |
| Fibrinogen, mg/dL | 460 (178–815) | - | - | 457 (210–777) | 0.15 |
| D-dimers, ng/mL | 2100 (758–22,327) | - | - | 1650 (1077–15,000) | 0.69 |
| Hemoglobin, g/dL | 10.1 (8.9–13.9) | - | - | 9.0 ((8.1–12.2) | 0.10 |
| Platelets, /mm3*103 | 210 (118–511) | - | - | 188 (112–410) | 0.18 |
| LDH, IU/L | 350 (155–515) | - | - | 311 (189–550) | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taccone, F.S.; Rinaldi, S.; Annoni, F.; Nobile, L.; Di Nardo, M.; Maccieri, J.; Aliberti, A.; Malfertheiner, M.V.; Marudi, A.; Broman, L.M.; et al. Safety and Effectiveness of Carbon Dioxide Removal CO2RESET Device in Critically Ill Patients. Membranes 2023, 13, 686. https://doi.org/10.3390/membranes13070686
Taccone FS, Rinaldi S, Annoni F, Nobile L, Di Nardo M, Maccieri J, Aliberti A, Malfertheiner MV, Marudi A, Broman LM, et al. Safety and Effectiveness of Carbon Dioxide Removal CO2RESET Device in Critically Ill Patients. Membranes. 2023; 13(7):686. https://doi.org/10.3390/membranes13070686
Chicago/Turabian StyleTaccone, Fabio Silvio, Simone Rinaldi, Filippo Annoni, Leda Nobile, Matteo Di Nardo, Jessica Maccieri, Anna Aliberti, Maximilan Valentin Malfertheiner, Andrea Marudi, Lars Mikael Broman, and et al. 2023. "Safety and Effectiveness of Carbon Dioxide Removal CO2RESET Device in Critically Ill Patients" Membranes 13, no. 7: 686. https://doi.org/10.3390/membranes13070686
APA StyleTaccone, F. S., Rinaldi, S., Annoni, F., Nobile, L., Di Nardo, M., Maccieri, J., Aliberti, A., Malfertheiner, M. V., Marudi, A., Broman, L. M., & Belliato, M. (2023). Safety and Effectiveness of Carbon Dioxide Removal CO2RESET Device in Critically Ill Patients. Membranes, 13(7), 686. https://doi.org/10.3390/membranes13070686







