Influence of Solid Retention Time on Membrane Fouling and Biogas Recovery in Anerobic Membrane Bioreactor Treating Sugarcane Industry Wastewater in Sahelian Climate
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Setup
2.2. Sampling and Analytical Methods
2.3. Operating Conditions
- VSS: concentration of volatile suspended solids;
- SS: concentration of suspended solids;
- CODin: COD influent;
- CODeff: COD effluent;
- : ion concentration in the influent;
- : ion concentration in the effluent;
- Volume CH4: methane volume per day;
- OLR: organic loading rate.
3. Results and Discussion
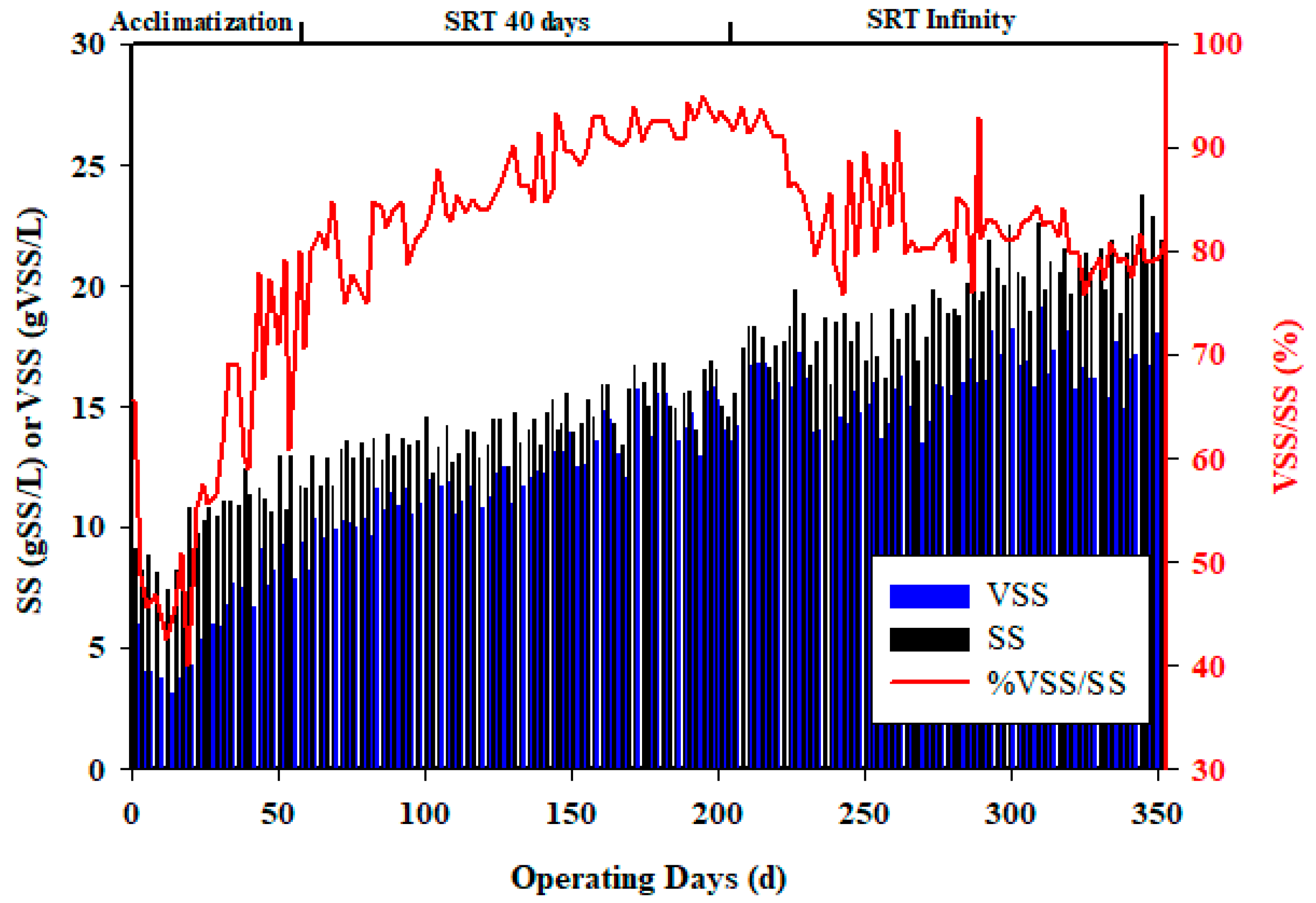
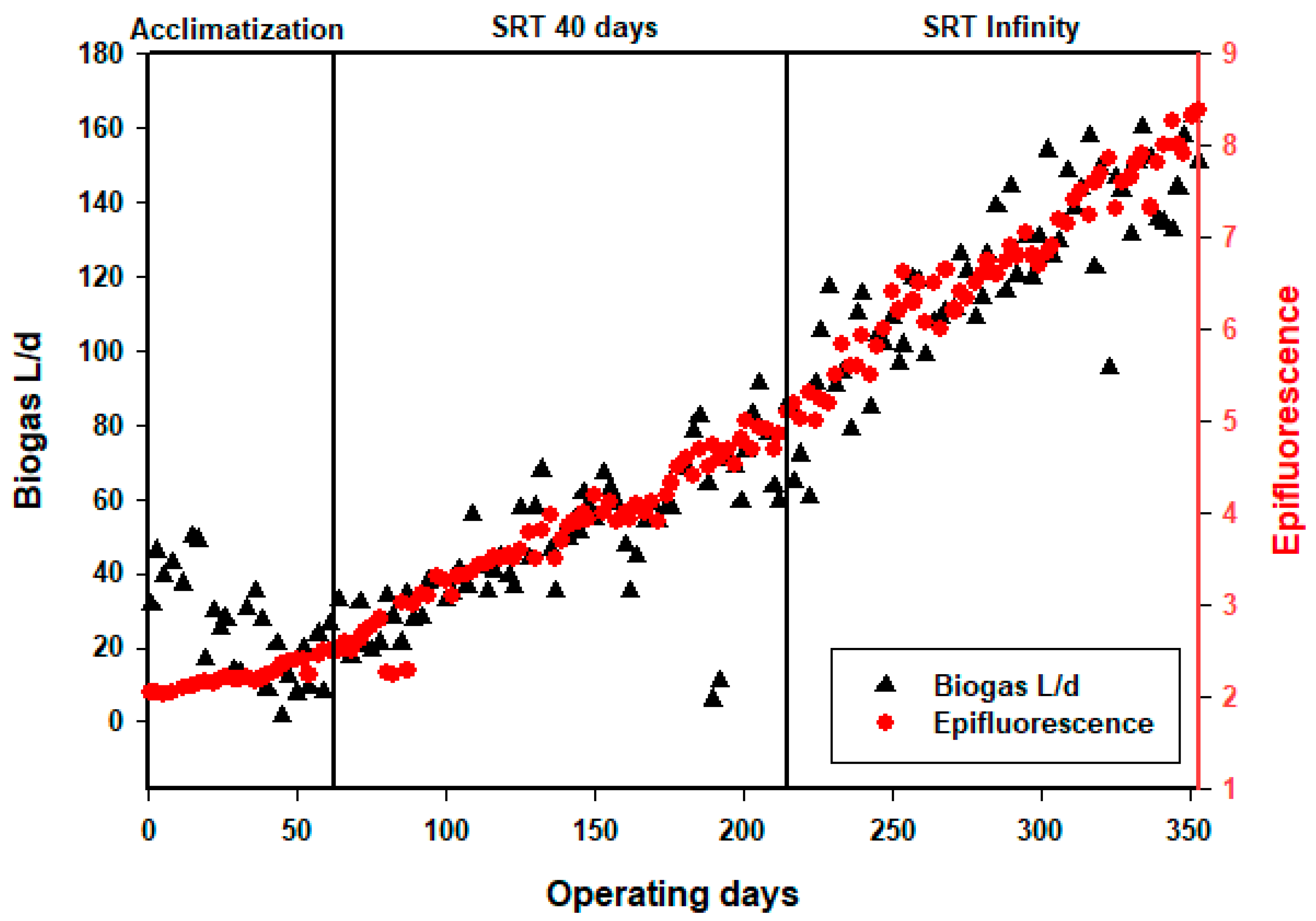
3.1. Biomass Acclimatization and Evolution
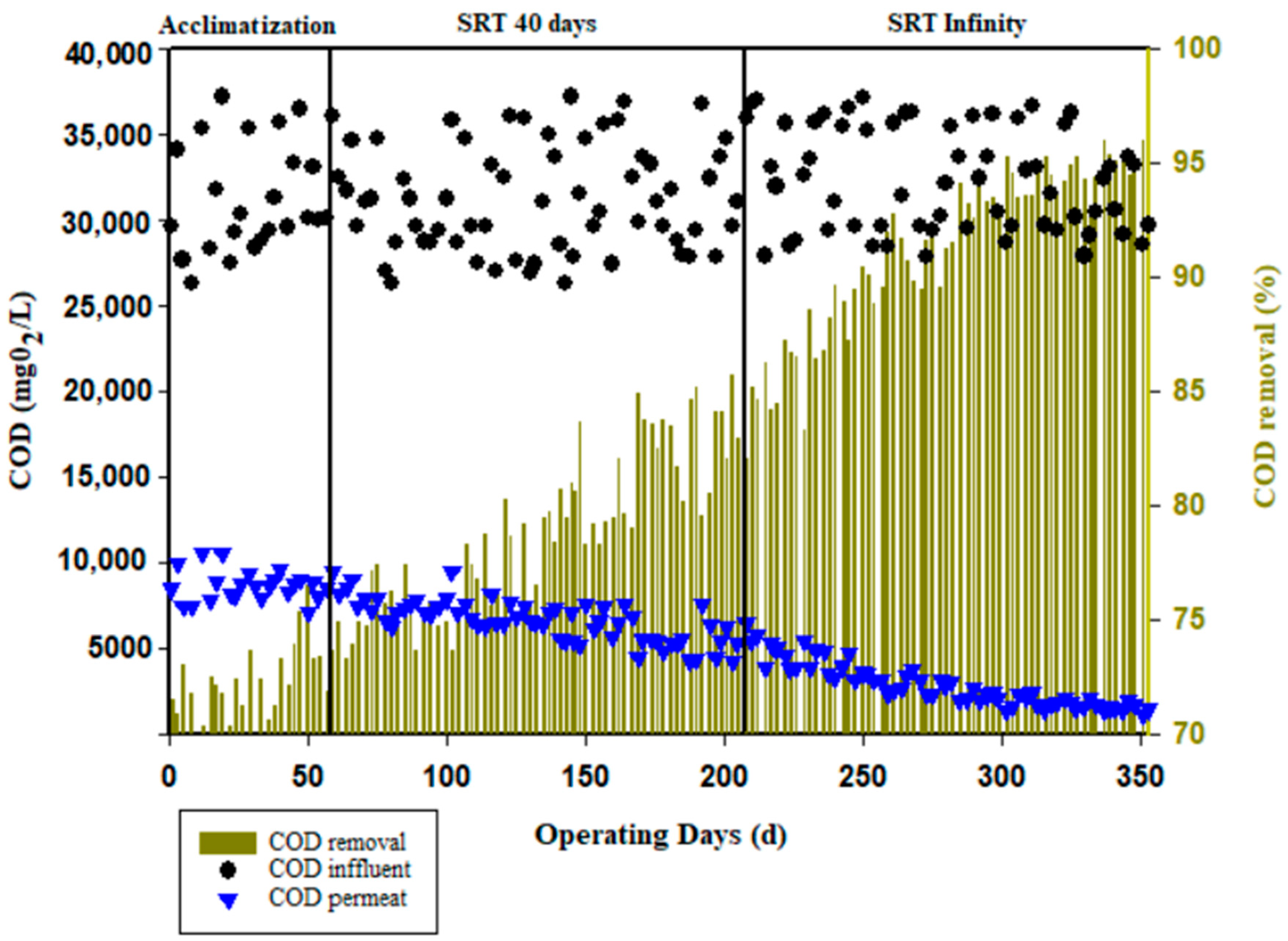
3.2. Treatment Efficiency
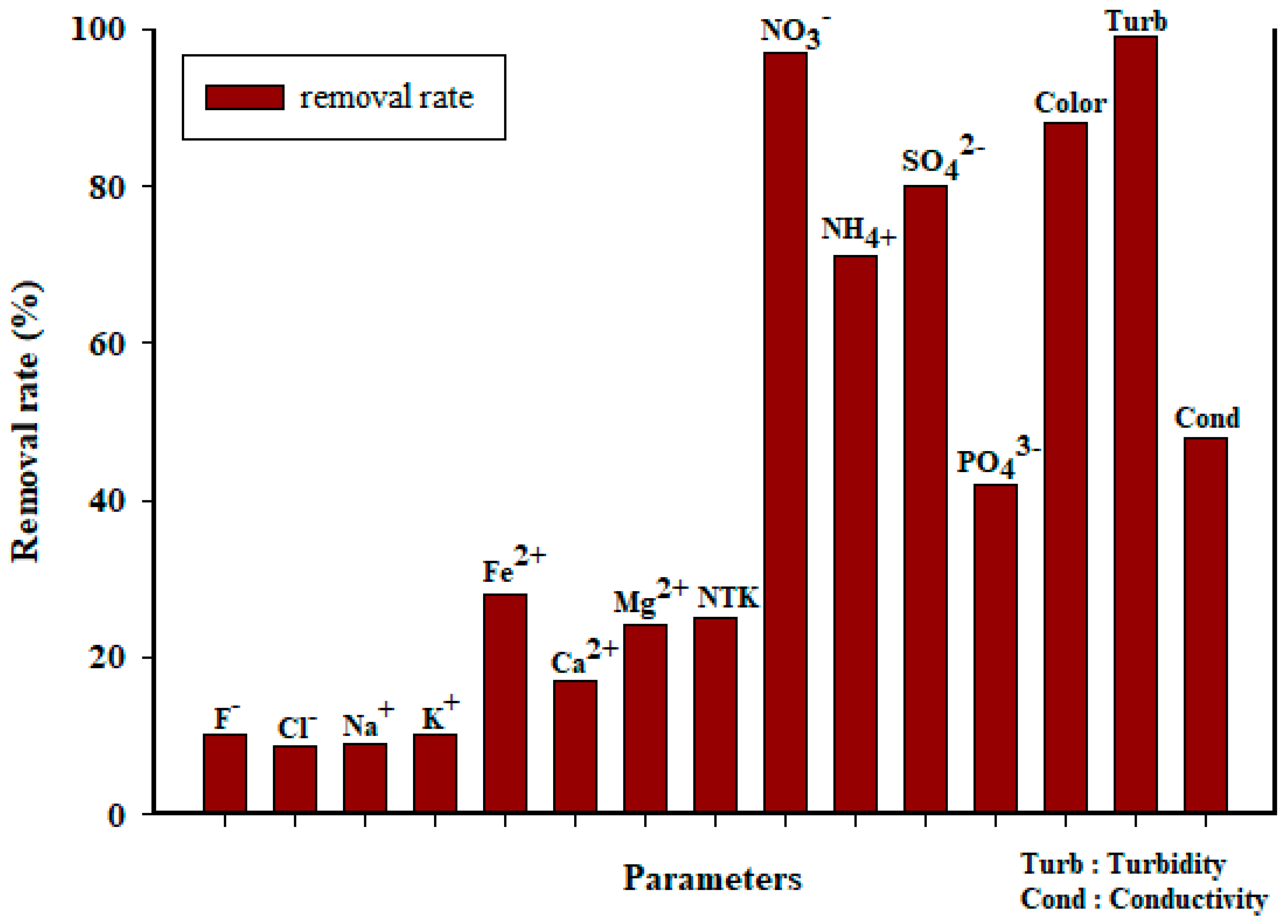
3.3. Water Quality
3.4. Biogas Production
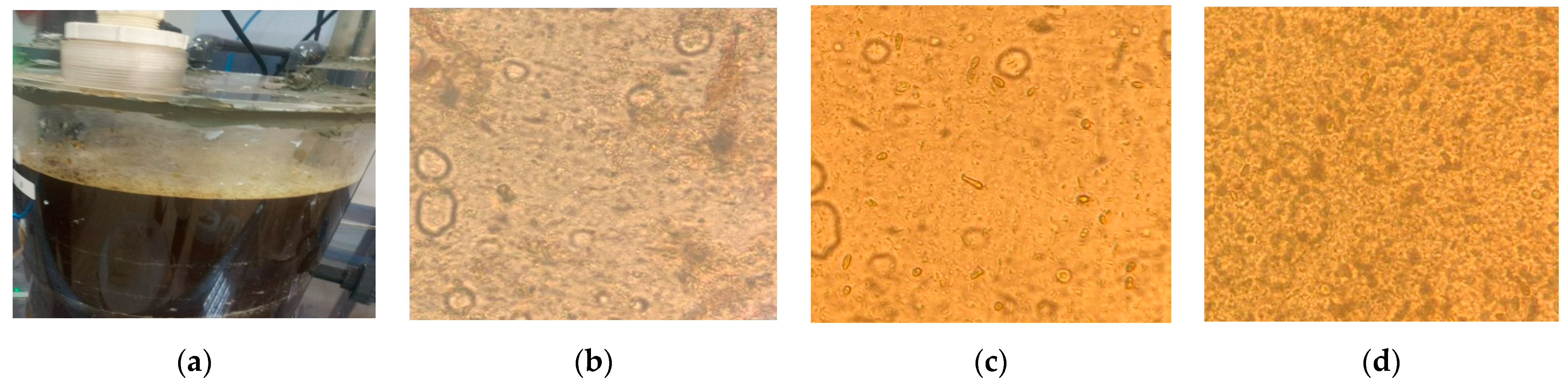
3.5. Biomass Investigation
3.6. Fouling and Membrane Filtration Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, A.; Silalertruksa, T.; Pongpat, P.; Gheewala, S.H. Efficiency Analysis of Sugarcane Production Systems in Thailand Using Data Envelopment Analysis. J. Clean. Prod. 2019, 238, 117877. [Google Scholar] [CrossRef]
- Cardoso, T.F.; Watanabe, M.D.B.; Souza, A.; Chagas, M.F.; Cavalett, O.; Morais, E.R.; Nogueira, L.A.H.; Leal, M.R.L.V.; Braunbeck, O.A.; Cortez, L.A.B.; et al. A Regional Approach to Determine Economic, Environmental and Social Impacts of Different Sugarcane Production Systems in Brazil. Biomass Bioenergy 2019, 120, 9–20. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Sadeghzadeh Hemayati, S.; Tabatabaei, M.; Aghbashlo, M.; Mahmoudi, S.B. A Review on Beet Sugar Industry with a Focus on Implementation of Waste-to-Energy Strategy for Power Supply. Renew. Sustain. Energy Rev. 2019, 103, 423–442. [Google Scholar] [CrossRef]
- Jacques, A.; Chaaya, N.; Beecher, K.; Ali, S.A.; Belmer, A.; Bartlett, S. The Impact of Sugar Consumption on Stress Driven, Emotional and Addictive Behaviors. Neurosci. Biobehav. Rev. 2019, 103, 178–199. [Google Scholar] [CrossRef]
- Alexander Bentley, R.; Ruck, D.J.; Fouts, H.N.U.S. Obesity as Delayed Effect of Excess Sugar. Econ. Hum. Biol. 2020, 36, 100818. [Google Scholar] [CrossRef]
- Kaab, A.; Sharifi, M.; Mobli, H.; Nabavi-Pelesaraei, A.; Chau, K. Use of Optimization Techniques for Energy Use Efficiency and Environmental Life Cycle Assessment Modification in Sugarcane Production. Energy 2019, 181, 1298–1320. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuang, X.; Ahmad, S.; Sung, S.; Ni, S.-Q. Biotreatment of High-Salinity Wastewater: Current Methods and Future Directions. World J. Microbiol. Biotechnol. 2020, 36, 37. [Google Scholar] [CrossRef]
- He, Q.; Song, J.; Zhang, W.; Gao, S.; Wang, H.; Yu, J. Enhanced Simultaneous Nitrification, Denitrification and Phosphorus Removal through Mixed Carbon Source by Aerobic Granular Sludge. J. Hazard. Mater. 2020, 382, 121043. [Google Scholar] [CrossRef]
- Show, K.-Y.; Yan, Y.; Yao, H.; Guo, H.; Li, T.; Show, D.-Y.; Chang, J.-S.; Lee, D.-J. Anaerobic Granulation: A Review of Granulation Hypotheses, Bioreactor Designs and Emerging Green Applications. Bioresour. Technol. 2020, 300, 122751. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Magalhães, N.C.; Assis, I.R.; Lange, L.C.; Amaral, M.C.S. Integrated Membrane-Based Processes to Obtain Organo-Mineral Fertilizer, Water, and Energy from Sugarcane Vinasse. Sep. Purif. Technol. 2022, 302, 122180. [Google Scholar] [CrossRef]
- Moreira, V.R.; Carpanez, T.G.; dos Santos, F.S.; Santos, L.S.; dos Santos, F.S.; França-Neta, L.S.; Lange, L.C.; Amaral, M.C.S. Circular Economy in Biorefineries: Scale-up of Anaerobic/Aerobic Membrane Bioreactors for Vinasse Recycling. J. Clean. Prod. 2022, 377, 134448. [Google Scholar] [CrossRef]
- Dhiman, S.; Balakrishnan, M.; Naddeo, V.; Ahsan, N. Anaerobic Membrane Bioreactor (AnMBR) Equipped with Waste-Based Ceramic Membranes: Relation with Life Cycle Cost (LCC). In Water-Energy-Nexus in the Ecological Transition: Natural-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability; Naddeo, V., Choo, K.-H., Ksibi, M., Eds.; Advances in Science Technology & Innovation; Springer International Publishing: Cham, Switzerland, 2022; pp. 245–247. ISBN 978-3-031-00808-5. [Google Scholar]
- Wang, K.M.; Martin Garcia, N.; Soares, A.; Jefferson, B.; McAdam, E.J. Comparison of Fouling between Aerobic and Anaerobic MBR Treating Municipal Wastewater. H2Open J. 2018, 1, 131–159. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, J.; Rong, C.; Wang, T.; Li, L.; Luo, Z.; Ji, J.; Hanaoka, T.; Sakemi, S.; Ito, M.; et al. Large Pilot-Scale Submerged Anaerobic Membrane Bioreactor for the Treatment of Municipal Wastewater and Biogas Production at 25 °C. Bioresour. Technol. 2021, 319, 124123. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Stadler, L.B.; Cao, L.; Love, N.G.; Raskin, L.; Skerlos, S.J. Navigating Wastewater Energy Recovery Strategies: A Life Cycle Comparison of Anaerobic Membrane Bioreactor and Conventional Treatment Systems with Anaerobic Digestion. Environ. Sci. Technol. 2014, 48, 5972–5981. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yang, S.; Li, Y.; Wen, W.; Wang, X.C.; Chen, R. Application of Anaerobic Membrane Bioreactors to Municipal Wastewater Treatment at Ambient Temperature: A Review of Achievements, Challenges, and Perspectives. Bioresour. Technol. 2018, 267, 756–768. [Google Scholar] [CrossRef]
- McKeown, R.M.; Hughes, D.; Collins, G.; Mahony, T.; O’Flaherty, V. Low-Temperature Anaerobic Digestion for Wastewater Treatment. Curr. Opin. Biotechnol. 2012, 23, 444–451. [Google Scholar] [CrossRef]
- Kiani Deh Kiani, M.; Parsaee, M.; Safieddin Ardebili, S.M.; Reyes, I.P.; Fuess, L.T.; Karimi, K. Different Bioreactor Configurations for Biogas Production from Sugarcane Vinasse: A Comprehensive Review. Biomass Bioenergy 2022, 161, 106446. [Google Scholar] [CrossRef]
- de Oliveira Lima, V.; de Barros, V.G.; Duda, R.M.; de Oliveira, R.A. Anaerobic Digestion of Vinasse and Water Treatment Plant Sludge Increases Methane Production and Stability of UASB Reactors. J. Environ. Manag. 2022, 327, 116451. [Google Scholar] [CrossRef]
- Ohimain, E.I.; Izah, S.C. A Review of Biogas Production from Palm Oil Mill Effluents Using Different Configurations of Bioreactors. Renew. Sustain. Energy Rev. 2017, 70, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Sawadogo, B.; Yacouba, K.; Wahab, A.; Lesage, G.; Zaviska, F.; Heran, M.; Karambiri, H. Anaerobic Membrane Bioreactor Coupled with Nanofiltration Applied to the Treatment of Beverage Industry Wastewater under Soudano-Sahelian Climatic Conditions. J. Membr. Sci. Res. 2022, 8. [Google Scholar] [CrossRef]
- Boureima, K.; Kam, S.; Ouedraogo, G.; Bathiebo, D. Etude de l’evolution Climatique Au Burkina Faso de 1983 a 2012: Cas Des Villes de Bobo Dioulasso, Ouagadougou et Dori. Arab. J. Earth Sci. 2017, 4, 50–59. [Google Scholar]
- Abuabdou, S.M.A.; Ahmad, W.; Aun, N.C.; Bashir, M.J.K. A Review of Anaerobic Membrane Bioreactors (AnMBR) for the Treatment of Highly Contaminated Landfill Leachate and Biogas Production: Effectiveness, Limitations and Future Perspectives. J. Clean. Prod. 2020, 255, 120215. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.-K.; Kwon, H.; Lee, S.H.; Lee, K.; Nahm, C.H.; Jo, S.J.; Oh, H.-S.; Park, P.-K.; Choo, K.-H.; et al. Crossing the Border between Laboratory and Field: Bacterial Quorum Quenching for Anti-Biofouling Strategy in an MBR. Environ. Sci. Technol. 2016, 50, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic Membrane Bioreactors for Wastewater Treatment: Novel Configurations, Fouling Control and Energy Considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef]
- Sato, Y.; Zhao, Y.-J.; Hori, T.; Aoyagi, T.; Inaba, T.; Aizawa, H.; Ogata, A.; Habe, H. Transition of Microbial Community Structures after Development of Membrane Fouling in Membrane Bioreactors (MBRs). AMB Express 2020, 10, 18. [Google Scholar] [CrossRef]
- Zulkefli, N.F.; Alias, N.H.; Jamaluddin, N.S.; Abdullah, N.; Abdul Manaf, S.F.; Othman, N.H.; Marpani, F.; Mat-Shayuti, M.S.; Kusworo, T.D. Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes 2022, 12, 26. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent Advances in Membrane Bioreactors (MBRs): Membrane Fouling and Membrane Material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Guo, G.; Zhang, T.; Qin, Y.; Hao, T.; Li, Y.-Y. Advanced Methanogenic Performance and Fouling Mechanism Investigation of a High-Solid Anaerobic Membrane Bioreactor (AnMBR) for the Co-Digestion of Food Waste and Sewage Sludge. Water Res. 2020, 187, 116436. [Google Scholar] [CrossRef]
- Nabi, M.; Liang, H.; Zhou, Q.; Cao, J.; Gao, D. In-Situ Membrane Fouling Control and Performance Improvement by Adding Materials in Anaerobic Membrane Bioreactor: A Review. Sci. Total Environ. 2023, 865, 161262. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in Membrane Bioreactors: An Updated Review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Kim, L.H.; Jung, Y.; Kim, S.-J.; Kim, C.-M.; Yu, H.-W.; Park, H.-D.; Kim, I.S. Use of Rhamnolipid Biosurfactant for Membrane Biofouling Prevention and Cleaning. Biofouling 2015, 31, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liang, H.; Xu, S.; Chuah, C.J.; Luo, X.; Wang, T.; Wang, J.; Li, G.; Snyder, S.A. Organic Matter Removal and Membrane Fouling Mitigation during Algae-Rich Surface Water Treatment by Powdered Activated Carbon Adsorption Pretreatment: Enhanced by UV and UV/Chlorine Oxidation. Water Res. 2019, 159, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Qu, F.; Liang, H.; Shao, S.; Han, Z.; Chang, H.; Du, X.; Li, G. Performance of Mesoporous Adsorbent Resin and Powdered Activated Carbon in Mitigating Ultrafiltration Membrane Fouling Caused by Algal Extracellular Organic Matter. Desalination 2014, 336, 129–137. [Google Scholar] [CrossRef]
- Dereli, R.K.; Ersahin, M.E.; Ozgun, H.; Ozturk, I.; Jeison, D.; van der Zee, F.; van Lier, J.B. Potentials of Anaerobic Membrane Bioreactors to Overcome Treatment Limitations Induced by Industrial Wastewaters. Bioresour. Technol. 2012, 122, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Gao, W.; Meng, F.; Liao, B.-Q.; Leung, K.-T.; Zhao, L.; Chen, J.; Hong, H. Membrane Bioreactors for Industrial Wastewater Treatment: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Petrosino, F.; Curcio, S.; Chakraborty, S.; De Luca, G. Enzyme Immobilization on Polymer Membranes: A Quantum and Molecular Mechanics Study. Computation 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Curcio, S.; Petrosino, F.; Morrone, M.; Luca, G.D. Interactions between Proteins and the Membrane Surface in Multiscale Modeling of Organic Fouling. J. Chem. Inf. Model. 2018, 58, 1815–1827. [Google Scholar] [CrossRef]
- Asiri, A.M.; Petrosino, F.; Pugliese, V.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Algieri, C.; Chakraborty, S. Synthesis and Characterization of Blended Cellulose Acetate Membranes. Polymers 2022, 14, 4. [Google Scholar] [CrossRef]
- Asiri, A.M.; Pugliese, V.; Petrosino, F.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Mukherjee, D.; Chakraborty, S. Photocatalytic Degradation of Textile Dye on Blended Cellulose Acetate Membranes. Polymers 2022, 14, 636. [Google Scholar] [CrossRef]
- De Vela, R.J. A Review of the Factors Affecting the Performance of Anaerobic Membrane Bioreactor and Strategies to Control Membrane Fouling. Rev. Environ. Sci. Bio/Technol. 2021, 20, 607–644. [Google Scholar] [CrossRef]
- Magalhães, N.C.; Silva, A.F.R.; Cunha, P.V.M.; Drewes, J.E.; Amaral, M.C.S. Role of Nanofiltration or Reverse Osmosis Integrated to Ultrafiltration-Anaerobic Membrane Bioreactor Treating Vinasse for the Conservation of Water and Nutrients in the Ethanol Industry. J. Water Process Eng. 2020, 36, 101338. [Google Scholar] [CrossRef]
- Dong, Q.; Parker, W.; Dagnew, M. Influence of SRT and HRT on Bioprocess Performance in Anaerobic Membrane Bioreactors Treating Municipal Wastewater. Water Environ. Res. 2016, 88, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Seco, A.; Mateo, O.; Zamorano-López, N.; Sanchis-Perucho, P.; Serralta, J.; Martí, N.; Borrás, L.; Ferrer, J. Exploring the Limits of Anaerobic Biodegradability of Urban Wastewater by AnMBR Technology. Environ. Sci. Water Res. Technol. 2018, 4, 1877–1887. [Google Scholar] [CrossRef]
- American Public Health Association. WEF (2005) Standard Methods for the Examination of Water and Wastewater; National Government Publication; American Public Health Association: Washington, DC, USA, 2007. [Google Scholar]
- NF T90-029. Available online: https://www.boutique.afnor.org/en-gb/standard/nf-t90029/water-quality-determination-of-dry-residues-at-105-c-and-180-c/fa120432/20118 (accessed on 8 May 2023).
- NFT 90-105-2. Water Quality—Dosage of Suspended Solids—Centrifugation Method 1997. Available online: https://infostore.saiglobal.com/en-us/Standards/NFT-90-105-2-1997-53668_SAIG_AFNOR_AFNOR_116016/ (accessed on 8 May 2023).
- Casu, S.; Crispino, N.A.; Farina, R.; Mattioli, D.; Ferraris, M.; Spagni, A. Wastewater Treatment in a Submerged Anaerobic Membrane Bioreactor. J. Environ. Sci. Health Part A 2012, 47, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Sobhey, M.; Badawy, M. Treatment of a Combined Dairy and Domestic Wastewater in an Up-Flow Anaerobic Sludge Blanket (UASB) Reactor Followed by Activated Sludge (AS System). Desalination 2008, 227, 167–177. [Google Scholar] [CrossRef]
- Patsios, S.I.; Michailidou, S.; Pasentsis, K.; Makris, A.M.; Argiriou, A.; Karabelas, A.J. Analysis of Microbial Community Dynamics during the Acclimatization Period of a Membrane Bioreactor Treating Table Olive Processing Wastewater. Appl. Sci. 2019, 9, 3647. [Google Scholar] [CrossRef] [Green Version]
- Sawadogo, B.; Konaté, Y.; Lesage, G.; Zaviska, F.; Monnot, M.; Heran, M.; Karambiri, H. Brewery Wastewater Treatment Using MBR Coupled with Nanofiltration or Electrodialysis: Biomass Acclimation and Treatment Efficiency. Water Sci. Technol. 2018, 77, 2624–2634. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of Organic Loading Rate on VFA Production, Organic Matter Removal and Microbial Activity of a Two-Stage Thermophilic Anaerobic Membrane Bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef]
- Elisabeth, A.C. Rôle des gaz Dissous dans la Digestion Anaérobie par voie Sèche de Déchets Ligno-Cellulosiques. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2015. [Google Scholar]
- Liu, X. Valorisation Energétique de la Biomasse Lignocellulosique par Digestion Anaérobie: Prétraitement Fongique Aérobie. Ph.D. Thesis, INSA de Lyon, Villeurbanne, France, 2015. [Google Scholar]
- Spagni, A.; Casu, S.; Grilli, S. Decolourisation of Textile Wastewater in a Submerged Anaerobic Membrane Bioreactor. Bioresour. Technol. 2012, 117, 180–185. [Google Scholar] [CrossRef]
- Maleki, E.; Bokhary, A.; Leung, K.; Liao, B.Q. Long-Term Performance of a Submerged Anaerobic Membrane Bioreactor Treating Malting Wastewater at Room Temperature (23 ± 1 °C). J. Environ. Chem. Eng. 2019, 7, 103269. [Google Scholar] [CrossRef]
- Vaquerizo, F.R.; Cruz-Salomon, A.; Valdovinos, E.R.; Pola-Albores, F.; Lagunas-Rivera, S.; Meza-Gordillo, R.; Ruiz Valdiviezo, V.M.; Simuta Champo, R.; Moreira-Acosta, J. Anaerobic Treatment of Vinasse from Sugarcane Ethanol Production in Expanded Granular Sludge Bed Bioreactor. J. Chem. Eng. Process Technol. 2017, 9, 3. [Google Scholar] [CrossRef]
- Santos, F.S.; Ricci, B.C.; França Neta, L.S.; Amaral, M.C.S. Sugarcane Vinasse Treatment by Two-Stage Anaerobic Membrane Bioreactor: Effect of Hydraulic Retention Time on Changes in Efficiency, Biogas Production and Membrane Fouling. Bioresour. Technol. 2017, 245, 342–350. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Yasin, M.; Khan, A.L.; Shahid, M.K.; Hossain, S.; Khan, Z.; Jamil, F.; Rafiq, S.; Bilad, M.R.; et al. Anaerobic Membrane Bioreactors for Biohydrogen Production: Recent Developments, Challenges and Perspectives. Bioresour. Technol. 2018, 269, 452–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahinkaya, E.; Isler, E.; Yurtsever, A.; Coban, I. Sulfidogenic Treatment of Acid Mine Drainage Using Anaerobic Membrane Bioreactor. J. Water Process Eng. 2019, 31, 100816. [Google Scholar] [CrossRef]
- Seo, H.; Cho, K.; Shin, J.; Lee, M.; Park, J.; Lee, B.C.; Song, K.G. Linking Process Performances and Core Microbial Community Structures in Anaerobic Membrane Bioreactor with Rotatory Disk (ARMBR) System Fed with High-Strength Food Waste Recycling Wastewater. Bioresour. Technol. 2019, 291, 121918. [Google Scholar] [CrossRef]
- Wang, S.; Ma, C.; Pang, C.; Hu, Z.; Wang, W. Membrane Fouling and Performance of Anaerobic Ceramic Membrane Bioreactor Treating Phenol- and Quinoline-Containing Wastewater: Granular Activated Carbon vs Polyaluminum Chloride. Environ. Sci. Pollut. Res. 2019, 26, 34167–34176. [Google Scholar] [CrossRef] [PubMed]
- Maleki, E. Psychrophilic Anaerobic Membrane Bioreactor (AnMBR) for Treating Malting Plant Wastewater and Energy Recovery. J. Water Process Eng. 2020, 34, 101174. [Google Scholar] [CrossRef]
- Pacheco-Ruiz, S.; Heaven, S.; Banks, C.J. Effect of Mean Cell Residence Time on Transmembrane Flux, Mixed-Liquor Characteristics and Overall Performance of a Submerged Anaerobic Membrane Bioreactor. Environ. Technol. 2017, 38, 1263–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamorano-López, N.; Moñino, P.; Borrás, L.; Aguado, D.; Barat, R.; Ferrer, J.; Seco, A. Influence of Food Waste Addition over Microbial Communities in an Anaerobic Membrane Bioreactor Plant Treating Urban Wastewater. J. Environ. Manag. 2018, 217, 788–796. [Google Scholar] [CrossRef]
- He, C.; Yang, C.; Yuan, S.; Hu, Z.; Wang, W. Effects of Sludge Retention Time on the Performance of Anaerobic Ceramic Membrane Bioreactor Treating High-Strength Phenol Wastewater. Archaea 2020, 2020, 8895321. [Google Scholar] [CrossRef]
- Nilusha, R.T.; Yu, D.; Zhang, J.; Wei, Y. Effects of Solids Retention Time on the Anaerobic Membrane Bioreactor with Yttria-Based Ceramic Membrane Treating Domestic Wastewater at Ambient Temperature. Membranes 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Idrus, S.; Che Man, H.; Nik Daud, N. Wastewater Treatment and Biogas Recovery Using Anaerobic Membrane Bioreactors (AnMBRs): Strategies and Achievements. Energies 2018, 11, 1675. [Google Scholar] [CrossRef] [Green Version]
- Fito, J.; Tefera, N.; Van Hulle, S.W.H. Sugarcane Biorefineries Wastewater: Bioremediation Technologies for Environmental Sustainability. Chem. Biol. Technol. Agric. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, P.G.; Dr Vaidia, N.G.; Dethe, S.B. Characterization and Comparative Study of Cane Sugar Industry WasteWater. Int. J. Chem. Phys. Sci. 2013, 2, 19–25. [Google Scholar]
- Mota, V.T.; Santos, F.S.; Amaral, M.C.S. Two-Stage Anaerobic Membrane Bioreactor for the Treatment of Sugarcane Vinasse: Assessment on Biological Activity and Filtration Performance. Bioresour. Technol. 2013, 146, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Durán, F.; Robles, Á.; Giménez, J.B.; Ferrer, J.; Ribes, J.; Serralta, J. Modeling the Anaerobic Treatment of Sulfate-Rich Urban Wastewater: Application to AnMBR Technology. Water Res. 2020, 184, 116133. [Google Scholar] [CrossRef]
- Gonçalves, M.M.M.; da Costa, A.C.A.; Leite, S.G.F.; Sant’Anna, G.L. Heavy Metal Removal from Synthetic Wastewaters in an Anaerobic Bioreactor Using Stillage from Ethanol Distilleries as a Carbon Source. Chemosphere 2007, 69, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Díaz, A.; Pereda-Reyes, I.; Dueñas-Moreno, J.; Véliz-Lorenzo, E.; Díaz-Marrero, M.A.; Menéndez-Gutiérrez, C.L.; Oliva-Merencio, D.; Zaiat, M. Combined treatment of vinasse by an upflow anaerobic filter-reactor and ozonation process. Braz. J. Chem. Eng. 2016, 33, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Parsaee, M.; Kiani Deh Kiani, M.; Karimi, K. A Review of Biogas Production from Sugarcane Vinasse. Biomass Bioenergy 2019, 122, 117–125. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane Gas Separation Technologies for Biogas Upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Sun, J.; Kosaki, Y.; Kawamura, K.; Watanabe, N. Operational Load Enhancement for an Anaerobic Membrane Bioreactor through Ethanol Fermentation Pretreatment of Food Waste. Energy Convers. Manag. 2021, 249, 114840. [Google Scholar] [CrossRef]
- Lovato, G.; Albanez, R.; Triveloni, M.; Ratusznei, S.M.; Rodrigues, J.A.D. Methane Production by Co-Digesting Vinasse and Whey in an AnSBBR: Effect of Mixture Ratio and Feed Strategy. Appl. Biochem. Biotechnol. 2019, 187, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, S.; Guo, Q.; Hong, Y.; Wu, P. Brewery Wastewater Treatment Using an Anaerobic Membrane Bioreactor. Biochem. Eng. J. 2016, 105, 321–331. [Google Scholar] [CrossRef]
- Svojitka, J.; Dvořák, L.; Studer, M.; Straub, J.O.; Frömelt, H.; Wintgens, T. Performance of an Anaerobic Membrane Bioreactor for Pharmaceutical Wastewater Treatment. Bioresour. Technol. 2017, 229, 180–189. [Google Scholar] [CrossRef]
- Balcıoğlu, G.; Yilmaz, G.; Gönder, Z.B. Evaluation of Anaerobic Membrane Bioreactor (AnMBR) Treating Confectionery Wastewater at Long-Term Operation under Different Organic Loading Rates: Performance and Membrane Fouling. Chem. Eng. J. 2021, 404, 126261. [Google Scholar] [CrossRef]
- Turker, M.; Dereli, R.K. Long Term Performance of a Pilot Scale Anaerobic Membrane Bioreactor Treating Beet Molasses Based Industrial Wastewater. J. Environ. Manag. 2021, 278, 111403. [Google Scholar] [CrossRef]
- Akram, A.; Stuckey, D.C. Biomass Acclimatisation and Adaptation during Start-up of a Submerged Anaerobic Membrane Bioreactor (SAMBR). Environ. Technol. 2008, 29, 1053–1065. [Google Scholar] [CrossRef]



| Parameter | Standard Method |
|---|---|
| Total solids (TS) | APHA 2540 B |
| Biochemical oxygen demand (BOD5) | APHA 5210 B |
| Chemical oxygen demand (COD) | APHA 5220—D |
| Microbial density | NF EN ISO 11348-1 |
| Suspended solids (SS) | AFNOR NFT 90-105 |
| Volatile suspended solids (VSS) | AFNOR NFT 90-029 |
| Absorbance | PBS ISO 3696:1987 |
| VFA (volatile fatty acids) | Biogas Titrator for FOS/TAC, Hach |
| Ferrous ion (Fe2+) | APHA 3500—B |
| Nitrate ions (NO3−) | APHA 4500—C |
| Phosphate ions (PO43−) | APHA 4500—B |
| Ammonium ions (NH4+) | APHA 4500—C |
| Magnesium ions (Mg2+) | APHA 3120B |
| Sodium ions (Na+) | Flame photometry method |
| Sulfate ions (SO42−) | APHA 4500—G |
| Chloride ions (Cl−) | APHA 4500—G |
| Calcium ions (Ca2+) | APHA 3500—B |
| Bicarbonate ions (HCO3−) | APHA 2320—B |
| Parameter | Minimum | Maximum | Average ± SD |
|---|---|---|---|
| Number of samples | 156 | ||
| pH | 6.8 | 7.5 | 7.1 ± 0.2 |
| T° | 21.5 | 42.3 | 31.9 ± 4.1 |
| Turbidity (NTU) | 1455 | 1966 | 1710 ± 94 |
| Conductivity(µs/cm) | 10,020 | 12,960 | 11,490 ± 688 |
| COD (mg/L) | 26,070 | 37,400 | 31,735 ± 2709 |
| BOD5 (mg/L) | 16,900 | 25,890 | 21,395 ± 2330 |
| COD/BOD5 | 1.48 | ||
| 1 COD/N/P | 100/0.2/0.01 | ||
| SS (mg/L) | 7360 | 8960 | 8160 ± 302 |
| VSS (mg/L) | 5010 | 6120 | 5565 ± 205 |
| VSS/SS | 68.2% | ||
| TS (mg/L) | 23,050 | 77,820 | 50,435 ± 8140 |
| Color | Brown | Brown | Brown |
| PO43− (mg/L) | 3.4 | 19.2 | 11.3 ± 3.7 |
| SO42− (mg/L) | 503 | 699 | 601 ± 42 |
| Mg2+ (mg/L) | 200 | 299 | 249 ± 23 |
| Ca2+ (mg/L) | 712 | 869 | 790 ± 39 |
| Fe2+ (mg/L) | 20.4 | 35.9 | 27.1 ± 3.7 |
| Total nitrogen (mg/L) | 20.2 | 103.4 | 61.8 ± 17.7 |
| NO3− (mg/L) | 1.2 | 14.1 | 7.6 ± 2.4 |
| NH4+ (mg/L) | 2.6 | 13.7 | 8.1 ± 2.6 |
| K+ (mg/L) | 16.6 | 93.2 | 54.9 ± 8.8 |
| F− (mg/L) | 20.5 | 68.8 | 44.6 ± 5.3 |
| HCO3− (mg/L) | 920 | 1085 | 1002 ± 29 |
| Cl− (mg/L) | 496 | 916 | 706 ± 147 |
| Na+ (mg/L) | 27.1 | 186.2 | 106.6 ± 12.1 |
| Hydraulic Conditions | |
|---|---|
| Reactor volume | 20 L |
| Hydraulic flow rate | 12 L·d−1 |
| Hydraulic retention time | 40 h |
| Permeate flux | 1.1 LMH |
| Biological conditions | |
| Temperature: 35 °C | |
| OLR: 15 to 22 gCOD/L/d | |
| Sludge extraction Run I (day 0 to day 62): no extraction Run II (day 62 to day 209): QW = 0.5 L·d−1, SRT = 40 d Run III (day 209 to day 353): no extraction | |
| Mass Balance | |||||
|---|---|---|---|---|---|
| Influent | Reactor | Permeate | Extraction | Biogas | |
| Phase 1 | 376 gCOD/d | 2.7 gVSS/d | 103.7 gCOD/d | 0 | 13.6 LCH4/d |
| Phase 2 | 368 gCOD/d | 0.825 gVSS/d | 85.4 gCOD/d | 7.5 gVSS/d | 42.5 LCH4/d |
| Phase 3 | 385 gCOD/d | 0.5 gVSS/d | 20.5 gCOD/d | 0 | 109.5 LCH4/d |
| Parameter | Unit | Phase I | Phase II | Phase III |
|---|---|---|---|---|
| Running period length | d | 0–63 | 63–209 | 209–353 |
| Volumetric organic load (Cv) | kgCOD·m−3·d−1 | 18.84 | 18.44 | 19.28 |
| Effluent concentration * | gCOD·m−3 | 8325 | 6304 | 1490 |
| COD removal rate | kgCOD·m−3·d−1 | 13.61 | 14.13 | 18.22 |
| Organic matter removal efficiency | % | 75% | 85% | 96% |
| Observed sludge production rate | kgVSS·m−3·d−1 | 0.135 | 0.416 | 0.025 |
| Conversion yield | kgVSS/kgCOD | 0.01 | 0.029 | 0.0013 |
| Methane conversion yield | LCH4·gCOD−1 | 0.05 | 0.15 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouhou Moussa, A.W.; Sawadogo, B.; Konate, Y.; Thianhoun, B.; Sidibe, S.d.S.; Heran, M. Influence of Solid Retention Time on Membrane Fouling and Biogas Recovery in Anerobic Membrane Bioreactor Treating Sugarcane Industry Wastewater in Sahelian Climate. Membranes 2023, 13, 710. https://doi.org/10.3390/membranes13080710
Nouhou Moussa AW, Sawadogo B, Konate Y, Thianhoun B, Sidibe SdS, Heran M. Influence of Solid Retention Time on Membrane Fouling and Biogas Recovery in Anerobic Membrane Bioreactor Treating Sugarcane Industry Wastewater in Sahelian Climate. Membranes. 2023; 13(8):710. https://doi.org/10.3390/membranes13080710
Chicago/Turabian StyleNouhou Moussa, Abdoul Wahab, Boukary Sawadogo, Yacouba Konate, Brony Thianhoun, Sayon dit Sadio Sidibe, and Marc Heran. 2023. "Influence of Solid Retention Time on Membrane Fouling and Biogas Recovery in Anerobic Membrane Bioreactor Treating Sugarcane Industry Wastewater in Sahelian Climate" Membranes 13, no. 8: 710. https://doi.org/10.3390/membranes13080710







