Improvement of MBBR-MBR Performance by the Addition of Commercial and 3D-Printed Biocarriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. MBBR-MBR Set-Up and Operating Conditions
2.2. Biofilm Extraction Method
2.3. Printing Methodology of the 3D-Printed Biocarriers with 13X Zeolite and Halloysite
2.4. Mechanical Properties of the 3D-Printed 13X-H Biocarriers
2.5. Determination of the Physicochemical Parameters
2.6. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.7. Bioinformatics
3. Results
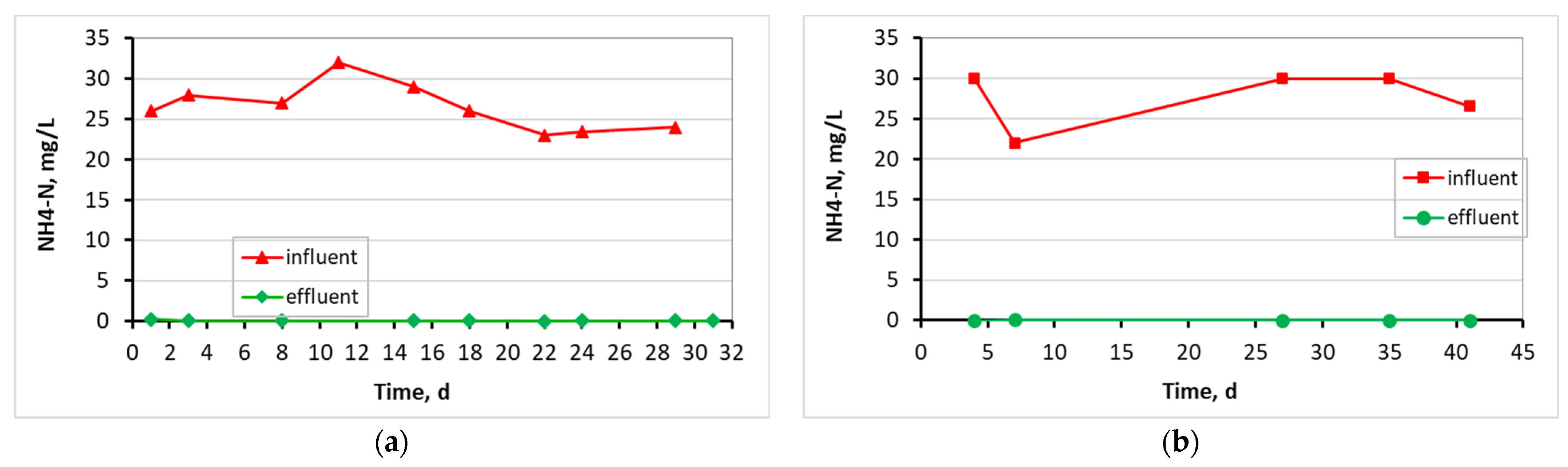
3.1. Evaluation of the MBBR-MBR Performance for the 3 Units
3.2. Biofilm Evaluation for the Kaldnes K1 Biocarriers
3.3. Biofilm Evaluation for the 3D-Printed 13X and Halloysite Biocarriers
3.4. Microbiome Analysis on Biofilm of Biocarriers via 16S rRNA Sequencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boltz, J.P.; Smets, B.F.; Rittmann, B.E.; van Loosdrecht, M.C.M.; Morgenroth, E.; Daigger, G.T. From biofilm ecology to reactors: A focused review. Water Sci. Technol. 2017, 75, 1753–1760. [Google Scholar] [CrossRef]
- Saidulu, D.; Majumder, A.; Gupta, A.K. A systematic review of moving bed biofilm reactor, membrane bioreactor, and moving bed membrane bioreactor for wastewater treatment: Comparison of research trends, removal mechanisms, and performance. J. Environ. Chem. Eng. 2021, 9, 106112. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; Monteoliva-García, A.; Martín-Pascual, J.; Munio, M.M.; García-Mesa, J.J.; Poyatos, J.M. Moving bed biofilm reactor as an alternative wastewater treatment process for nutrient removal and recovery in the circular economy model. Bioresour. Technol. 2020, 299, 122631. [Google Scholar] [CrossRef]
- Liu, J.; Sun, F.; Zhang, P.; Zhou, Y. Integrated powdered activated carbon and quorum quenching strategy for biofouling control in industrial wastewater membrane bioreactor. J. Clean. Prod. 2021, 279, 123551. [Google Scholar] [CrossRef]
- Kawan, J.A.; Abu Hasan, H.; Suja, F.; Jaafar, O.; Abd-Rahman, R. A review on sewage treatment and polishing using moving bed bioreactor (MBBR). J. Eng. Sci. Technol. 2016, 11, 1098–1120. [Google Scholar] [CrossRef]
- Banti, D.C.; Samaras, P.; Tsioptsias, C.; Zouboulis, A.; Mitrakas, M. Mechanism of SMP aggregation within the pores of hydrophilic and hydrophobic MBR membranes and aggregates detachment. Sep. Purif. Technol. 2018, 202, 119–129. [Google Scholar] [CrossRef]
- Banti, D.C.; Karayannakidis, P.D.; Samaras, P.; Mitrakas, M.G. An innovative bioreactor set-up that reduces membrane fouling by adjusting the filamentous bacterial population. J. Memb. Sci. 2017, 542, 430–438. [Google Scholar] [CrossRef]
- Gkotsis, P.; Banti, D.; Pritsa, A.; Mitrakas, M.; Samaras, P.; Peleka, E.; Zouboulis, A. Effect of operating conditions on membrane fouling in pilot-scale mbrs; filaments growth, diminishing dissolved oxygen and recirculation rate of the activated sludge. Membranes 2021, 11, 490. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Memb. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Banti, D.; Mitrakas, M.; Fytianos, G.; Tsali, A.; Samaras, P. Combined effect of colloids and SMP on membrane fouling in MBRs. Membranes 2020, 10, 118. [Google Scholar] [CrossRef]
- Mcquarriev, J.P.; Boltz, J.P. Moving Bed Biofilm Reactor Technology; Process Applications, Design, and Performance. Water Environ. Res. 2010, 83, 560–575. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, S.Q.; Shen, Y.; Yang, J.X.; Yan, P.; Chen, Y.P.; Li, J.; Guo, J.S.; Duan, X.M.; Fang, F.; et al. A novel bio-carrier fabricated using 3D Printing technique for wastewater treatment. Sci. Rep. 2015, 5, srep12400. [Google Scholar] [CrossRef]
- Tang, B.; Song, H.; Bin, L.; Huang, S.; Zhang, W.; Fu, F.; Zhao, Y.; Chen, Q. Bioresource Technology Determination of the profile of DO and its mass transferring coefficient in a biofilm reactor packed with semi-suspended bio-carriers. Bioresour. Technol. 2017, 241, 54–62. [Google Scholar] [CrossRef]
- Lee, S.; Badoux, G.O.; Wu, B.; Chong, T.H. Enhancing performance of biocarriers facilitated gravity-driven membrane (GDM) reactor for decentralized wastewater treatment: Effect of internal recirculation and membrane packing density. Sci. Total Environ. 2021, 762, 144104. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, H.; Zhang, M.; Liu, Y. A novel single-stage ceramic membrane moving bed biofilm reactor coupled with reverse osmosis for reclamation of municipal wastewater to NEWater-like product water. Chemosphere 2021, 268, 128836. [Google Scholar] [CrossRef] [PubMed]
- Barwal, A.; Chaudhary, R. To study the performance of biocarriers in moving bed biofilm reactor ( MBBR ) technology and kinetics of biofilm for retrofitting the existing aerobic treatment systems: A review. Sci. Bio. Technol. 2014, 13, 285–299. [Google Scholar] [CrossRef]
- Felföldi, T.; Jurecska, L.; Vajna, B.; Barkács, K.; Makk, J.; Cebe, G.; Szabó, A.; Záray, G.; Márialigeti, K. Texture and type of polymer fiber carrier determine bacterial colonization and biofilm properties in wastewater treatment. Chem. Eng. J. 2015, 264, 824–834. [Google Scholar] [CrossRef]
- Elliott, O.; Gray, S.; Mcclay, M.; Nassief, B.; Nunnelley, A.; Ekong, J.; Kardel, K.; Khoshkhoo, A.; Proaño, G.; David, M.; et al. Design and Manufacturing of High Surface Area 3D-Printed Media for Moving Bed Bioreactors for Wastewater Treatment. J. Contemp. Water Res. Educ. 2017, 160, 144–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, H.H.; Wheeler, J.J.; Tang, S.; Jiang, X. Emerging investigator series: Emerging biotechnologies in wastewater treatment: From biomolecular engineering to multiscale integration. Environ. Sci. Water Res. Technol. 2020, 6, 1967–1985. [Google Scholar] [CrossRef]
- Tang, B.; Zhao, Y.; Bin, L.; Huang, S.; Fu, F. Bioresource Technology Variation of the characteristics of bio fi lm on the semi-suspended bio-carrier produced by a 3D printing technique: Investigation of a whole growing cycle. Bioresour. Technol. 2017, 244, 40–47. [Google Scholar] [CrossRef]
- Chioti, A.G.; Tsioni, V.; Patsatzis, S.; Filidou, E.; Banti, D.; Samaras, P.; Economou, E.A.; Kostopoulou, E.; Sfetsas, T. Characterization of biofilm microbiome formation developed on novel 3D-printed zeolite biocarriers during aerobic and anaerobic digestion processes. Fermentation 2022, 8, 746. [Google Scholar] [CrossRef]
- Mandakhalikar, K.D.; Rahmat, J.N.; Chiong, E.; Neoh, K.G.; Shen, L.; Tambyah, P.A. Extraction and quantification of biofilm bacteria: Method optimized for urinary catheters. Sci. Rep. 2018, 8, 8069. [Google Scholar] [CrossRef] [PubMed]
- Bernholc, J.; Brenner, D.; Buongiorno Nardelli, M.; Meunier, V.; Roland, C. Mechanical and electrical properties of nanotubes. Annu. Rev. Mater. Sci. 2002, 32, 347–375. [Google Scholar] [CrossRef]
- Economou, E.A.; Koltsakidis, S.; Dalla, I.; Middelkoop, V. Novel 3D Printed biocarriers from aluminosilicate materials. Materials 2023, 16, 4826. [Google Scholar] [CrossRef]
- Gleadall, A. FullControl GCode Designer: Open-source software for unconstrained design in additive manufacturing. Addit. Manuf. 2021, 46, 102109. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Hwang, B.K.; Kim, J.H.; Ahn, C.H.; Lee, C.H.; Song, J.Y.; Ra, Y.H. Effect of disintegrated sludge recycling on membrane permeability in a membrane bioreactor combined with a turbulent jet flow ozone contactor. Water Res. 2010, 44, 1833–1840. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a lineart photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Eikelboom, D.H. Process Control of Activated Sludge Plants by Microscopic Investigation; IWA Publishing: Zutphen, The Netherlands, 2000. [Google Scholar]
- Banti, D.C.; Mitrakas, M.; Samaras, P. Membrane fouling controlled by adjustment of biological treatment parameters in step-aerating MBR. Membranes 2021, 11, 553. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Benjamin, C.; McMurdie, P.; Rosen, M.; Han, A.; Johnson, A.; Holmes, S. DADA2:High resolution sample inference from Illumina amplicon data. Encycl. Med. Immunol. 2020, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic. Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Borea, L.; Naddeo, V.; Belgiorno, V. Frontiers in Wastewater Treatment and Modelling—An Electro Moving Bed Membrane Bioreactore (eMB-MBR) as a Novel Technology for Wastewater Treatment and Reuse; Mannina, G., Ed.; Springer: Dordrecht, The Netherlands, 2017; ISBN 9783319584201. [Google Scholar]
- Abdelfattah, A.; Hossain, M.I.; Cheng, L. High-strength wastewater treatment using microbial biofilm reactor: A critical review. World J. Microbiol. Biotechnol. 2020, 36, 75. [Google Scholar] [CrossRef] [PubMed]
- Shreve, M.J.; Brennan, R.A. Trace organic contaminant removal in six full-scale integrated fixed-film activated sludge (IFAS) systems treating municipal wastewater. Water Res. 2019, 151, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Yuan, P.; Liu, D.; Du, P.; Recycle, R. Developments in Clay Science Chapter 8—Surface Modifications of Halloysite; Elsevier: Amsterdam, The Netherlands, 2016; Volume 7, pp. 167–201. [Google Scholar]
- Zhang, W.; Wang, L.; Su, Y.; Liu, Z.; Du, C. Indium oxide/Halloysite composite as highly efficient adsorbent for tetracycline Removal: Key roles of hydroxyl groups and interfacial interaction. Appl. Surf. Sci. 2021, 566, 150708. [Google Scholar] [CrossRef]
- Yi, K.; Huang, J.; Li, X.; Li, S.; Pang, H.; Liu, Z.; Zhang, W.; Liu, S.; Liu, C.; Shu, W. Long-term impacts of polyethylene terephthalate (PET) microplastics in membrane bioreactor. J. Environ. Manag. 2022, 323, 116234. [Google Scholar] [CrossRef]
- Mazioti, A.A.; Koutsokeras, L.E.; Constantinides, G.; Vyrides, I. Untapped potential of moving bed biofilm reactors with different biocarrier types for bilge water treatment: A laboratory-scale study. Water 2021, 13, 1810. [Google Scholar] [CrossRef]
- Zhou, Y.; Kiely, P.D.; Kibbee, R.; Ormeci, B. Effect of polymeric support material on biofilm development, bacterial population, and wastewater treatment performance in anaerobic fixed-film systems. Chemosphere 2021, 264, 128477. [Google Scholar] [CrossRef]
- Qin, S.; Wainaina, S.; Liu, H.; Soufiani, A.M.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Taherzadeh, M.J. Microbial dynamics during anaerobic digestion of sewage sludge combined with food waste at high organic loading rates in immersed membrane bioreactors. Fuel 2021, 303, 121276. [Google Scholar] [CrossRef]
- Gerber, E.; Bernard, R.; Castang, S.; Chabot, N.; Coze, F.; Dreux-Zigha, A.; Hauser, E.; Hivin, P.; Joseph, P.; Lazarelli, C.; et al. Deinococcus as new chassis for industrial biotechnology: Biology, physiology and tools. J. Appl. Microbiol. 2015, 119, 1–10. [Google Scholar] [CrossRef]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Esposito, G.; Guibaud, G.; van Hullebusch, E.D. Potential Use of Waste-to-Bioenergy By-Products in Bioremediation of Total Petroleum Hydrocarbons (TPH)-Contaminated Soils; Springer: Berlin, Germany, 2020; ISBN 9783030403485. [Google Scholar]
- Tong, J.; Cui, L.; Wang, D.; Wang, X.; Liu, Z. Assessing the performance and microbial structure of biofilms in membrane aerated biofilm reactor for high p-nitrophenol concentration treatment. J. Environ. Chem. Eng. 2022, 10, 108635. [Google Scholar] [CrossRef]
- Wang, X.; Gao, C.; Jin, P.; Zhang, Y.; Xie, Y.; Chen, T.; Zhang, A. Nitrogen removal performance and bacterial community in a full-scale modified Orbal oxidation ditch with internal nitrate recycle and biocarriers. J. Water Process Eng. 2021, 40, 101791. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Wang, D.; Huang, Y.X.; Yuan, S.; Meng, F. Simultaneous methanogenesis and denitrification coupled with nitrifying biofilm for high-strength wastewater treatment: Performance and microbial mechanisms. Water Res. 2022, 225, 119163. [Google Scholar] [CrossRef] [PubMed]





















| Material | Paste Content | Zeolite/Clay Percentage | |
|---|---|---|---|
| Zeolite | 13X | 50% | 89% |
| Inorganic binder | Halloysite nanotubes | 6% | 11% |
| Colloidal silica | Ludox AS-40 | 16% | |
| Water | 27% | ||
| Organic binder | Methyl cellulose | 1% |
| t, d | Dry Mass of Biofilm, mg | MLSS, mg/L |
|---|---|---|
| 6 | 3.2 | 40 |
| 20 | 3.5 | 240 |
| 32 | 4.6 | 360 |
| 41 | 2.9 | 20 |
| t, d | Dry Mass, mg | MLSS, mg/L |
|---|---|---|
| 11 | 4980 | 863 |
| 14 | 5426 | 1875 |
| 24 | 5210 | 1038 |
| 28 | 5711 | 1250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banti, D.C.; Samaras, P.; Kostopoulou, E.; Tsioni, V.; Sfetsas, T. Improvement of MBBR-MBR Performance by the Addition of Commercial and 3D-Printed Biocarriers. Membranes 2023, 13, 690. https://doi.org/10.3390/membranes13080690
Banti DC, Samaras P, Kostopoulou E, Tsioni V, Sfetsas T. Improvement of MBBR-MBR Performance by the Addition of Commercial and 3D-Printed Biocarriers. Membranes. 2023; 13(8):690. https://doi.org/10.3390/membranes13080690
Chicago/Turabian StyleBanti, Dimitra C., Petros Samaras, Eleni Kostopoulou, Vassiliki Tsioni, and Themistoklis Sfetsas. 2023. "Improvement of MBBR-MBR Performance by the Addition of Commercial and 3D-Printed Biocarriers" Membranes 13, no. 8: 690. https://doi.org/10.3390/membranes13080690
APA StyleBanti, D. C., Samaras, P., Kostopoulou, E., Tsioni, V., & Sfetsas, T. (2023). Improvement of MBBR-MBR Performance by the Addition of Commercial and 3D-Printed Biocarriers. Membranes, 13(8), 690. https://doi.org/10.3390/membranes13080690











