Precise Filtration of Chronic Myeloid Leukemia Cells by an Ultrathin Microporous Membrane with Backflushing to Minimize Fouling
Abstract
:1. Introduction
2. Method
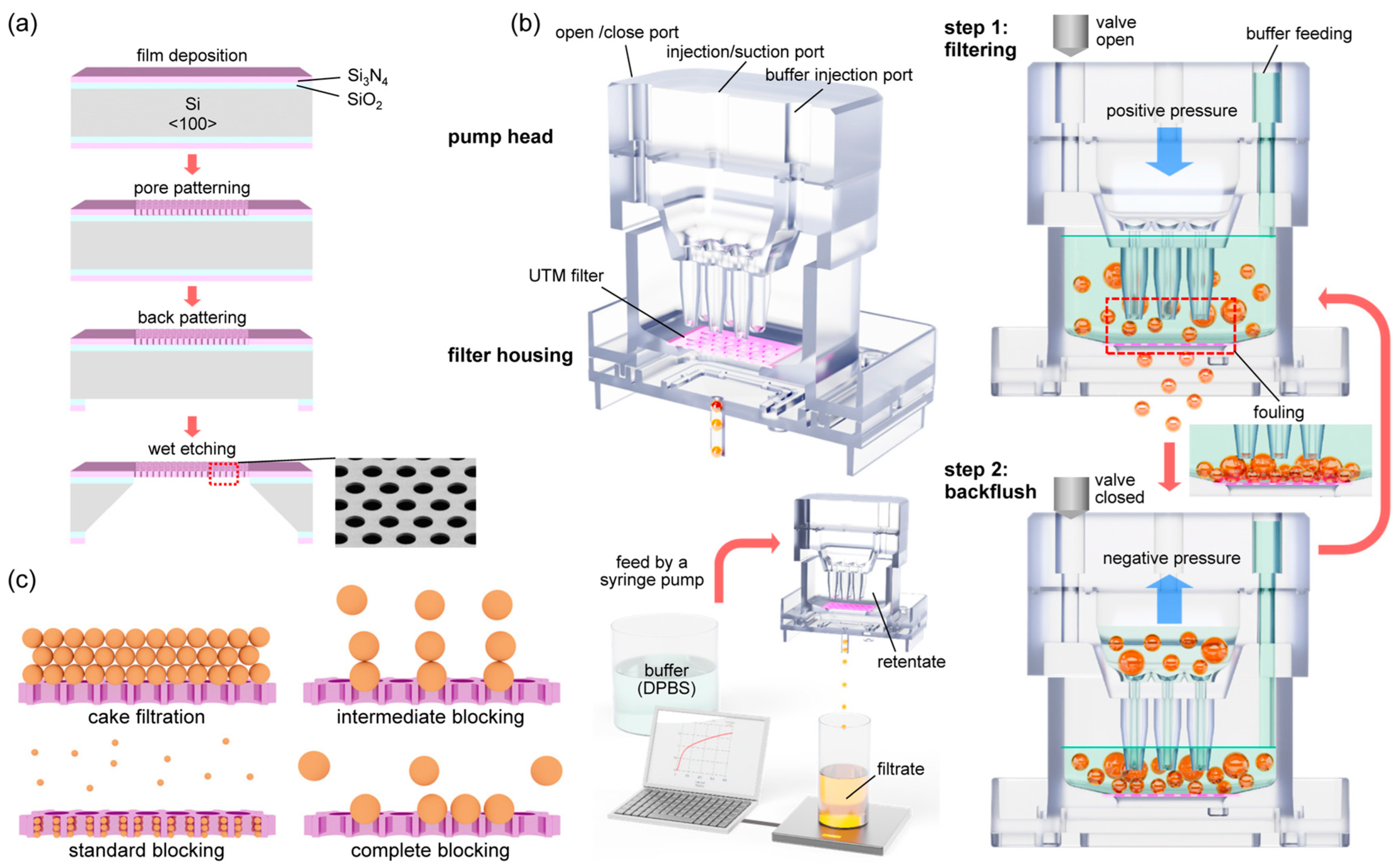
2.1. Fabrication Processes of Ultra-Thin Porous Membrane (UTM) Filter
2.2. Operation of the Filtering System with Backflush
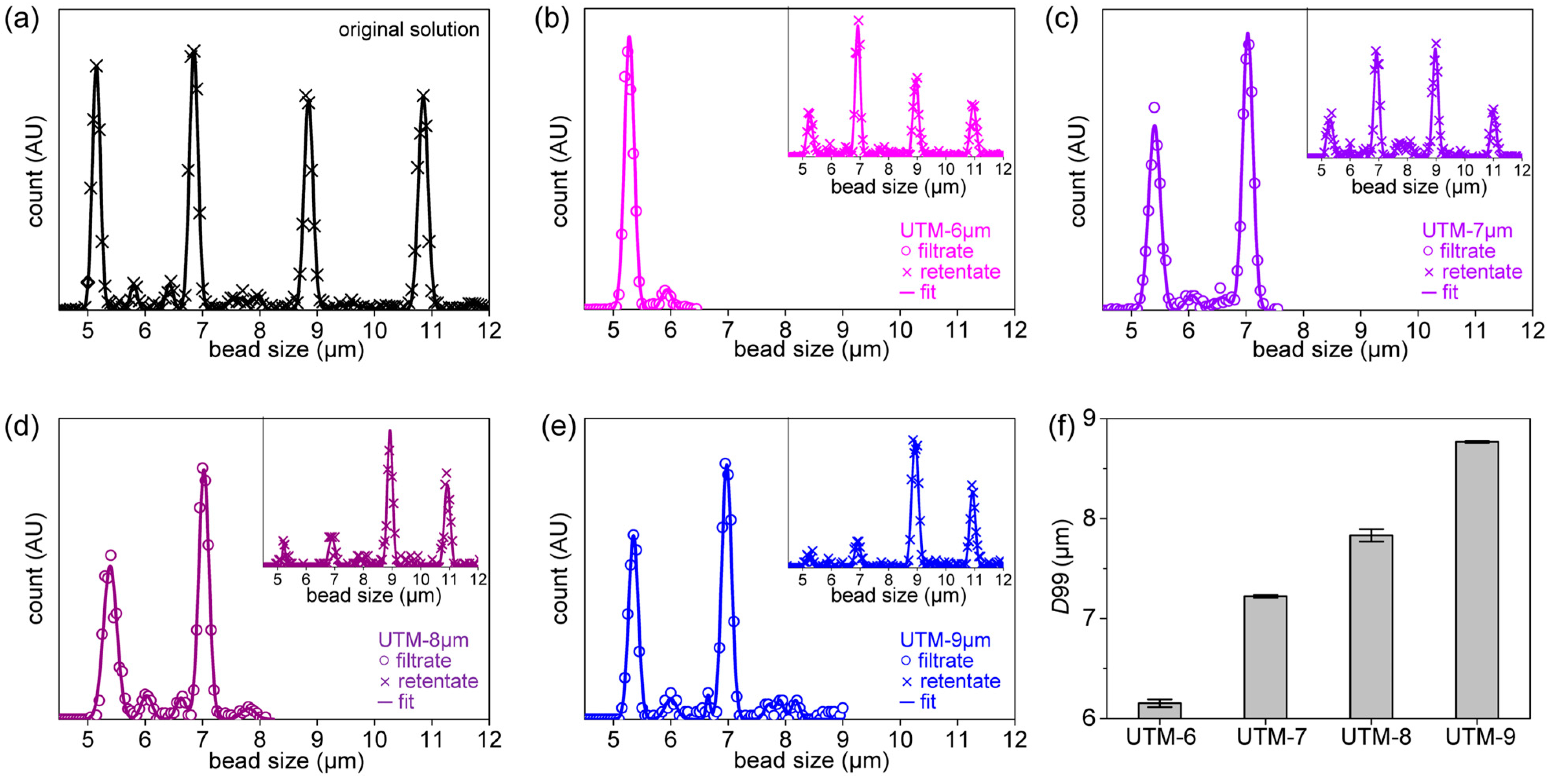
2.3. Filtering Rate Measurements and Fitting to the Blocking Laws
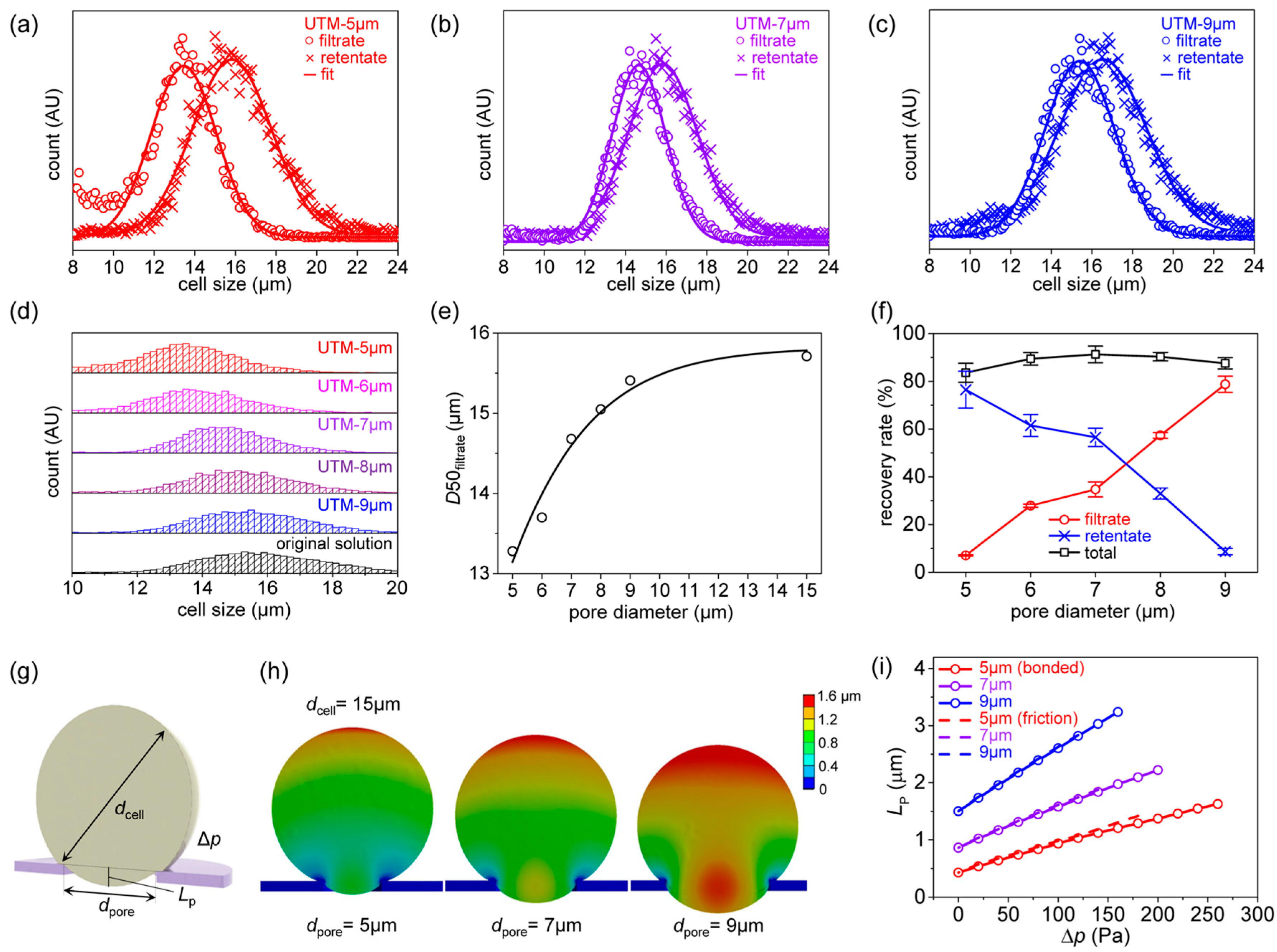
2.4. Filtration Processes of Polystyrene (PS) Beads and CML (K562) Cells
2.5. Determination of Size Distribution and the Recovery Rate
2.6. Observation of Structural Characteristics of UTM Filters
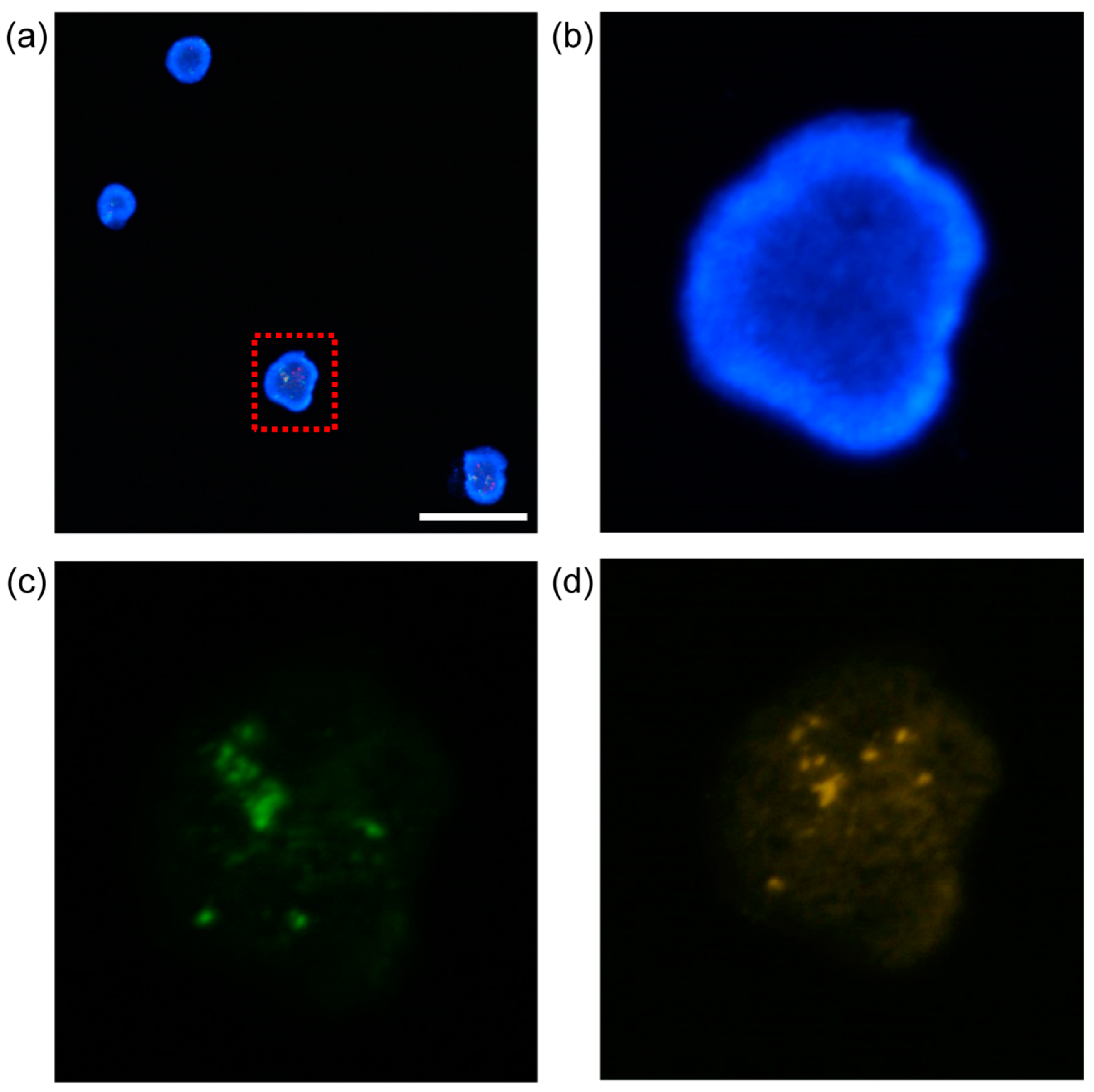
2.7. Procedure of Fluorescence Microscopy Observation of K562 Cells
2.8. Finite Element Analysis (FEA)
3. Theory
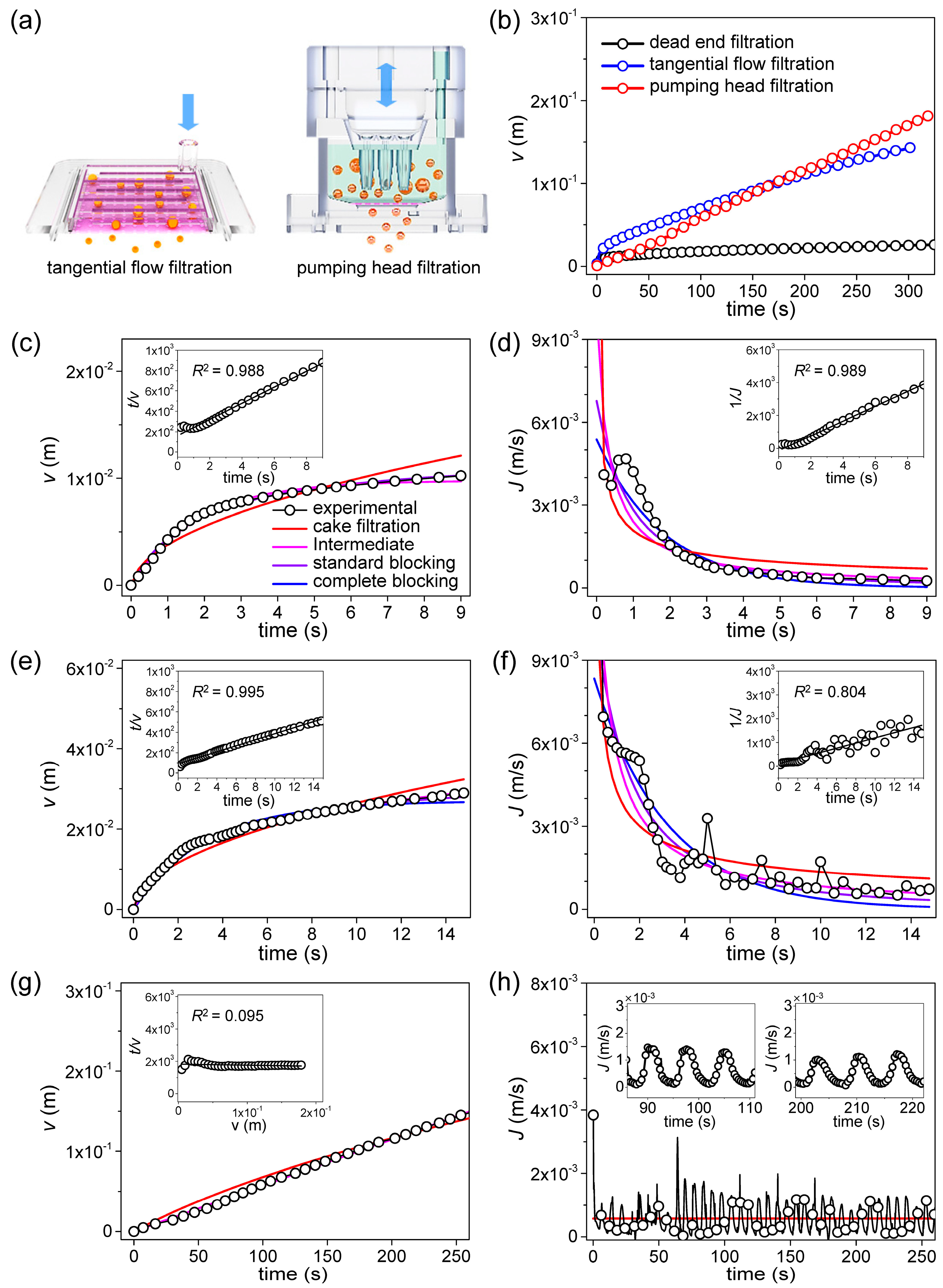
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Horade, M.; Tsai, C.H.D.; Ito, H.; Kaneko, M. Red Blood Cell Responses during a Long-Standing Load in a Microfluidic Constriction. Micromachines 2017, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.L.; Chung, J.; Lee, K.; Balaj, L.; Min, C.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Lee, H.; Weissleder, R. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 2015, 6, 6999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rho, J.; Chung, J.; Im, H.; Liong, M.; Shao, H.L.; Castro, C.M.; Weissleder, R.; Lee, H. Magnetic Nanosensor for Detection and Profiling of Erythrocyte-Derived Microvesicles. Acs Nano 2013, 7, 11227–11233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, W.; Kim, H.; Bae, P.K.; Park, J.; Kim, J. Oscillatory flow-assisted efficient target enrichment with small volumes of sample by using a particle-based microarray device. Biosens. Bioelectron. 2019, 131, 280–286. [Google Scholar] [CrossRef]
- Kim, M.S.; Sim, T.S.; Kim, Y.J.; Kim, S.S.; Jeong, H.; Park, J.M.; Moon, H.S.; Kim, S.I.; Gurel, O.; Lee, S.S.; et al. SSA-MOA: A novel CTC isolation platform using selective size amplification (SSA) and a multi-obstacle architecture (MOA) filter. Lab. Chip. 2012, 12, 5282. [Google Scholar]
- Stott, S.L.; Hsu, C.H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef]
- Murlidhar, V.; Zeinali, M.; Grabauskiene, S.; Ghannad-Rezaie, M.; Wicha, M.S.; Simeone, D.M.; Ramnath, N.; Reddy, R.M.; Nagrath, S. A Radial Flow Microfluidic Device for Ultra-High-Throughput Affinity-Based Isolation of Circulating Tumor Cells. Small 2014, 10, 4895–4904. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Luo, B.; Jiang, P.P.; Zhou, X.X.; Lan, F.; Yi, Q.Y.; Wu, Y. Immuno-modified superparamagnetic nanoparticles via host-guest interactions for high-purity capture and mild release of exosomes. Nanoscale 2018, 10, 14280–14289. [Google Scholar] [CrossRef]
- Podenphant, M.; Ashley, N.; Koprowska, K.; Mir, K.U.; Zalkovskij, M.; Bilenberg, B.; Bodmer, W.; Kristensen, A.; Marie, R. Separation of cancer cells from white blood cells by pinched flow fractionation. Lab. Chip. 2015, 15, 4598–4606. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Han, D.; Park, M.C.; Mun, J.; Choi, J.; Chun, H.; Kim, S.; Hong, J.W. Separation of extracellular nanovesicles and apoptotic bodies from cancer cell culture broth using tunable microfluidic systems. Sci. Rep. 2017, 7, 9907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Shao, H.L.; Weissleder, R.; Lee, H. Acoustic Purification of Extracellular Microvesicles. Acs Nano 2015, 9, 2321–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Mao, Z.M.; Peng, Z.L.; Zhou, L.L.; Chen, Y.C.; Huang, P.H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.G.; Kong, M.Q.; Zhou, S.; Sheng, Y.F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.J.; Demirci, U.; et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017, 7, 46224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.K.; Sunkara, V.; Park, J.; Kim, T.H.; Han, J.R.; Kim, C.J.; Choi, H.I.; Kim, Y.K.; Cho, Y.K. Exodisc for Rapid, Size-Selective, and Efficient Isolation and Analysis of Nanoscale Extracellular Vesicles from Biological Samples. ACS Nano 2017, 11, 1360–1370. [Google Scholar] [CrossRef]
- Phuong, N.T.; Andisetiawan, A.; Lam, D.V.; Kim, J.H.; Choi, D.S.; Whang, K.H.; Nham, J.; Lee, Y.J.; Yoo, Y.E.; Yoon, J.S. Nano sand filter with functionalized nanoparticles embedded in anodic aluminum oxide templates. Sci. Rep. 2016, 6, 37673. [Google Scholar] [CrossRef] [Green Version]
- Mehendale, N.; Sharma, O.; Pandey, S.; Paul, D. Clogging-free continuous operation with whole blood in a radial pillar device (RAPID). Biomed. Microdevices 2018, 20, 75. [Google Scholar] [CrossRef]
- Wunsch, B.H.; Smith, J.T.; Gifford, S.M.; Wang, C.; Brink, M.; Bruce, R.L.; Austin, R.H.; Stolovitzky, G.; Astier, Y. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol. 2016, 11, 936–940. [Google Scholar] [CrossRef]
- Choi, J.C.; Hyun, J.C.; Yang, S. On-chip Extraction of Intracellular Molecules in White Blood Cells from Whole Blood. Sci. Rep. 2015, 5, 15167. [Google Scholar] [CrossRef] [Green Version]
- Sabirova, A.; Pisig, F.; Rayapuram, N.; Hirt, H.; Nunes, S.P. Nanofabrication of Isoporous Membranes for Cell Fractionation. Sci. Rep. 2020, 10, 6138. [Google Scholar] [CrossRef] [Green Version]
- Mireles, M.; Soule, C.W.; Dehghani, M.; Gaborski, T.R. Use of nanosphere self-assembly to pattern nanoporous membranes for the study of extracellular vesicles. Nanoscale Adv. 2020, 2, 4427–4436. [Google Scholar] [CrossRef]
- Mazzocchi, A.R.; Man, A.J.; DesOrmeaux, J.P.S.; Gaborski, T.R. Porous Membranes Promote Endothelial Differentiation of Adipose-Derived Stem Cells and Perivascular Interactions. Cell. Mol. Bioeng. 2014, 7, 369–378. [Google Scholar] [CrossRef]
- Ohanessian, K.; Monnot, M.; Moulin, P.; Ferrasse, J.H.; Barca, C.; Soric, A.; Boutin, O. Dead-end and crossflow ultrafiltration process modelling: Application on chemical mechanical polishing wastewaters (vol 158, pg 164, 2020). Chem. Eng. Res. Des. 2020, 162, 38. [Google Scholar] [CrossRef]
- Enten, A.C.; Leipner, M.P.I.; Bellavia, M.C.; King, L.E.; Sulchek, T.A. Optimizing Flux Capacity of Dead-end Filtration Membranes by Controlling Flow with Pulse Width Modulated Periodic Backflush. Sci. Rep. 2020, 10, 896. [Google Scholar] [CrossRef] [Green Version]
- Karabacak, N.M.; Spuhler, P.S.; Fachin, F.; Lim, E.J.; Pai, V.; Ozkumur, E.; Martel, J.M.; Kojic, N.; Smith, K.; Chen, P.I.; et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014, 9, 694–710. [Google Scholar] [CrossRef] [Green Version]
- Hui, T.H.; Zhou, Z.L.; Qian, J.; Lin, Y.; Ngan, A.H.W.; Gao, H. Volumetric Deformation of Live Cells Induced by Pressure-Activated Cross-Membrane Ion Transport. Phys. Rev. Lett. 2014, 113, 118101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iritani, E.; Katagiri, N. Developments of Blocking Filtration Model in Membrane Filtration. Kona Powder Part J. 2016, 33, 179–202. [Google Scholar] [CrossRef] [Green Version]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Almandoz, C.; Pagliero, C.; Ochoa, A.; Marchese, J. Corn syrup clarification by microfiltration with ceramic membranes. J. Membrane Sci. 2010, 363, 87–95. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.Q.; Xi, N.; Wang, Y.C.; Dong, Z.L.; Xiao, X.B.; Zhang, W.J. Atomic force microscopy imaging and mechanical properties measurement of red blood cells and aggressive cancer cells. Sci. China Life Sci. 2012, 55, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.H.; Crawford, K.; Turbyfield, C.; Lam, W.; Alexeev, A.; Sulchek, T. Microfluidic cellular enrichment and separation through differences in viscoelastic deformation. Lab. Chip. 2015, 15, 532–540. [Google Scholar] [CrossRef]
- Li, Y.; Deng, J.X.; Zhou, J.; Li, X.E. Elastic and viscoelastic mechanical properties of brain tissues on the implanting trajectory of sub-thalamic nucleus stimulation. J. Mater. Sci.-Mater. Med. 2016, 27, 163. [Google Scholar] [CrossRef]
- Available online: https://www.memsnet.org/material/siliconnitridesi3n4film/ (accessed on 5 April 2023).
- Sun, X.H.; Wang, K.; Wu, H.A.; Chen, J.; Long, R. Finite element simulation of a viscoelastic cell entering a cylindrical channel: Effects of frictional contact. Mech. Mater. 2022, 167, 104263. [Google Scholar] [CrossRef]
- Ruth, B.F. Studies in Filtration III. Derivation of General Filtration Equations. Ind. Eng. Chem. 1935, 27, 708–723. [Google Scholar] [CrossRef]
- Ruth, B.F. Correlating Filtration Theory with Industrial Practice. Ind. Eng. Chem. 1946, 38, 564–571. [Google Scholar] [CrossRef]
- Hermans, P.H.; Bredée, H.L. Principles of the mathematical treatment of constant-pressure filtration. J. Soc. Chem. Ind. 1936, 55, 1–4. [Google Scholar]
- Grace, H.P. Resistance and Compressibility of Filter Cake. Chemical Engineering Progress. Chem. Eng. Prog. 1953, 49, 303–318. [Google Scholar]
- Grace, H.P. Structure and performance of filter media. II. Performance of filter media in liquid service. AIChE J. 1956, 2, 316–336. [Google Scholar] [CrossRef]
- Hermia, J. Constant pressure blocking filtration laws—Application to power-law non-newtonian fluids. Trans. Inst. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Mogab, C.J. The loading effect in plasma etching. J. Electrochem. Soc. 1977, 124, 1262–1268. [Google Scholar] [CrossRef]
- Wolfrum, B.; Mourzina, Y.; Sommerhage, F.; Offenhausser, A. Suspended nanoporous membranes as interfaces for neuronal biohybrid systems. Nano Lett. 2006, 6, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiss, O.; Bianchi, I.; Senaldi, C.; Bucher, G.; Verleysen, E.; Waegeneers, N.; Brassinne, F.; Mast, J.; Loeschner, K.; Vidmar, J.; et al. Particle size analysis of pristine food-grade titanium dioxide and E 171 in confectionery products: Interlaboratory testing of a single-particle inductively coupled plasma mass spectrometry screening method and confirmation with transmission electron microscopy. Food Control 2021, 120, 107550. [Google Scholar] [CrossRef] [PubMed]
- Amosa, M.K. Towards sustainable membrane filtration of palm oil mill effluent: Analysis of fouling phenomena from a hybrid PAC-UF process. Appl. Water Sci. 2017, 7, 3365–3375. [Google Scholar]
- Hwang, K.J.; Hsu, Y.L.; Tung, K.L. Effect of particle size on the performance of cross-flow microfiltration. Adv. Powder Technol. 2006, 17, 189–206. [Google Scholar] [CrossRef]
- Druker, B.J.; Tamura, S.; Buchdunger, E.; Ohno, S.; Segal, G.M.; Fanning, S.; Zimmermann, J.; Lydon, N.B. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996, 2, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet Classification with Deep Convolutional Neural Networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Lecun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- ABAQUS Analysis User’s Manual, Version 6.6; ABAQUS Inc.: Palo Alto, CA, USA, 2006.
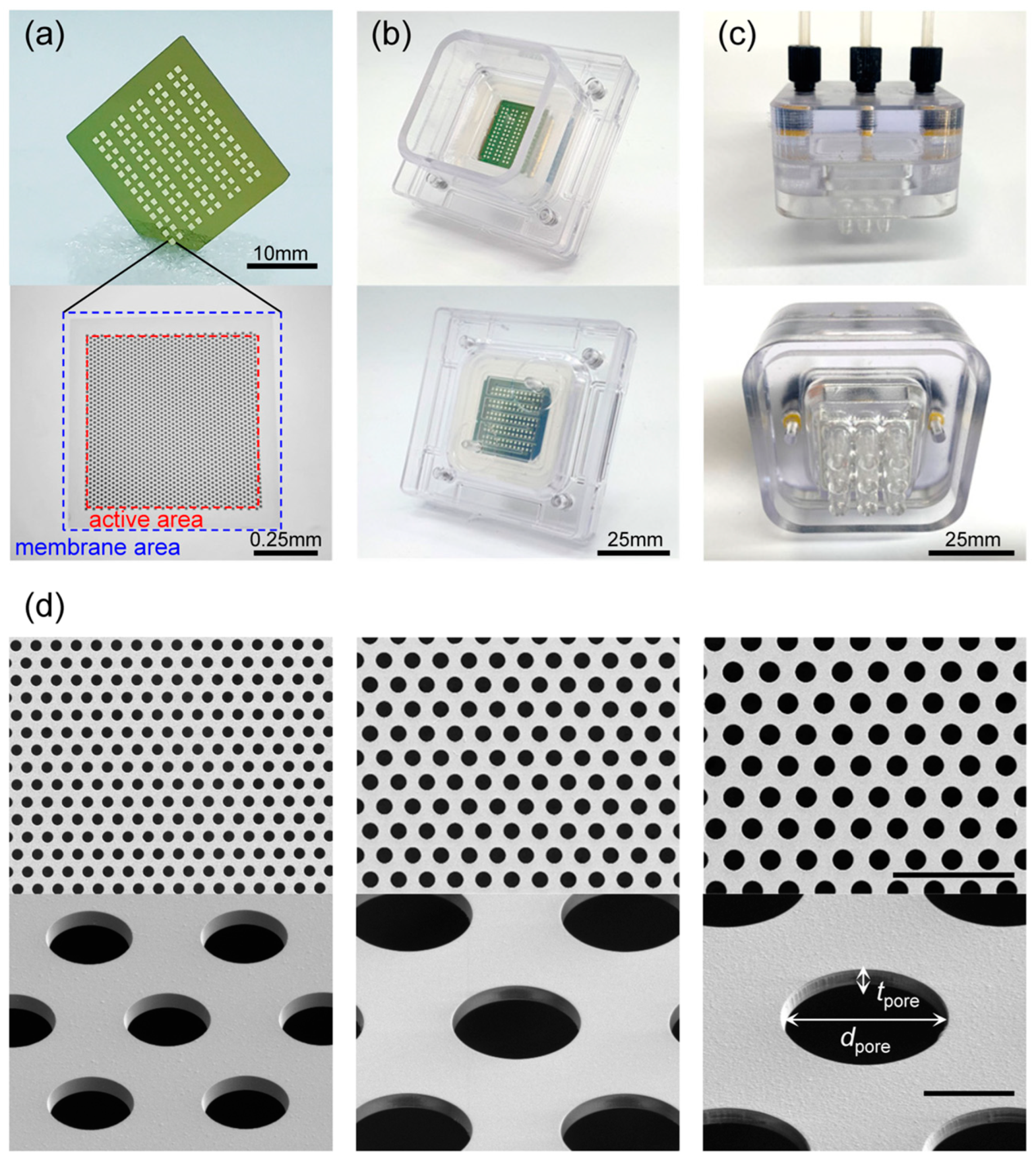
| Designed Pore Dia. (μm) | Actual Pore Dia. (μm) | Designed Porosity (%) | Actual Porosity (%) | Active Area (mm2) | Thickness (μm) | |
|---|---|---|---|---|---|---|
| UTM-5 | 5 | 4.82 ± 0.13 | 32.6 | 30.3 | 54.9 | 0.89 |
| UTM-6 | 6 | 5.87 ± 0.01 | 32.5 | 31.1 | 63.7 | 0.89 |
| UTM-7 | 7 | 6.83 ± 0.06 | 32.5 | 31.0 | 63.7 | 0.89 |
| UTM-8 | 8 | 7.60 ± 0.04 | 32.6 | 29.6 | 63.7 | 0.89 |
| UTM-9 | 9 | 9.00 ± 0.06 | 32.6 | 32.6 | 63.7 | 0.89 |
| Filtrate | K562 | UTM-5 | UTM-6 | UTM-7 | UTM-8 | UTM-9 | UTM-15 |
|---|---|---|---|---|---|---|---|
| D10 | 13.4 | 10.0 | 11.0 | 13.0 | 12.7 | 13.3 | 13.6 |
| D50 | 15.7 | 13.3 | 13.7 | 14.7 | 15.1 | 15.4 | 15.7 |
| D90 | 18.6 | 15.4 | 15.9 | 16.6 | 17.4 | 17.8 | 18.4 |
| D95 | 19.4 | 16.1 | 16.7 | 17.4 | 18.1 | 18.5 | 19.3 |
| D99 | 22.2 | 17.5 | 18.2 | 18.7 | 19.7 | 20.4 | 22.4 |
| K562 | UTM-5 | UTM-6 | UTM-7 | UTM-8 | UTM-9 | |
|---|---|---|---|---|---|---|
| D10 | 13.4 | 13.5 | 13.7 | 13.8 | 13.4 | 13.6 |
| D50 | 15.7 | 15.8 | 15.9 | 15.8 | 16.0 | 16.6 |
| D90 | 18.6 | 18.9 | 18.8 | 18.5 | 18.9 | 20.0 |
| D95 | 19.4 | 19.7 | 19.8 | 19.5 | 20.0 | 21.6 |
| D99 | 22.2 | 22.4 | 22.8 | 23.2 | 23.6 | 25.5 |
| Filtration Method | Blocking Model | Fitting Result | |||
|---|---|---|---|---|---|
| Jo (m/s) | Blocking Constant | Fitting Method 1: RMSE | Fitting Method 2: R2 | ||
| Dead end filtration | cake filtration | 2.46 × 10−2 | 3.24 × 105 (m−2s) | 2.50 × 10−3 | 0.658 |
| intermediate | 1.26 × 10−2 | 3.67 × 102 (m−1) | 3.56 × 10−4 | 0.989 | |
| standard | 4.82 × 10−3 | 1.35 × 102 (m−1) | 7.12 × 10−4 | 0.988 | |
| complete | 3.26 × 10−3 | 2.45 × 10−1 (s−1) | 1.25 × 10−3 | 0.873 | |
| Tangential flow filtration | cake filtration | 9.62 × 10−2 | 3.89 × 104 (m−2s) | 2.68 × 10−3 | 0.893 |
| intermediate | 1.06 × 10−2 | 8.91 × 101 (m−1) | 1.16 × 10−3 | 0.804 | |
| standard | 6.09 × 10−3 | 4.17 × 101 (m−1) | 2.12 × 10−3 | 0.995 | |
| complete | 4.32 × 10−3 | 1.08 × 10−1 (s−1) | 3.06 × 10−3 | 0.736 | |
| Pumping head filtration | cake filtration | 5.28 × 10−4 | 1.09 × 10−6 (m−2s) | 1.24 × 10−3 | 0.095 |
| intermediate | 5.28 × 10−4 | 1.07 × 10−10 (m−1) | 1.24 × 10−3 | 0.011 | |
| standard | 5.74 × 10−4 | 2.00 × 10−14 (m−1) | 1.24 × 10−3 | 0.079 | |
| complete | 5.74 × 10−4 | 1.41 × 10−12 (s−1) | 1.24 × 10−3 | 0.028 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Hong, J.; Lee, J.; Lee, C.; Kim, T.; Jeong, Y.; Kim, K.; Jung, I. Precise Filtration of Chronic Myeloid Leukemia Cells by an Ultrathin Microporous Membrane with Backflushing to Minimize Fouling. Membranes 2023, 13, 707. https://doi.org/10.3390/membranes13080707
Lee J, Hong J, Lee J, Lee C, Kim T, Jeong Y, Kim K, Jung I. Precise Filtration of Chronic Myeloid Leukemia Cells by an Ultrathin Microporous Membrane with Backflushing to Minimize Fouling. Membranes. 2023; 13(8):707. https://doi.org/10.3390/membranes13080707
Chicago/Turabian StyleLee, Jaehyuk, Jeongpyo Hong, Jungwon Lee, Changgyu Lee, Tony Kim, Young Jeong, Kwanghee Kim, and Inhwa Jung. 2023. "Precise Filtration of Chronic Myeloid Leukemia Cells by an Ultrathin Microporous Membrane with Backflushing to Minimize Fouling" Membranes 13, no. 8: 707. https://doi.org/10.3390/membranes13080707
APA StyleLee, J., Hong, J., Lee, J., Lee, C., Kim, T., Jeong, Y., Kim, K., & Jung, I. (2023). Precise Filtration of Chronic Myeloid Leukemia Cells by an Ultrathin Microporous Membrane with Backflushing to Minimize Fouling. Membranes, 13(8), 707. https://doi.org/10.3390/membranes13080707






