Simulation of the Membrane Process of CO2 Capture from Flue Gas via Commercial Membranes While Accounting for the Presence of Water Vapor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Commercial Gas Separation Membranes: Materials and Manufacturers
2.2. Modeling of Membrane Process of CO2 Capture from Flue Gas
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | area of membrane, m2 |
| a | coordinate of membrane area, m2 |
| J | flow rate, mol/s |
| P | permeability coefficient, barrer |
| p | pressure, bar |
| Q | permeance, mol/(m2∙s∙kPa) |
| y | molar fraction, mol% |
| Greek letter | |
| α | selectivity of polymer/membrane |
| θ | stage cut, % |
| θi | recovery of component i, % |
| Superscript | |
| 0 | initial value |
| F | feed |
| P | permeate |
| R | retentate |
| Subscript | |
| i | component |
Appendix A
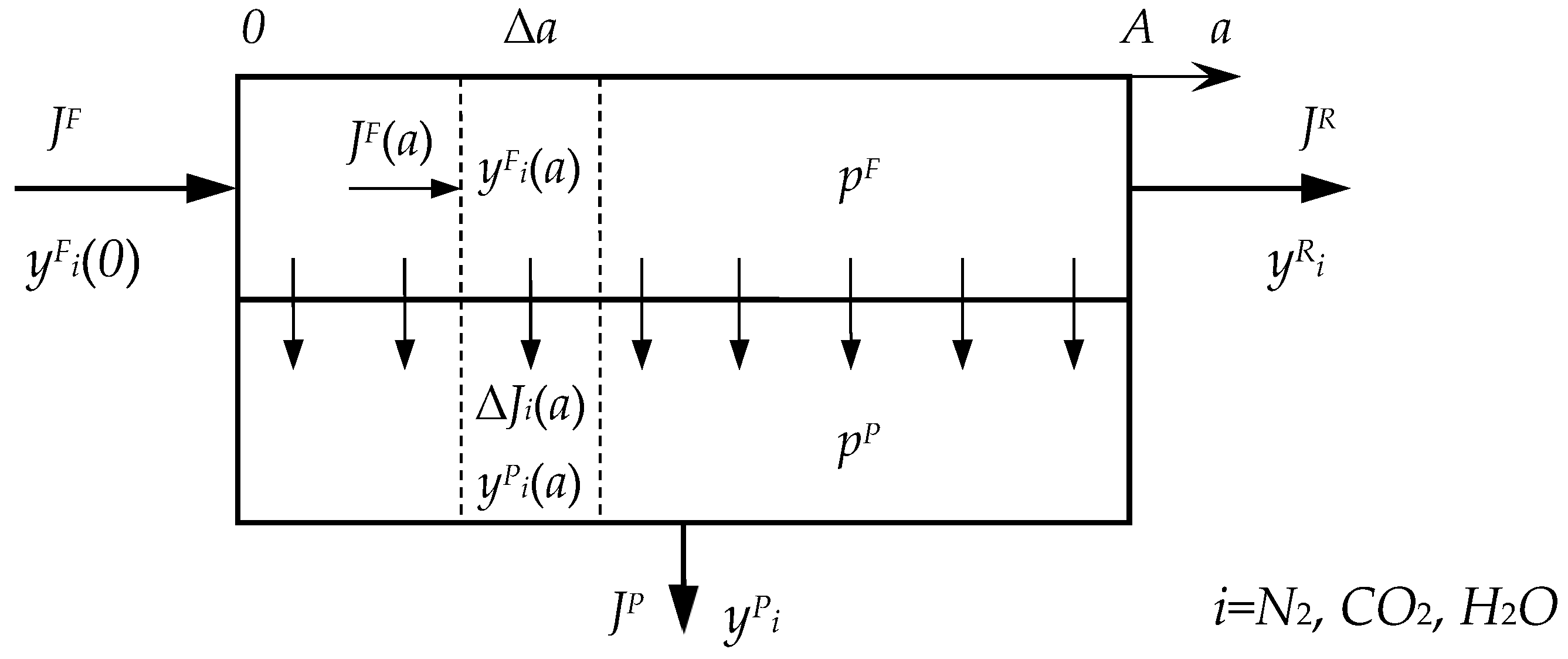
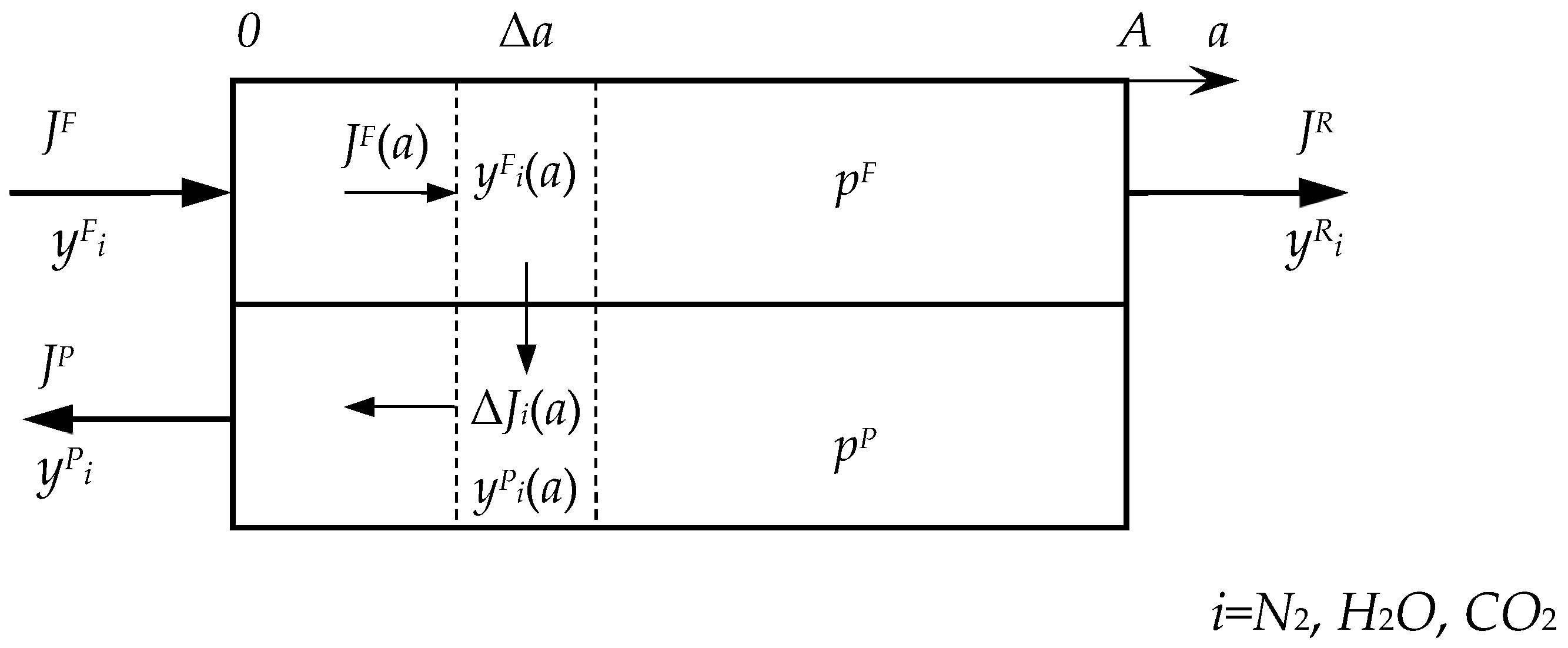
References
- Olivier, J.; Janssens-Maenhout, G.; Peters, J. Trends in Global CO2 Emissions: 2012 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2012. [Google Scholar]
- Membrane Technology & Research. Available online: https://www.mtrinc.com/our-business/carbon-capture/coal-fired-power-plants/ (accessed on 5 April 2023).
- Beckwith, R. Carbon capture and storage: A mixed review. J. Pet. Technol. 2011, 63, 42–45. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Li, Q.; Wu, H.; Wang, Z.; Wang, J. Analysis and optimal design of membrane processes for flue gas CO2 capture. Sep. Purif. Technol. 2022, 298, 121584. [Google Scholar] [CrossRef]
- Brunetti, A.; Scuraa, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Y.; Ho, W.S. Recent Progress in the Engineering of Polymeric Membranes for CO2 Capture from Flue Gas. Membranes 2020, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Hägg, M.-B.; Lindbråthen, A.; He, X.; Nodeland, S.G.; Cantero, T. Pilot Demonstration-reporting on CO2 Capture from a Cement Plant Using Hollow Fiber Process. Energy Procedia 2017, 114, 6150–6165. [Google Scholar] [CrossRef]
- He, X.; Chen, D.; Liang, Z.; Yang, F. Insight and Comparison of Energy-efficient Membrane Processes for CO2 Capture from Flue Gases in Power Plant and Energy-intensive Industry. Carbon Capture Sci. Technol. 2022, 2, 100020. [Google Scholar] [CrossRef]
- STC “Vladipor”. Available online: http://www.vladipor.ru/ (accessed on 15 April 2023).
- Teplyakov, V.V.; Shalygin, M.G.; Kozlova, A.A.; Netrusov, A.I. Composite Membranes with a Polyvinyltrimethylsilane Skin Layer for Separation of Water–Alcohol Mixtures. Pet. Chem. 2018, 58, 949–957. [Google Scholar] [CrossRef]
- Puri, P.S. Commercial Applications of Membranes in Gas Separations. In Membrane Engineering for the Treatment of Gases, Volume 1: Gas-separation Problems with Membranes; Drioli, E., Barbieri, G., Eds.; The Royal Society of Chemistry: London, UK, 2011; pp. 215–244. [Google Scholar]
- Evonik. Available online: https://corporate.evonik.com/en/product/PR_52037843?name=P84-Fibres (accessed on 15 April 2023).
- PermSelect. Available online: https://www.permselect.com/membranes (accessed on 16 April 2023).
- Kozlova, A.A.; Shalygin, M.G.; Teplyakov, V.V. On the Relationship between the Permeability Parameters of Gases and Vapors of C1-C4 Aliphatic Alcohols in Hydrophobic Polymeric Membranes. Int. J. Membr. Sci. Technol. 2016, 3, 56–63. [Google Scholar]
- Metz, S.J.; van de Ven, W.J.C.; Potreck, J.; Mulder, M.H.V.; Wessling, M. Transport of water vapor and inert gas mixtures through highly selective and highly permeable polymer membranes. J. Membr. Sci. 2005, 251, 29–41. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Levin, I.S.; Belov, N.A.; Nikiforov, R.Y.; Chirkov, S.V.; Bezgin, D.A.; Ryzhikh, V.E.; Kostina, J.V.; Shantarovich, V.P.; Grunin, L.Y. Features of the Gas-Permeable Crystalline Phase of Poly-2,6-dimethylphenylene Oxide. Polymers 2022, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.A. Polymers for gas separations: The next decade. J. Membr. Sci. 1994, 1, 1–65. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; Membrane Technology and Research, Inc.: Menlo Park, CA, USA, 2000; pp. 309–328. [Google Scholar]
- Schnell, H. Linear Aromatic Polyesters of Carbonic Acid. Ind. Eng. Chem. 1959, 51, 157–160. [Google Scholar] [CrossRef]
- Qiao, X.; Chung, T.-S.; Pramoda, K. Fabrication and characterization of BTDA-TDI/MDI (P84) co-polyimide membranes for the pervaporation dehydration of isopropanol. J. Membr. Sci. 2005, 264, 176–189. [Google Scholar] [CrossRef]
- Zhmakin, V.V.; Teplyakov, V.V. Permeability of C1–C3 Hydrocarbons through MDK Membranes under Nonisothermal Conditions at Lower Temperatures. Pet. Chem. 2016, 56, 335–343. [Google Scholar] [CrossRef]
- Teplyakov, V.V.; Shalygin, M.G.; Kozlova, A.A.; Chistyakov, A.V.; Tsodikov, M.V.; Netrusov, A.I. Membrane Technology in Bioconversion of Lignocellulose to Motor Fuel Components. Pet. Chem. 2017, 57, 747–762. [Google Scholar] [CrossRef]
- Merkel, T.C.; Wei, X.; He, Z.; White, L.S.; Wijmans, J.G.; Baker, R.W. Selective Exhaust Gas Recycle with Membranes for CO2 Capture from Natural Gas Combined Cycle Power Plants. Ind. Eng. Chem. Res. 2013, 52, 1150–1159. [Google Scholar] [CrossRef]

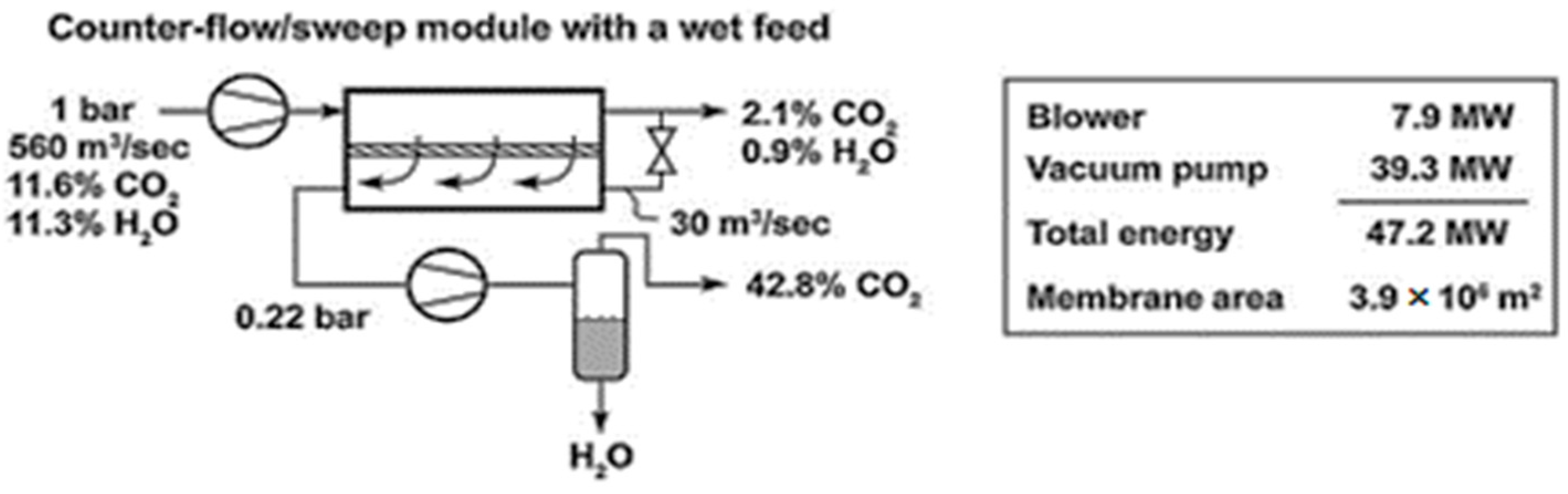
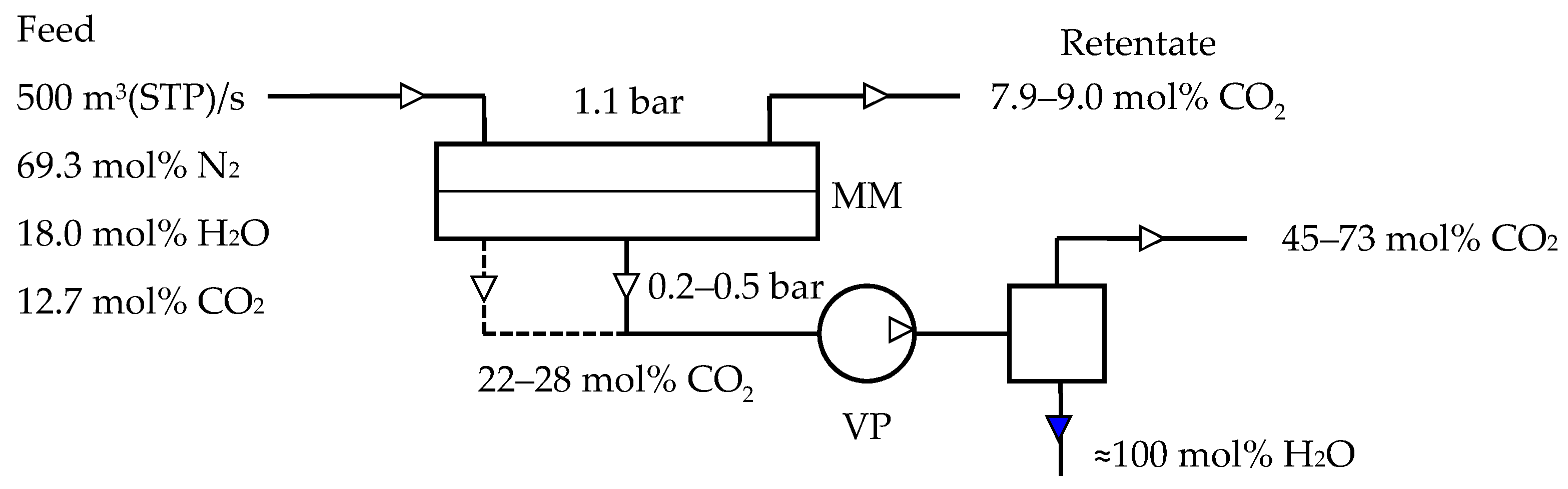

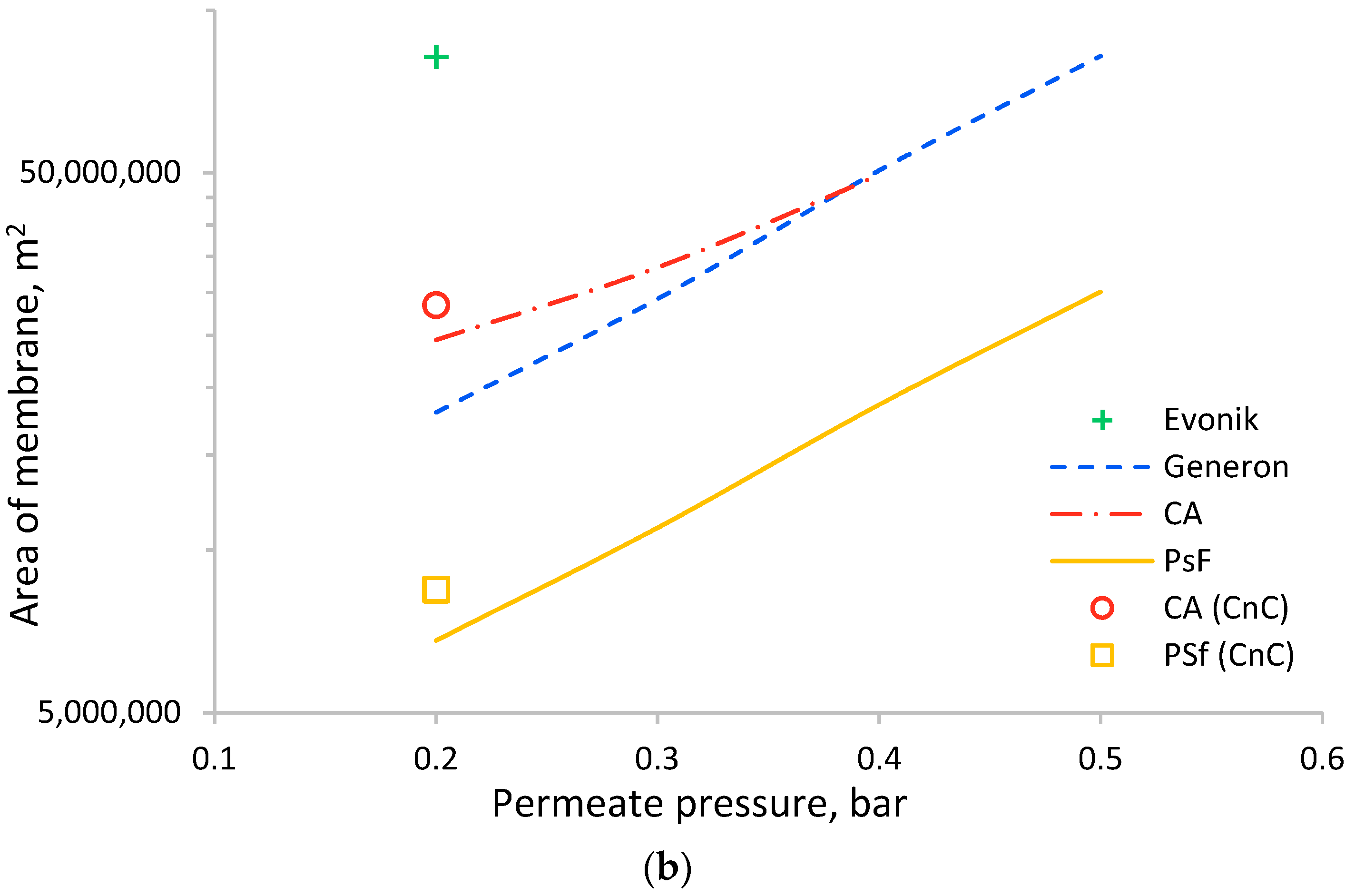
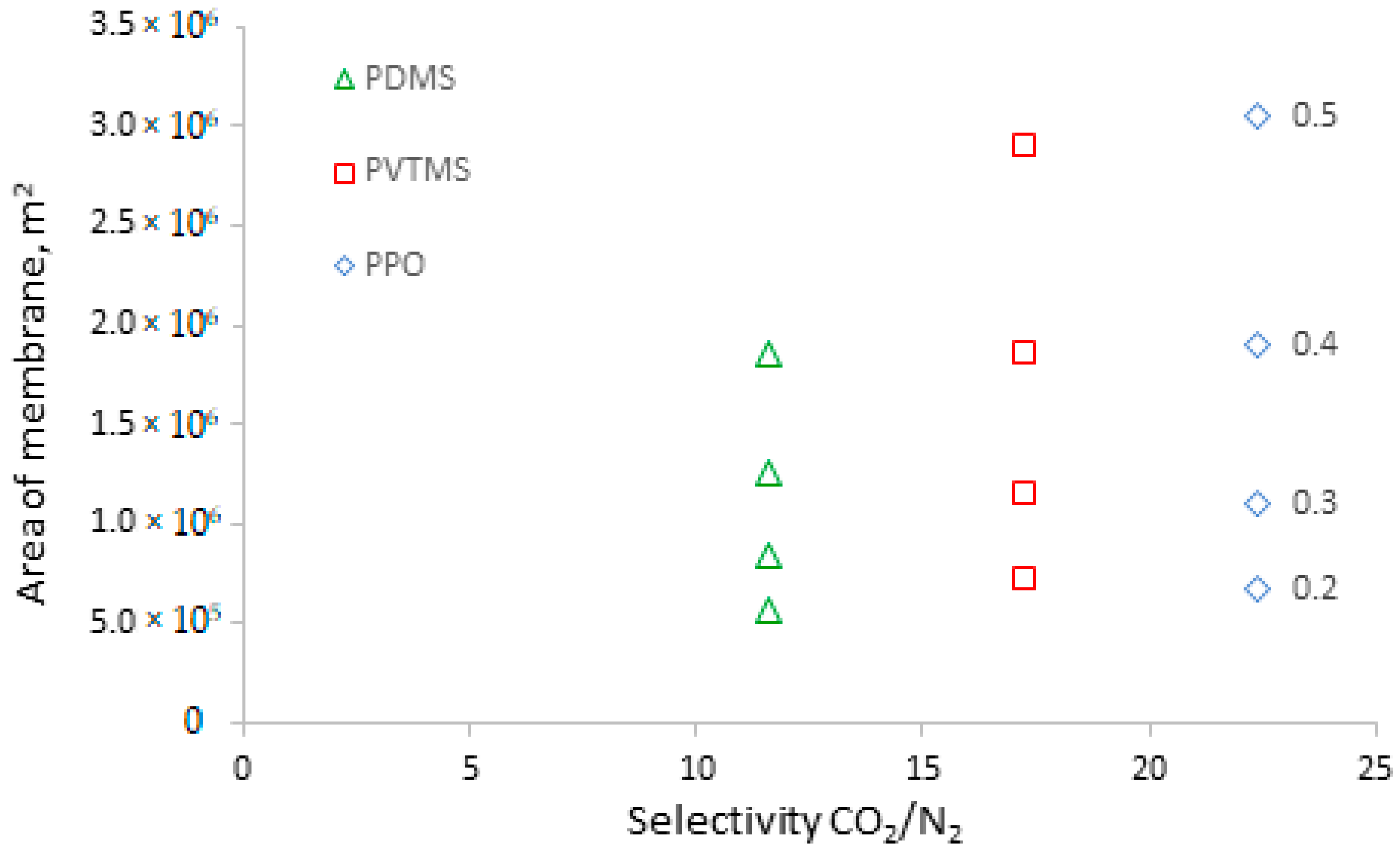
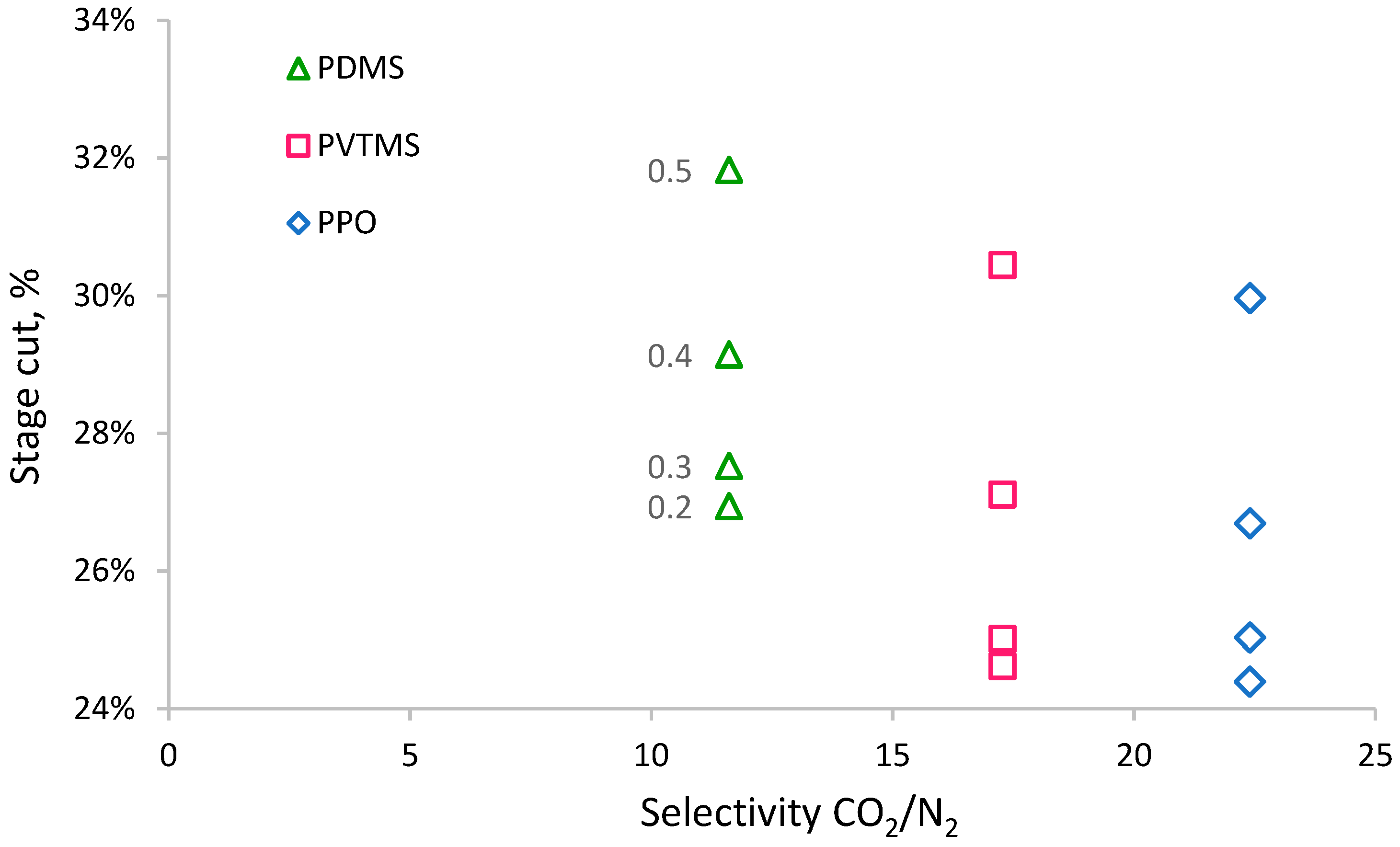

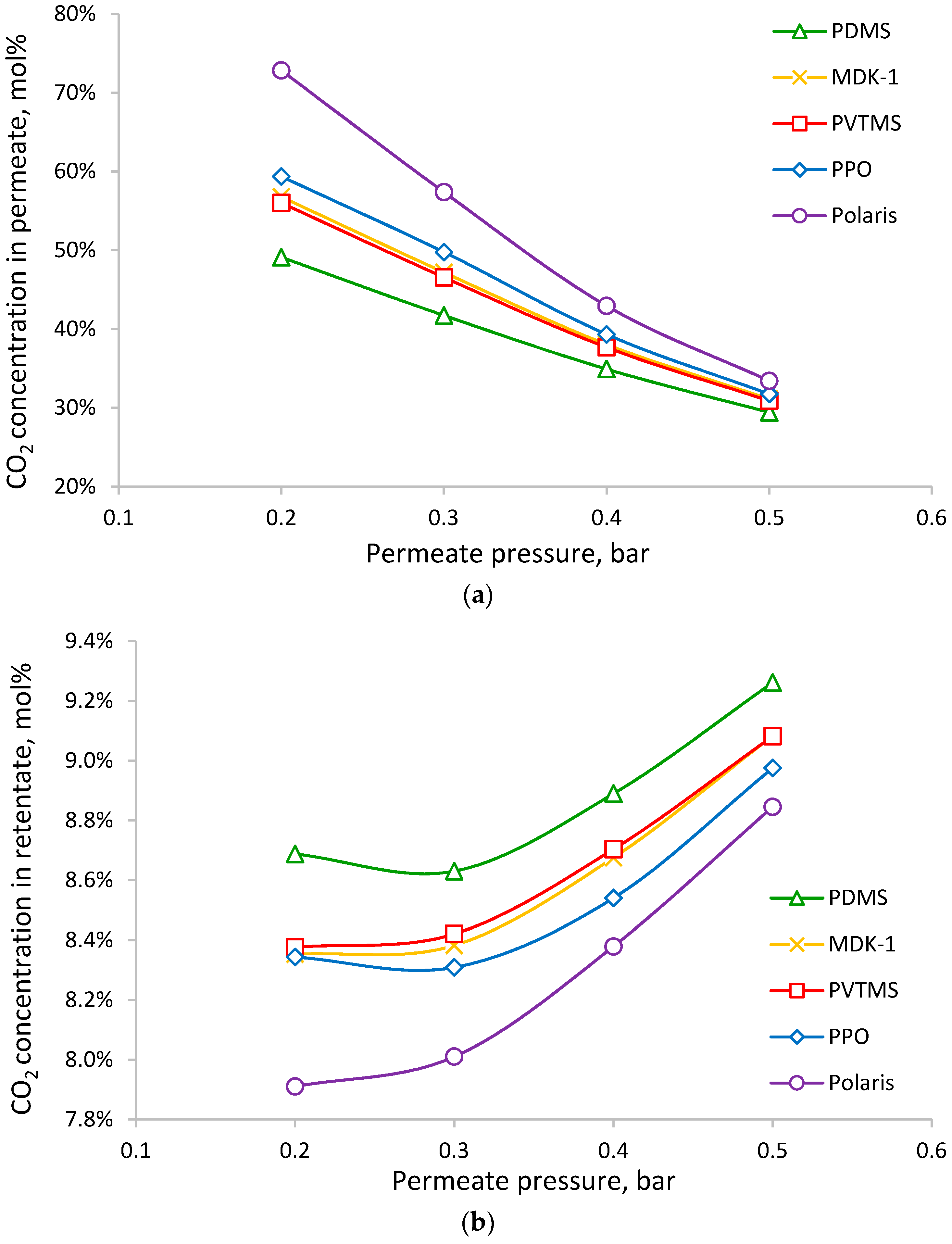

| Manufacturer | Commercial Name | Polymer | Ref. |
|---|---|---|---|
| STC “Vladipor”, Vladimir, Russia | MDK-1 | PDMS-based copolymer | [10] |
| NPO PJSC “Cryogenmash”, Balashikha, Russia | PVTMS | PVTMS | [11] |
| Parker Hannifin, Cleveland, OH, USA | Parker | PPO | [12] |
| UOP (A Honeywell Company), Charlotte, NC, USA Schlumberger, Houston, TX, USA | Separex, Cynara | CA | [12] |
| Air products, Allentown, PA, USA | Prizm | PSf | [12] |
| MTR, 39630 Eureka Dr, Newark, CA, USA | Polaris Gen-2 | - | [12] |
| Generon, 16250 Tomball Parkway Houston, TX, USA | Generon | TBPC | [12] |
| Evonik, Essen, Germany | Sepuran | P84 | [13] |
| Polymer | P (CO2), Barrer | P (N2), Barrer | P (H2O), Barrer | α (CO2/N2) | α (H2O/N2) | Ref. |
|---|---|---|---|---|---|---|
| PDMS | 3250 | 280 | 36,000 | 11.6 | 129 | [14] |
| PVTMS | 190 | 11.0 | 1450 | 17.3 | 132 | [15] |
| PPO | 56.0 | 2.50 | 4060 | 22.4 | 1620 | [16,17] |
| CA | 2.40 | 0.250 | 6800 | 9.60 | 27,200 | [16,18] |
| PSf | 5.60 | 0.250 | 2000 | 22.4 | 8000 | [16,19] |
| TBPC | 4.23 | 0.182 | 795 | 23.2 | 4370 | [20] |
| P84 | 1.20 | 0.0240 | 1840 | 50.0 | 2080 | [21] |
| Parameters | Values |
|---|---|
| Flue gas feed flow rate, m3 (STP)/h | 1,800,000 |
| Initial flue gas composition, mol%: | |
| N2 | 69.3 |
| CO2 | 12.7 |
| H2O | 18.0 |
| Feed pressure (absolute), bar | 1.1 |
| Permeate pressure (absolute), bar | 0.2–0.5 |
| Temperature, °C | 40 |
| Polymer Membrane | Selective Layer Thickness, μm | Q (CO2)∙106, mol/(m2∙s∙kPa) | Q (N2)∙106, mol/(m2∙s∙kPa) | Q (H2O)∙106, mol/(m2∙s∙kPa) |
|---|---|---|---|---|
| PDMS | 3.0 | 362 | 31.2 | 4010 |
| MDK-1 * | n/a | 203 | 11.2 | 2040 |
| PVTMS | 0.2 | 318 | 18.4 | 2430 |
| PPO | 0.05 | 375 | 16.7 | 27,200 |
| CA | 0.10 | 7.99 | 0.790 | 22,700 |
| PSf | 0.05 | 37.5 | 1.70 | 13,400 |
| Polaris Gen-2 | n/a | 737 | 14.8 | 1470 ** |
| TBPC | 0.10 | 13.8 | 0.590 | 2660 |
| P84 | 0.10 | 4.05 | 0.0790 | 6270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroshnichenko, D.; Shalygin, M.; Bazhenov, S. Simulation of the Membrane Process of CO2 Capture from Flue Gas via Commercial Membranes While Accounting for the Presence of Water Vapor. Membranes 2023, 13, 692. https://doi.org/10.3390/membranes13080692
Miroshnichenko D, Shalygin M, Bazhenov S. Simulation of the Membrane Process of CO2 Capture from Flue Gas via Commercial Membranes While Accounting for the Presence of Water Vapor. Membranes. 2023; 13(8):692. https://doi.org/10.3390/membranes13080692
Chicago/Turabian StyleMiroshnichenko, Daria, Maxim Shalygin, and Stepan Bazhenov. 2023. "Simulation of the Membrane Process of CO2 Capture from Flue Gas via Commercial Membranes While Accounting for the Presence of Water Vapor" Membranes 13, no. 8: 692. https://doi.org/10.3390/membranes13080692
APA StyleMiroshnichenko, D., Shalygin, M., & Bazhenov, S. (2023). Simulation of the Membrane Process of CO2 Capture from Flue Gas via Commercial Membranes While Accounting for the Presence of Water Vapor. Membranes, 13(8), 692. https://doi.org/10.3390/membranes13080692








