Current and Potential Applications of Green Membranes with Nanocellulose
Abstract
:1. Introduction
2. Types of Nanocellulose
3. Applications of Nanocellulose-Based Membranes
3.1. Desalination and Wastewater Treatment
3.2. Gas Separation
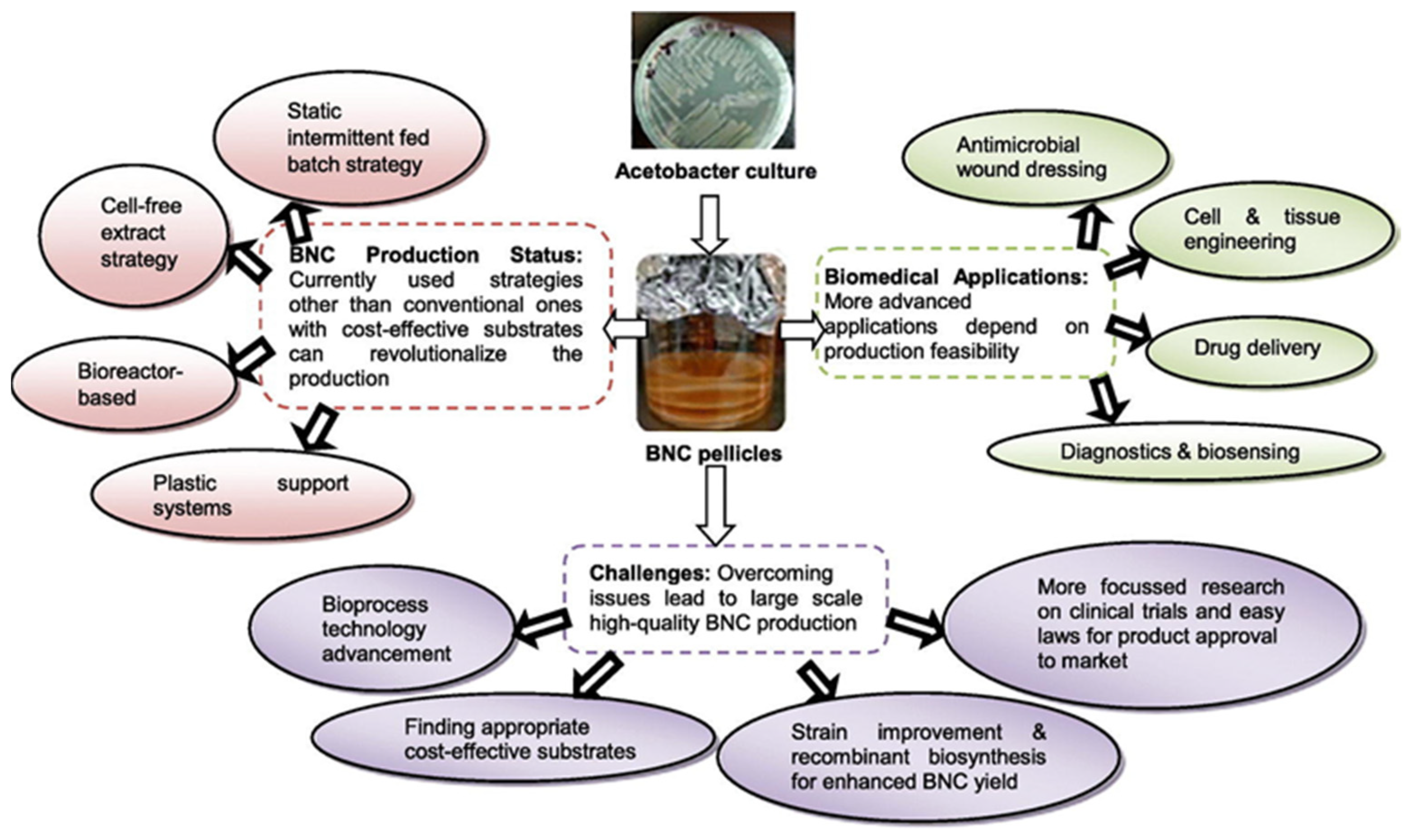
3.3. Biomedical Applications
4. Future of Nanocellulose
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, T.S.; Muhammadaly, S.A.; Kanoth, B.P.; Joseph, T.; Dominic, M.D.C.; George, N.; Balachandrakurupp, V.; John, H. Isolation of high crystalline nanocellulose from Mimosa pudica plant fibres with potential in packaging applications. Packag. Technol. Sci. 2021, 35, 163–174. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Nanocellulose as a green and sustainable emerging material in energy applications: A review. Polym. Adv. Technol. 2017, 28, 1583–1594. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, C.; Huang, Y.; Wu, M. Chapter 3: Surface Modification of Nanocellulose. Nanocellulose 2021, 65–92. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. Nanocellulose: Preparation methods and applications. In Cellulose-Reinforced Nanofibre Composites: Production, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–276. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Naz, S.; Ali, J.S.; Zia, M. Nanocellulose isolation characterization and applications: A journey from non-remedial to biomedical claims. Bio Design Manuf. 2019, 2, 187–212. [Google Scholar] [CrossRef]
- Vineeth, S.K.; Gadhave, R.V.; Gadekar, P.T. Chemical Modification of Nanocellulose in Wood Adhesive. Open J. Polym. Chem. 2019, 9, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Köse, K.; Mavlan, M.; Youngblood, J.P. Applications and impact of nanocellulose based adsorbents. Cellulose 2020, 27, 2967–2990. [Google Scholar] [CrossRef]
- Kumar, N.; Kardam, A.; Rajawat, D.S.; Jain, V.K. Suman Carboxymethyl nanocellulose stabilized nano zero-valent iron: An effective method for reduction of hexavalent chromium in wastewater. Mater. Res. Express 2019, 6, 1150f3. [Google Scholar] [CrossRef]
- Rai, G.K.; Singh, V. Study of fabrication and analysis of nanocellulose reinforced polymer matrix composites. Mater. Today Proc. 2021, 38, 85–88. [Google Scholar] [CrossRef]
- Mahardika, M.; Amelia, D.; Azril; Syafri, E. Applications of nanocellulose and its composites in bio packaging-based starch. Mater. Today Proc. 2023, 74, 415–418. [Google Scholar] [CrossRef]
- Valentini, F.; Dorigato, A.; Rigotti, D.; Pegoretti, A. Polyhydroxyalkanoates/Fibrillated Nanocellulose Composites for Additive Manufacturing. J. Polym. Environ. 2019, 27, 1333–1341. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498. [Google Scholar] [CrossRef]
- Tan, K.; Heo, S.; Foo, M.; Chew, I.M.; Yoo, C. An insight into nanocellulose as soft condensed matter: Challenge and future prospective toward environmental sustainability. Sci. Total. Environ. 2019, 650, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.-X.; Liu, Y.; Zhou, N.; Guo, D.-Q.; Ma, M.-G. Multifunctional nanocellulose-based composites for potential environmental applications. Cellulose 2023, 30, 39–60. [Google Scholar] [CrossRef]
- Zeng, H.; Hao, H.; Wang, X.; Shao, Z. Chitosan-based composite film adsorbents reinforced with nanocellulose for removal of Cu(II) ion from wastewater: Preparation, characterization, and adsorption mechanism. Int. J. Biol. Macromol. 2022, 213, 369–380. [Google Scholar] [CrossRef]
- Yin, Z.; Li, M.; Li, Z.; Deng, Y.; Xue, M.; Chen, Y.; Ou, J.; Lei, S.; Luo, Y.; Xie, C. A harsh environment resistant robust Co(OH)2@stearic acid nanocellulose-based membrane for oil-water separation and wastewater purification. J. Environ. Manag. 2023, 342, 118127. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Vineis, P.; Chan, Q.; Khan, A. Climate change impacts on water salinity and health. J. Epidemiol. Glob. Health 2011, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Torstensen, J.Ø.; Helberg, R.M.; Deng, L.; Gregersen, O.W.; Syverud, K. PVA/nanocellulose nanocomposite membranes for CO2 separation from flue gas. Int. J. Greenh. Gas Control 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Jaekel, E.E.; Kluge, S.; Tröger-Müller, S.; Tutuş, M.; Filonenko, S. Tunable Gas Permeation Behavior in Self-Standing Cellulose Nanocrystal-Based Membranes. ACS Sustain. Chem. Eng. 2022, 10, 12895–12905. [Google Scholar] [CrossRef]
- Mithra, S.N.; Ahankari, S. Nanocellulose-based membranes for CO2 separation from biogas through the facilitated transport mechanism: A review. Mater. Today Sustain. 2022, 19, 100191. [Google Scholar] [CrossRef]
- Casadei, R.; Firouznia, E.; Baschetti, M.G. Effect of Mobile Carrier on the Performance of PVAm–Nanocellulose Facilitated Transport Membranes for CO2 Capture. Membranes 2021, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dutta, S.D.; Lim, K.-T. Nanocellulose-based polymer hybrids and their emerging applications in biomedical engineering and water purification. RSC Adv. 2019, 9, 19143–19162. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Naficy, S.; Chandrawati, R.; Dehghani, F. Nanocellulose for Sensing Applications. Adv. Mater. Interfaces 2019, 6, 1900424. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.d.L.T.M.; Teixeira, J.A. Nanocellulose Production: Exploring the Enzymatic Route and Residues of Pulp and Paper Industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef]
- Chen, M.; Ma, Q.; Zhu, J.Y.; Alonso, D.M.; Runge, T. GVL pulping facilitates nanocellulose production from woody biomass. Green Chem. 2019, 21, 5316–5325. [Google Scholar] [CrossRef] [Green Version]
- Charreau, H.; Cavallo, E.; Foresti, M.L. Patents involving nanocellulose: Analysis of their evolution since 2010. Carbohydr. Polym. 2020, 237, 116039. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-Based Composite Materials Used in Drug Delivery Systems. Polymers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef] [PubMed]
- Derami, H.G.; Gupta, P.; Gupta, R.; Rathi, P.; Morrissey, J.J.; Singamaneni, S. Palladium Nanoparticle-Decorated Mesoporous Polydopamine/Bacterial Nanocellulose as a Catalytically Active Universal Dye Removal Ultrafiltration Membrane. ACS Appl. Nano Mater. 2020, 3, 5437–5448. [Google Scholar] [CrossRef]
- Numata, Y.; Sakata, T.; Furukawa, H.; Tajima, K. Bacterial cellulose gels with high mechanical strength. Mater. Sci. Eng. C 2015, 47, 57–62. [Google Scholar] [CrossRef]
- El-Hoseny, S.M.; Basmaji, P.; de Olyveira, G.M.; Costa, L.M.M.; Alwahedi, A.M.; Oliveira, J.D.D.C.; Francozo, G.B. Natural ECM-Bacterial Cellulose Wound Healing—Dubai Study. J. Biomater. Nanobiotechnol. 2015, 6, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Lindström, T.; Sharma, P.S.; Chi, K.; Hsiao, B.S. Nanocellulose for Sustainable Water Purification. Chem. Rev. 2022, 122, 8936–9031. [Google Scholar] [CrossRef]
- Saud, A.; Saleem, H.; Zaidi, S.J. Progress and Prospects of Nanocellulose-Based Membranes for Desalination and Water Treatment. Membranes 2022, 12, 462. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Sirohi, R. Nanocellulose-based products for sustainable applications-recent trends and possibilities. Rev. Environ. Sci. Bio/Technol. 2020, 19, 779–806. [Google Scholar] [CrossRef]
- Chung, N.H.; Van Binh, N.; Dien, L.Q. Preparation of nanocellulose acetate from bleached hardwood pulp and its application for seawater desalination. Vietnam J. Chem. 2020, 58, 281–286. [Google Scholar] [CrossRef]
- Rezaei-DashtArzhandi, M.; Sarrafzadeh, M.H.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Wong, K.C.; Mohamed, M.A. Enhancing the desalination performance of forward osmosis membrane through the incorporation of green nanocrystalline cellulose and halloysite dual nanofillers. J. Chem. Technol. Biotechnol. 2020, 95, 2359–2370. [Google Scholar] [CrossRef]
- Sijabat, E.K.; Nuruddin, A.; Aditiawati, P.; Purwasasmita, B.S. Synthesis and Characterization of Bacterial Nanocellulose from Banana Peel for Water Filtration Membrane Application. J. Physics Conf. Ser. 2019, 1230, 012085. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Zhang, X.; Chen, T.; Ye, Z.; Rahaman, S. High performance nanocomposite nanofiltration membranes with polydopamine-modified cellulose nanocrystals for efficient dye/salt separation. Desalination 2021, 521, 115385. [Google Scholar] [CrossRef]
- Mohammed, S.; Hegab, H.M.; Ou, R.; Liu, S.; Ma, H.; Chen, X.; Sridhar, T.; Wang, H. Effect of oxygen plasma treatment on the nanofiltration performance of reduced graphene oxide/cellulose nanofiber composite membranes. Green Chem. Eng. 2020, 2, 122–131. [Google Scholar] [CrossRef]
- Güzel, F.; Yakut, H.; Topal, G. Determination of kinetic and equilibrium parameters of the batch adsorption of Mn(II), Co(II), Ni(II) and Cu(II) from aqueous solution by black carrot (Daucus carota L.) residues. J. Hazard. Mater. 2008, 153, 1275–1287. [Google Scholar] [CrossRef]
- Aoudi, B.; Boluk, Y.; El-Din, M.G. Recent advances and future perspective on nanocellulose-based materials in diverse water treatment applications. Sci. Total. Environ. 2022, 843, 156903. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Pang, H.; Lu, Y.; Li, Z.; Kang, H.; Wang, M.; Zhang, S.; Li, J. Wood-inspired nanocellulose aerogel adsorbents with excellent selective pollutants capture, superfast adsorption, and easy regeneration. J. Hazard. Mater. 2021, 415, 125612. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Sehaqui, H.; De Larraya, U.P.; Liu, P.; Pfenninger, N.; Mathew, A.P.; Zimmermann, T.; Tingaut, P. Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose 2014, 21, 2831–2844. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Leitch, M.E.; Li, C.; Ikkala, O.; Mauter, M.S.; Lowry, G.V. Bacterial Nanocellulose Aerogel Membranes: Novel High-Porosity Materials for Membrane Distillation. Environ. Sci. Technol. Lett. 2016, 3, 85–91. [Google Scholar] [CrossRef]
- Xu, T.; Jiang, Q.; Ghim, D.; Liu, K.-K.; Sun, H.; Derami, H.G.; Wang, Z.; Tadepalli, S.; Jun, Y.-S.; Zhang, Q.; et al. Catalytically Active Bacterial Nanocellulose-Based Ultrafiltration Membrane. Small 2018, 14, e1704006. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, S.; Ghim, D.; Jiang, Q.; Singamaneni, S.; Jun, Y.-S. A thermally engineered polydopamine and bacterial nanocellulose bilayer membrane for photothermal membrane distillation with bactericidal capability. Nano Energy 2021, 79, 105353. [Google Scholar] [CrossRef]
- Morsy, A.; Mahmoud, A.S.; Soliman, A.; Ibrahim, H.; Fadl, E. Improved anti-biofouling resistances using novel nanocelluloses/cellulose acetate extracted from rice straw based membranes for water desalination. Sci. Rep. 2022, 12, 4386. [Google Scholar] [CrossRef]
- Chen, C.; Kuang, Y.; Hu, L. Challenges and Opportunities for Solar Evaporation. Joule 2019, 3, 683–718. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Xu, Y.; Ma, X.; Tian, Z.; Zhang, C.; Han, J.; Han, X.; He, S.; Duan, G.; Li, Y. Cellulose-based Interfacial Solar Evaporators: Structural Regulation and Performance Manipulation. Adv. Funct. Mater. 2023, 33, 2302351. [Google Scholar] [CrossRef]
- Jiang, Q.; Derami, H.G.; Ghim, D.; Cao, S.; Jun, Y.-S.; Singamaneni, S. Polydopamine-filled bacterial nanocellulose as a biodegradable interfacial photothermal evaporator for highly efficient solar steam generation. J. Mater. Chem. A 2017, 5, 18397–18402. [Google Scholar] [CrossRef]
- Janakiram, S.; Ansaloni, L.; Jin, S.-A.; Yu, X.; Dai, Z.; Spontak, R.J.; Deng, L. Humidity-responsive molecular gate-opening mechanism for gas separation in ultraselective nanocellulose/IL hybrid membranes. Green Chem. 2020, 22, 3546–3557. [Google Scholar] [CrossRef]
- Janakiram, S.; Yu, X.; Ansaloni, L.; Dai, Z.; Deng, L. Manipulation of Fibril Surfaces in Nanocellulose-Based Facilitated Transport Membranes for Enhanced CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 33302–33313. [Google Scholar] [CrossRef]
- Venturi, D.; Chrysanthou, A.; Dhuiège, B.; Missoum, K.; Baschetti, M.G. Arginine/Nanocellulose Membranes for Carbon Capture Applications. Nanomaterials 2019, 9, 877. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Shen, Y.; Wang, L.; Chen, J.; Lu, Y. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges. Appl. Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Ho, N.A.D.; Leo, C. A review on the emerging applications of cellulose, cellulose derivatives and nanocellulose in carbon capture. Environ. Res. 2021, 197, 111100. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in Advanced Functional Material Applications of Nanocellulose. Processes 2018, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 2011, 18, 1097–1111. [Google Scholar] [CrossRef]
- Ibrahim, H.; Sazali, N.; Salleh, W.N.W.; Abidin, M.N.Z. A short review on recent utilization of nanocellulose for wastewater remediation and gas separation. Mater. Today Proc. 2021, 42, 45–49. [Google Scholar] [CrossRef]
- Dai, Z.; Ottesen, V.; Deng, J.; Helberg, R.M.L.; Deng, L. A Brief Review of Nanocellulose Based Hybrid Membranes for CO2 Separation. Fibers 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-N.; Saito, T.; Fujisawa, S.; Fukuzumi, H.; Isogai, A. Ultrastrong and High Gas-Barrier Nanocellulose/Clay-Layered Composites. Biomacromolecules 2012, 13, 1927–1932. [Google Scholar] [CrossRef]
- Sun, Y.; Chu, Y.; Wu, W.; Xiao, H. Nanocellulose-based lightweight porous materials: A review. Carbohydr. Polym. 2021, 255, 117489. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Jiang, H.; Wang, X.; Zhang, T.; Yao, Y. High CO2 adsorption by amino-modified bio-spherical cellulose nanofibres aerogels. Environ. Chem. Lett. 2018, 16, 605–614. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Jiang, H.; Wang, X. Aminosilane-grafted spherical cellulose nanocrystal aerogel with high CO2 adsorption capacity. Environ. Sci. Pollut. Res. 2019, 26, 16716–16726. [Google Scholar] [CrossRef]
- Valencia, L.; Rosas-Arbelaez, W.; Aguilar-Sanchez, A.; Mathew, A.P.; Palmqvist, A.E.C. Bio-based Micro-/Meso-/Macroporous Hybrid Foams with Ultrahigh Zeolite Loadings for Selective Capture of Carbon Dioxide. ACS Appl. Mater. Interfaces 2019, 11, 40424–40431. [Google Scholar] [CrossRef] [PubMed]
- Valencia, L.; Abdelhamid, H.N. Nanocellulose leaf-like zeolitic imidazolate framework (ZIF-L) foams for selective capture of carbon dioxide. Carbohydr. Polym. 2019, 213, 338–345. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Ma, Y.; Guo, H.; Wei, J.; Wang, B.; Jiang, X.; Deng, L. Nanocellulose Crystal-Enhanced Hybrid Membrane for CO2 Capture. Ind. Eng. Chem. Res. 2022, 61, 9067–9076. [Google Scholar] [CrossRef]
- Venturi, D.; Grupkovic, D.; Sisti, L.; Baschetti, M.G. Effect of humidity and nanocellulose content on Polyvinylamine-nanocellulose hybrid membranes for CO2 capture. J. Membr. Sci. 2018, 548, 263–274. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Yu, Q.; Helberg, R.M.L.; Janakiram, S.; Ansaloni, L.; Deng, L. Fabrication and Evaluation of Bio-Based Nanocomposite TFC Hollow Fiber Membranes for Enhanced CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 10874–10882. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, S.; Manukyan, L.; Mihranyan, A. Protein–Nanocellulose Interactions in Paper Filters for Advanced Separation Applications. Langmuir 2017, 33, 4729–4736. [Google Scholar] [CrossRef]
- dos Santos, C.A.; dos Santos, G.R.; Soeiro, V.S.; dos Santos, J.R.; Rebelo, M.D.A.; Chaud, M.V.; Gerenutti, M.; Grotto, D.; Pandit, R.; Rai, M.; et al. Bacterial nanocellulose membranes combined with nisin: A strategy to prevent microbial growth. Cellulose 2018, 25, 6681–6689. [Google Scholar] [CrossRef]
- Sampaio, L.M.; Padrão, J.; Faria, J.; Silva, J.P.; Silva, C.J.; Dourado, F.; Zille, A. Laccase immobilization on bacterial nanocellulose membranes: Antimicrobial, kinetic and stability properties. Carbohydr. Polym. 2016, 145, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ong, K.K.; Noor, S.A.M.; Halim, N.A.; Shah, N.A.A.; Jamal, S.H.; Janudin, N.; Misenan, M.S.M. Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes. Polymers 2021, 13, 3249. [Google Scholar] [CrossRef]
- Abba, M.; Ibrahim, Z.; Chong, C.S.; Zawawi, N.A.; Kadir, M.R.A.; Yusof, A.H.M.; Razak, S.I.A. Transdermal Delivery of Crocin Using Bacterial Nanocellulose Membrane. Fibers Polym. 2019, 20, 2025–2031. [Google Scholar] [CrossRef]
- Pachuau, L.S. A Mini Review on Plant-based Nanocellulose: Production, Sources, Modifications and Its Potential in Drug Delivery Applications. Mini-Rev. Med. Chem. 2015, 15, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Ullah, M.W.; Liang, X.; Yang, G. Chapter 5: Recent Developments in Synthesis, Properties, and Biomedical Applications of Cellulose-Based Hydrogels. Nanocellulose 2021, 121–153. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, N.; Sun, Y.; Shao, J.; Liu, Q.; Zhuang, X.; Twebaze, C.B. Nanocellulose aerogels from banana pseudo-stem as a wound dressing. Ind. Crop. Prod. 2023, 194, 116383. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Arun, K.B.; Binod, P.; Pugazhendhi, A.; Awasthi, M.K.; Gnansounou, E.; Pandey, A.; Sindhu, R. Promising eco-friendly biomaterials for future biomedicine: Cleaner production and applications of Nanocellulose. Environ. Technol. Innov. 2021, 24, 101855. [Google Scholar] [CrossRef]
- Eldhose, M.; Roy, R.; George, C.; Joseph, A. Sensing and Biosensing Applications of Nanocellulose. In Handbook of Biopolymers; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1007–1032. [Google Scholar] [CrossRef]
- Roshkovan, L.; Singhal, S.; Katz, I.S.; Galperin-Aizenberg, M. Multimodality imaging of Surgicel®, an important mimic of post-operative complication in the thorax. BJR Open 2021, 3, 20210031. [Google Scholar] [CrossRef]
- Queirós, E.C.; Pinheiro, S.P.; Pereira, J.E.; Prada, J.; Pires, I.; Dourado, F.; Parpot, P.; Gama, M. Hemostatic Dressings Made of Oxidized Bacterial Nanocellulose Membranes. Polysaccharides 2021, 2, 80–99. [Google Scholar] [CrossRef]
- Curvello, R.; Mendoza, L.; McLiesh, H.; Manolios, J.; Tabor, R.F.; Garnier, G. Nanocellulose Hydrogel for Blood Typing Tests. ACS Appl. Bio Mater. 2019, 2, 2355–2364. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Lasrado, D.; Ahankari, S.; Kar, K. Nanocellulose-based polymer composites for energy applications—A review. J. Appl. Polym. Sci. 2020, 137, 48959. [Google Scholar] [CrossRef]
- Yang, H.; Gueskine, V.; Berggren, M.; Engquist, I. Cross-Linked Nanocellulose Membranes for Nanofluidic Osmotic Energy Harvesting. ACS Appl. Energy Mater. 2022, 5, 15740–15748. [Google Scholar] [CrossRef]
- Farid, M.; Purniawan, A.; Susanti, D.; Priyono, S.; Ardhyananta, H.; Rahmasita, M.E. Nanocellulose based polymer composite for acoustical materials. AIP Conf. Proc. 2018, 1945, 020025. [Google Scholar] [CrossRef]
- De Amorim, J.D.P.; De Souza, K.C.; Duarte, C.R.; Da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; De Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zheng, Z.; Liu, H.; Zhu, L.; Yang, M.; Chen, Y. Nanocellulose-based membranes for highly efficient molecular separation. Chem. Eng. J. 2023, 451, 138711. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, Y.; Liza, A.A.; Yang, G.; Sipponen, M.H.; Guo, J.; Li, H. Nanocellulose: A review on preparation routes and applications in functional materials. Cellulose 2023, 30, 4115–4147. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N., Jr. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Pires, J.R.; Souza, V.G.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production—Current knowledge and future prospects. Ind. Crop. Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Shi, G.; Zhuo, H.; Ali, M.A.; Jamróz, E.; Zhang, H.; Zhong, L.; Peng, X. Advanced Flexible Materials from Nanocellulose. Adv. Funct. Mater. 2023, 33, 2214245. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.; Veiga, M.; Antunes, F.; Pintado, M.; Sèbe, G.; Coma, V.; et al. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]

| Structural Properties | Mechanical Properties | ||||
|---|---|---|---|---|---|
| Nanocellulose Type | Length (nm) | Diameter (nm) | Crystallinity (%) | Young’s Modulus (GPa) | Tensile Strength (GPa) |
| CNCs | 100–500 | 3–50 | ~90 | 50–140 | 8–10 |
| CNFs | ≥103 | 3–60 | 50–90 | 50–160 | 0.8–1 |
| BC | ≥103 | 20–100 | 84–89 | 78 | 0.2–2 |
| Nanocellulose-Based Membrane Material | Summary of Results | Ref. |
|---|---|---|
| Polydopamine-modified Cellulose nanocrystals (CNCs) |
| [44] |
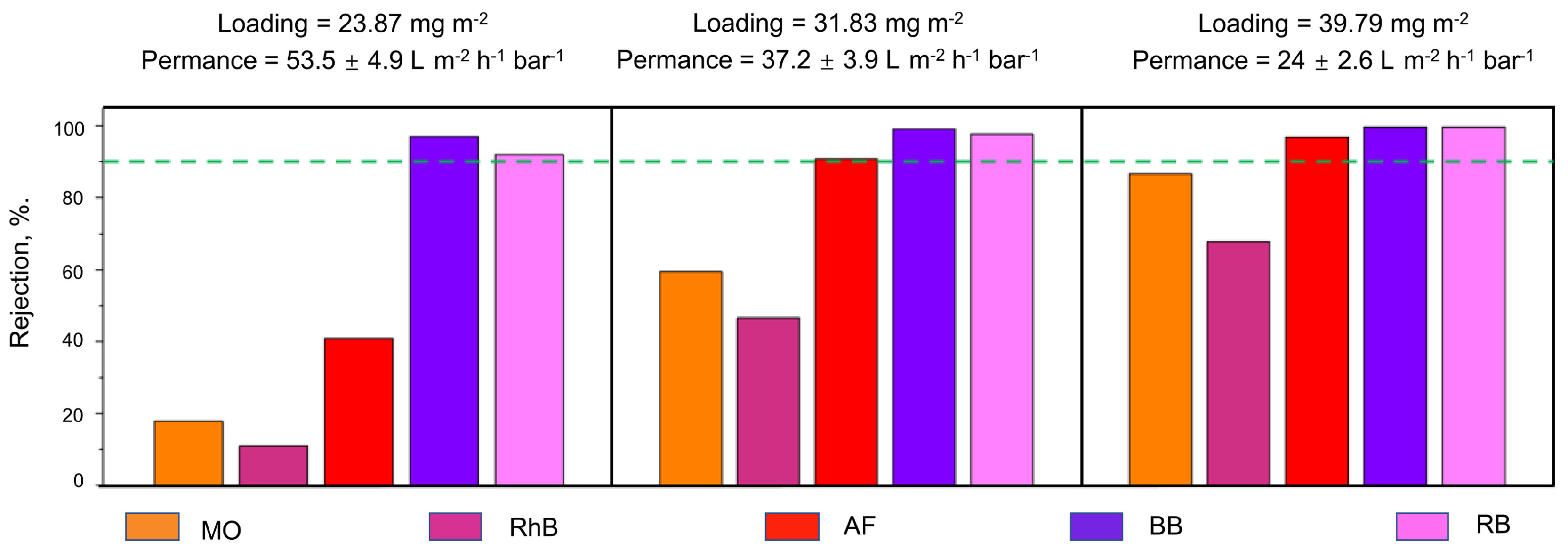
| Reduced graphene oxide (RGO)/cellulose nanofibrils (CNFs) | Varying rGO loading at a fixed 1:1 ratio of rGO to CNF and testing of MO, RhB, AF, BB and RB dyes:
| [45] |
| Negatively charged carboxylated CNF with trimethylolpropane-tris-(2-methyl-1-aziridine) propionate and graphene oxide | Excellent adsorbent of numerous cations of heavy metals, including Pb2+, Cd2+ and Cu2+ | [48] |
| Tetramethylpiperidine-1-oxyl (TEMPO)-oxidized nanocellulose | Adsorption capabilities for divalent cations | [50] |
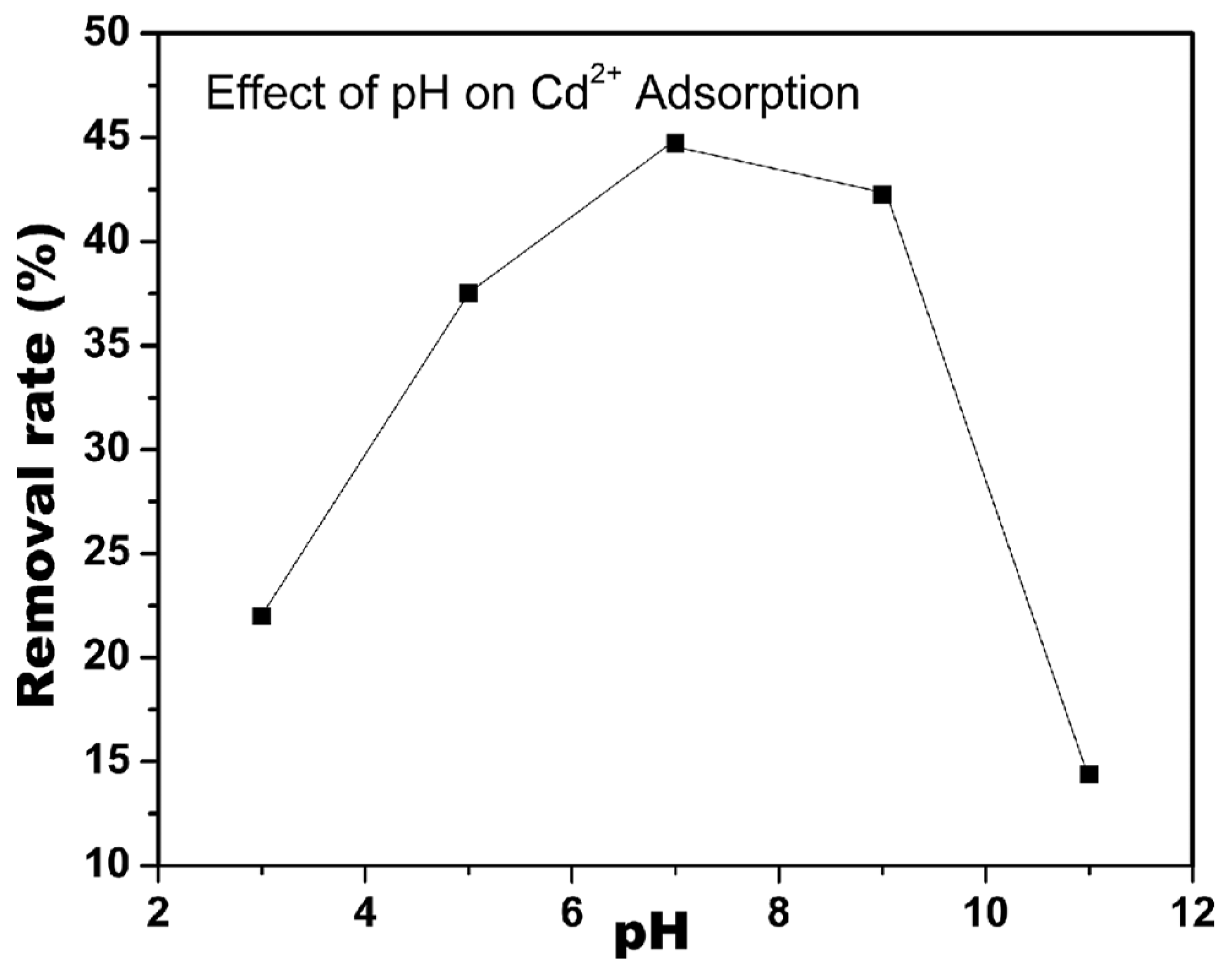
| CNF modified by nitro-oxidation | Adsorption of Cd2+ was optimal at pH 7 and decreased in acidic and basic conditions | [51] |
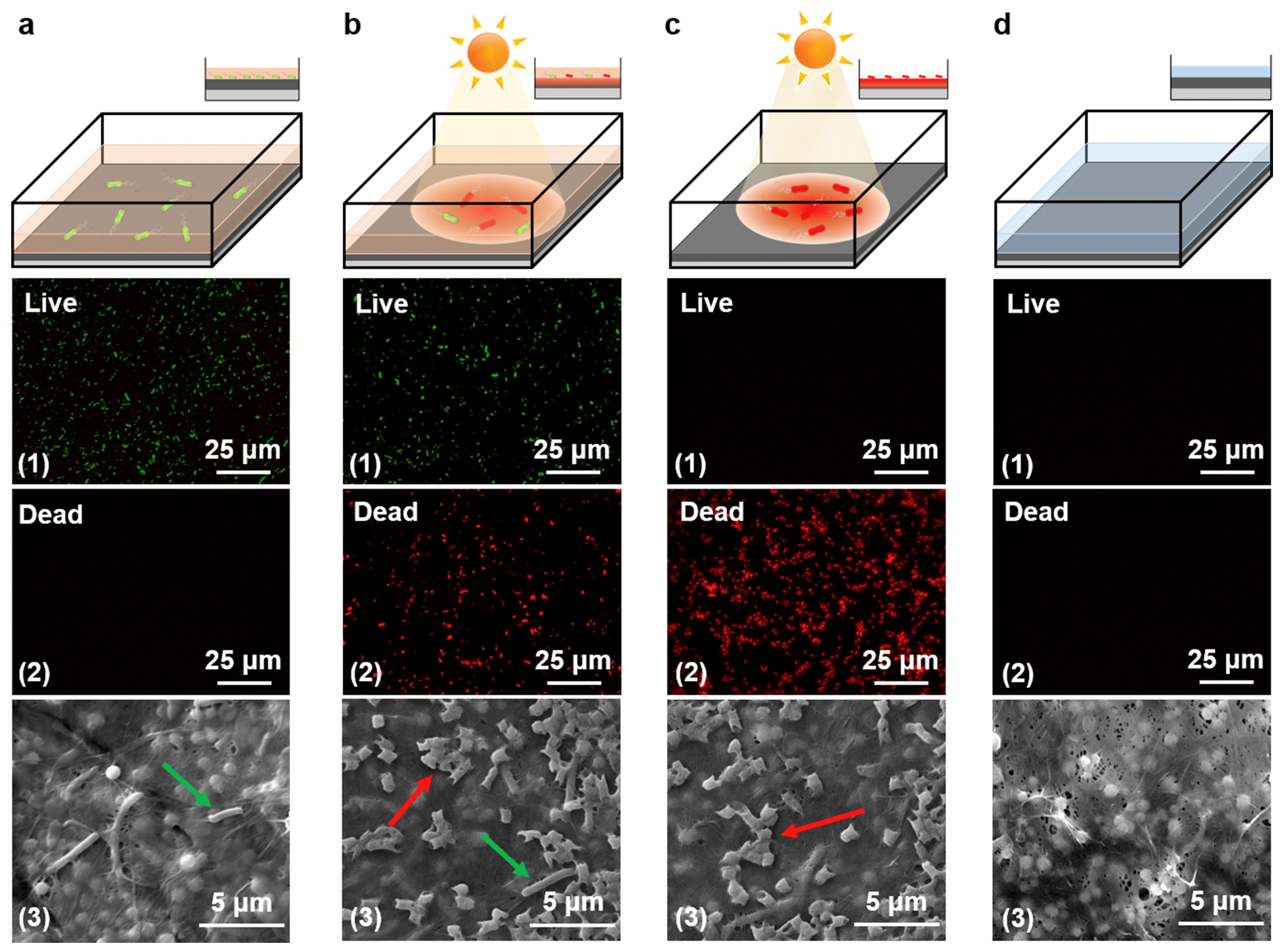
| Polydopamine (PDA) particles and bacterial nanocellulose |
| [54] |
| Nanocellulose acetate (NCA) |
| [55] |
| Microporous network of cellulose composite | High evaporation rate and salt resistance within evaporator | [57] |
| BNC loaded with a high concentration of polydopamine (PDA) | Solar steam generation efficiency of 78% within evaporator | [58] |
| Nanocellulose-Based Membrane Material | Results | Ref. |
|---|---|---|
| Modified spherical cellulose nanofibril (CNF) hydrogel | CO2 adsorption capacity of 1.28–1.78 mmol/g at high temperature | [70] |
| N-(2-aminoethyl)-3 aminopropylmethyldimethoxysilane modified spherical cellulose nanocrystal (CNC) aerogel | CO2 adsorption capacity of 1.68 mmol/g | [71] |
| Silicalite-1 zeolite modified hybrid CNF-gelatin foam | Adsorption up to 1.2 mmol CO2/g | [72] |
| Hybrid CNF-gelatin foam with a zeolitic imidazolate metal-organic framework (ZIF) | Greater CO2 adsorption and selectivity over nitrogen | [73] |
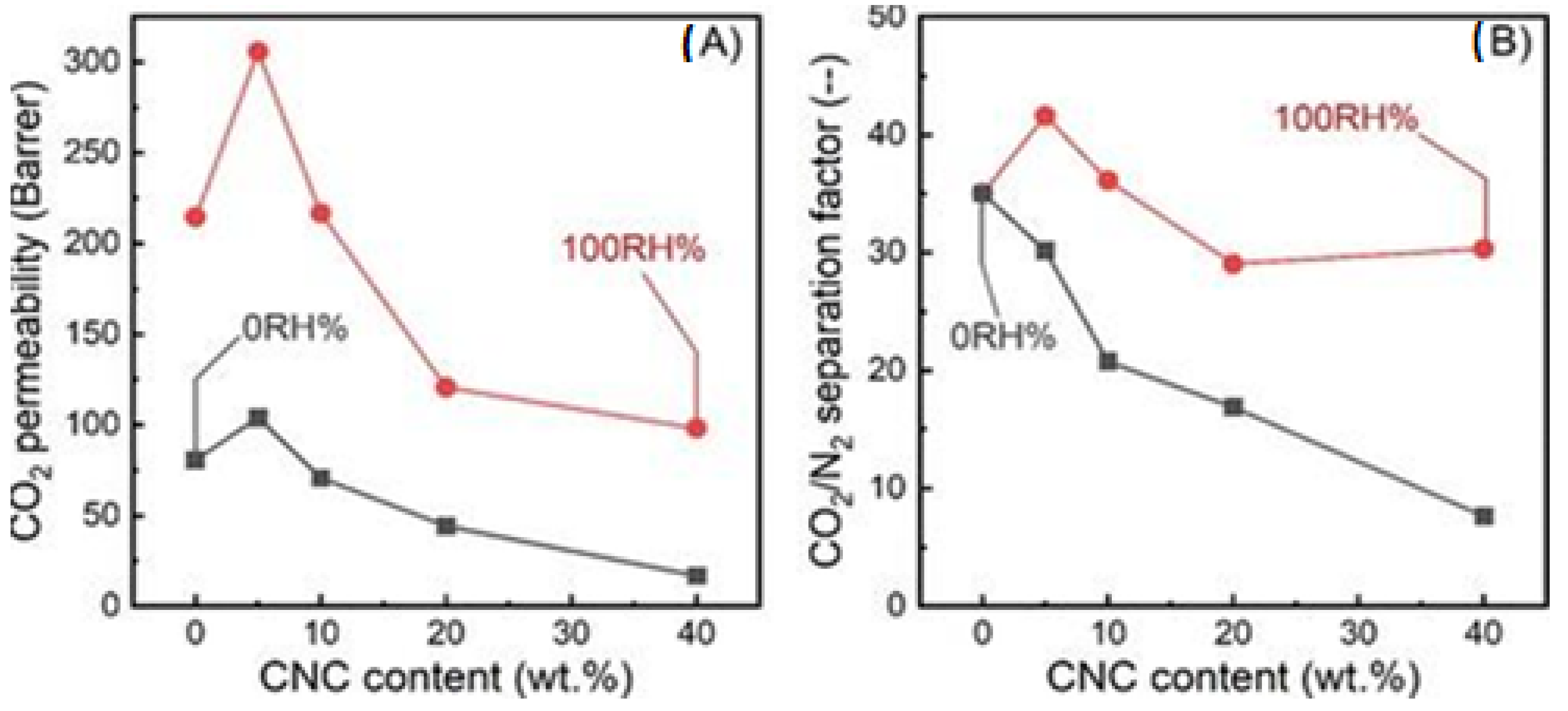
| Pebax/CNC hybrid membranes | Under dry conditions, 5 wt% CNC loading resulted in:
| [74] |
| Pebax/CNC hybrid membranes | Under humid conditions, 5 wt% CNC loading led to improvement of:
| [74] |
| 30% nano-fibrilated cellulose (NFC) into a polyvinylamine membrane | CO2 permeability of 187 Barrer, CO2/N2 selectivity of 100%, and CO2/CH4 selectivity of 22% at 80% relative humidity | [75] |
| Hybrid nanocellulose (80%)/polyvinyl alcohol (PVA) membranes | 80 wt% CNC/PVA membranes exhibited a 65% increase in CO2 permeance compared to the pure PVA membrane. (80 wt% CNF/PVA membrane had a 15% increase in CO2 permeance with respect to the pure PVA membrane.) | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitodas, S.; Skehan, M.; Liu, H.; Shah, R. Current and Potential Applications of Green Membranes with Nanocellulose. Membranes 2023, 13, 694. https://doi.org/10.3390/membranes13080694
Nitodas S, Skehan M, Liu H, Shah R. Current and Potential Applications of Green Membranes with Nanocellulose. Membranes. 2023; 13(8):694. https://doi.org/10.3390/membranes13080694
Chicago/Turabian StyleNitodas, Stefanos (Steve), Meredith Skehan, Henry Liu, and Raj Shah. 2023. "Current and Potential Applications of Green Membranes with Nanocellulose" Membranes 13, no. 8: 694. https://doi.org/10.3390/membranes13080694






