Nanomembranes-Affiliated Water Remediation: Chronology, Properties, Classification, Challenges and Future Prospects
Abstract
:1. Introduction
2. Chronology of Nanomembranes
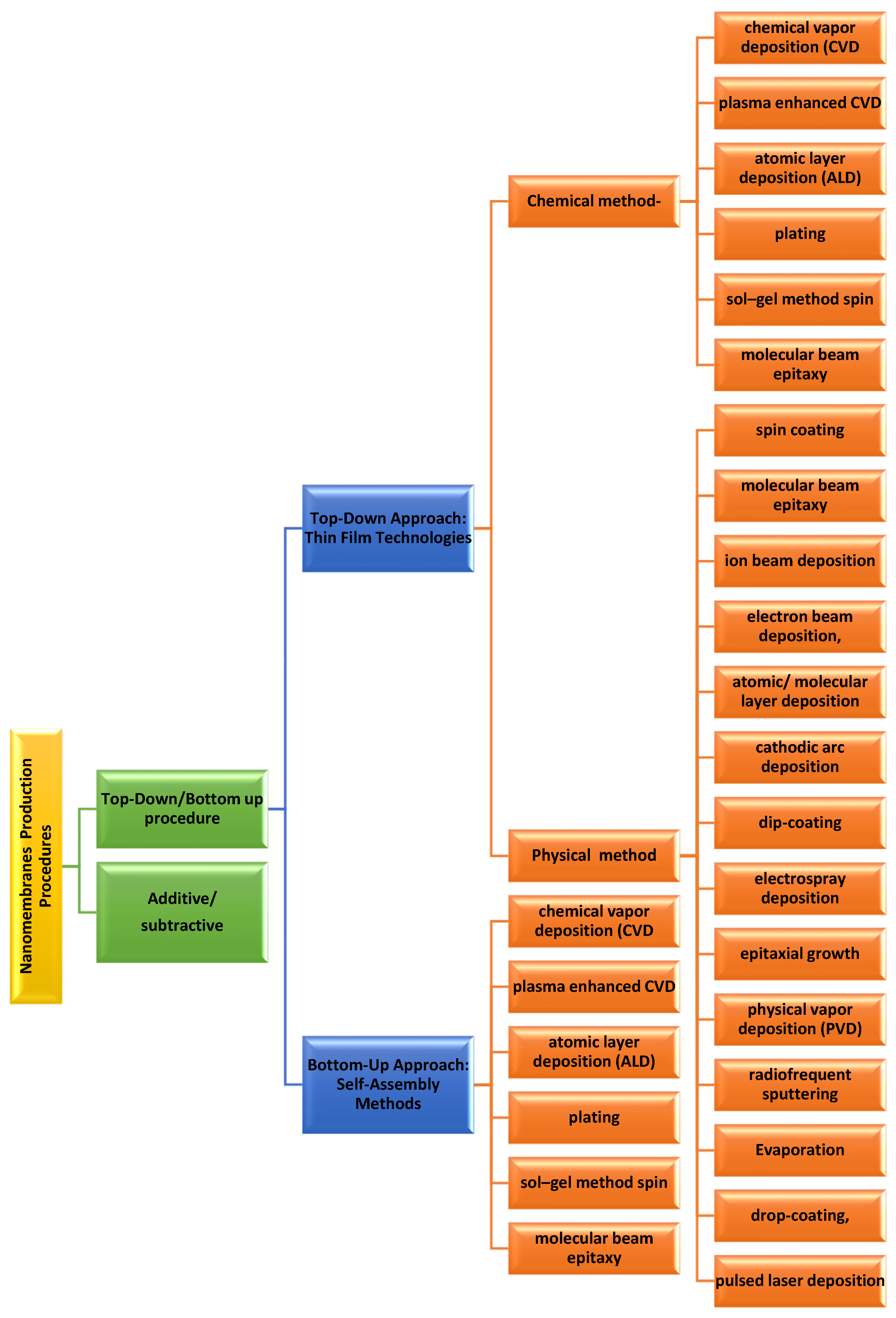
3. Nanomembrane Production Methods
3.1. Top–Down Approach
3.2. Bottom–Up Approach: The Following Are the Common Types of Bottom–Up Approaches [45]
3.2.1. Self-Assembly Approach
3.2.2. Langmuir–Blodget Method
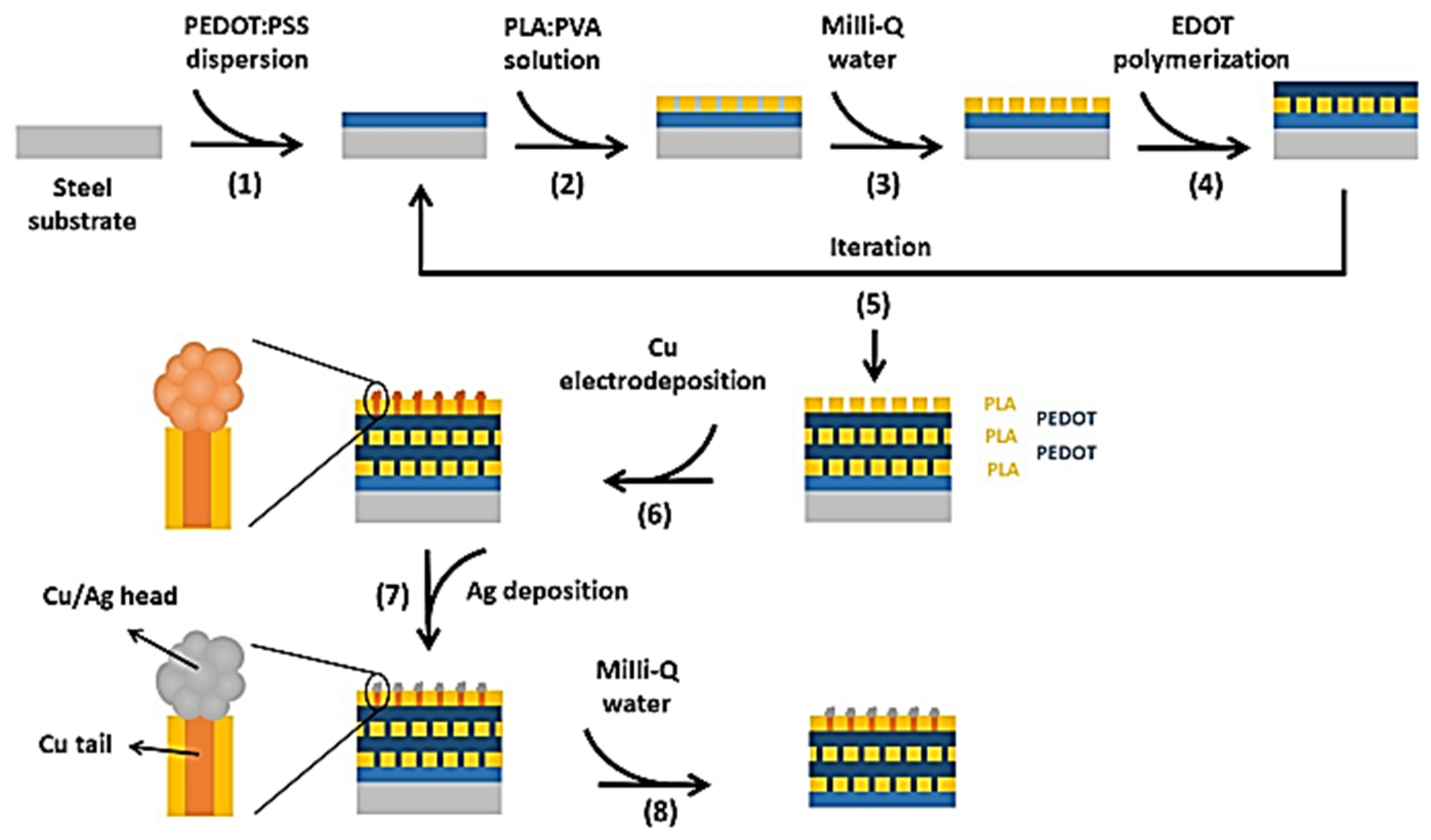
3.2.3. Layer-by-Layer Self-Assembly
3.2.4. Block Copolymer Self-Assembly
3.2.5. Sol–Gel Process
3.2.6. Dip-Coating
3.2.7. Drop-Coating
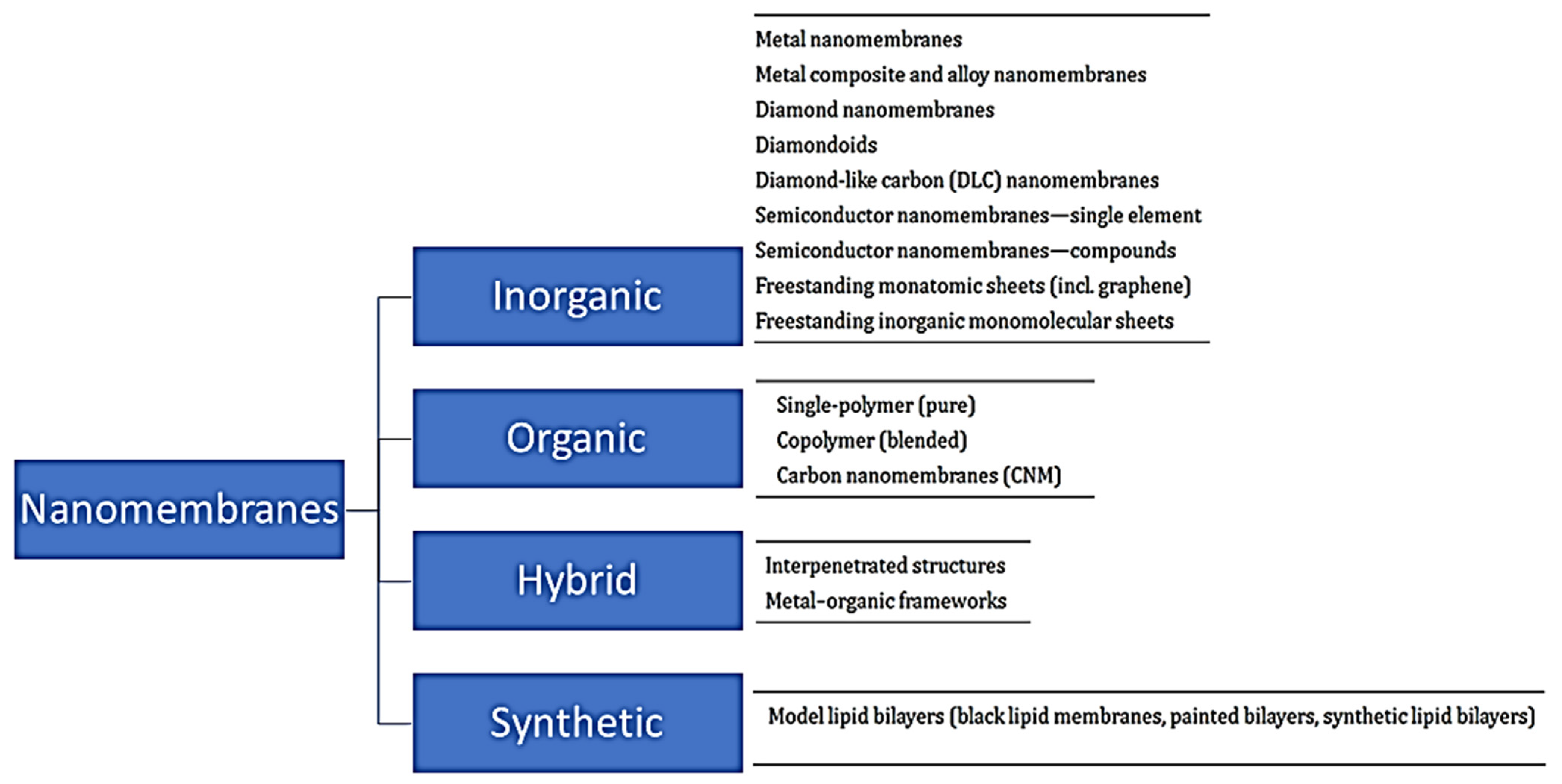
4. Types of Nanomembranes and Their Water Remediation Applications
4.1. Organic Nanomembranes
Carbon Nanomembranes

4.2. Hybrid (Inorganic/Organic) Nanomembranes
4.3. Inorganic Nanomembranes
4.4. Synthetic Biological Nanomembranes
5. Characteristics of Nanomembranes Attributed with Water Purification
5.1. Electrical Properties
5.2. Adsorption
5.3. Photocatalysis
5.4. Antimicrobial Activity
5.5. Chlorine Resistance
6. Challenges with Nanomembrane-Enhanced Water Remediation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Puri, N.; Gupta, A. Water remediation using titanium and zinc oxide nanomaterials through disinfection and photo catalysis process: A review. Environ. Res. 2023, 227, 115786. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Puri, N.; Gupta, A.; Mishra, A. Recent advances on nano-adsorbents and nanomembranes for the remediation of water. J. Clean. Prod. 2021, 322, 129051. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, S.; Zhang, X.; Wen, B.; Su, Z. The future of freshwater access: Functional material-based nano-membranes for desalination. Mater. Today Energy 2021, 22, 100856. [Google Scholar] [CrossRef]
- Naskar, J.; Boatemaa, M.A.; Rumjit, N.P.; Thomas, G.; George, P.J.; Lai, C.W.; Mousavi, S.M.; Wong, Y.H. Recent Advances of Nanotechnology in Mitigating Emerging Pollutants in Water and Wastewater: Status, Challenges, and Opportunities. Water Air Soil Pollut. 2022, 233, 156. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Namasivayam, S.K.R.; Prakash, P.; Babu, V.; Paul, E.J.; Bharani, R.A.; Kumar, J.A.; Kavisri, M.; Moovendhan, M. Aquatic biomass cellulose fabrication into cellulose nanocomposite and its application in water purification. J. Clean. Prod. 2023, 396, 136386. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Liu, X.; Song, Y. Study on the Changes in the Microcosmic Environment in Forward Osmosis Membranes to Reduce Membrane Resistance. Membranes 2022, 12, 1203. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Liu, X.; Song, Y. Influence of Membrane Fouling and Reverse Salt Flux on Membrane Impedance of Forward Osmosis Microbial Fuel Cell. Membranes 2022, 12, 1165. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Hermanowicz, S.W. Influence of water transport characteristics on membrane internal conductive structure in forward osmosis microbial fuel cell. J. Mol. Liq. 2023, 380, 121704. [Google Scholar] [CrossRef]
- Park, C.H.; Choi, O. Electrochemical energy-generating desalination system using a pressure-driven ion-selective nanomembrane. Nano Energy 2022, 94, 106939. [Google Scholar] [CrossRef]
- Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanostructures for water purification: Graphene and beyond. Nanoscale 2016, 8, 15115–15131. [Google Scholar] [CrossRef]
- Okello, V.A. Environmental Detoxification Using Polyamic Acid Powder and Nanomembranes. In Proceedings of the Sustainable Use of Water Resources: Proceedings of the Expert Workshop, Kisumu, Kenya, 3–8 July 2016; Cuvillier Verlag: Göttingen, Germany, 2017. [Google Scholar]
- Langmuir, I. Mechanical properties of monomolecular films. J. Frankl. Inst. 1934, 218, 143–171. [Google Scholar] [CrossRef]
- Blodgett, K.B. Monomolecular films of fatty acids on glass. J. Am. Chem. Soc. 1934, 56, 495. [Google Scholar] [CrossRef]
- Polymeropoulos, E.E.; Sagiv, J. Electrical conduction through adsorbed monolayers. J. Chem. Phys. 1978, 69, 1836–1847. [Google Scholar] [CrossRef]
- Sagiv, J. Organized monolayers by adsorption. 1. Formation and structure of oleophobic mixed monolayers on solid surfaces. J. Am. Chem. Soc. 1980, 102, 92–98. [Google Scholar] [CrossRef]
- Nuzzo, R.G.; Allara, D.L. Adsorption of bifunctional organic disulfides on gold surfaces. J. Am. Chem. Soc. 1983, 105, 4481–4483. [Google Scholar] [CrossRef]
- Gaikar, P.; Sangale, S.; Wadhawa, G. The Langmuir-Blodgett method for metal oxide nanostructures. In Solution Methods for Metal Oxide Nanostructures; Elsevier: Amsterdam, The Netherlands, 2023; pp. 369–392. [Google Scholar]
- Dubois, L.H.; Nuzzo, R.G. Synthesis, structure, and properties of model organic surfaces. Annu. Rev. Phys. Chem. 1992, 43, 437–463. [Google Scholar] [CrossRef]
- Finklea, H.O. Electrochemistry of organized monolayers of thiols and related molecules on electrodes. Electroanal. Chem. 1996, 19, 110–337. [Google Scholar]
- Oliveira, O.N., Jr.; Caseli, L.; Ariga, K. The past and the future of Langmuir and Langmuir–Blodgett films. Chem. Rev. 2022, 122, 6459–6513. [Google Scholar] [CrossRef]
- Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 2000, 65, 151–257. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Zharnikov, M.; Grunze, M. Spectroscopic characterization of thiol-derived self-assembling monolayers. J. Phys. Condens. Matter 2001, 13, 11333. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.A.; Gago, J.M.; Torrelles, X.; Salvarezza, R.C. Surface characterization of sulfur and alkanethiol self-assembled monolayers on Au (111). J. Phys. Condens. Matter 2006, 18, R867. [Google Scholar] [CrossRef]
- Woodruff, D.P. The interface structure of n-alkylthiolate self-assembled monolayers on coinage metal surfaces. Phys. Chem. Chem. Phys. 2008, 10, 7211–7221. [Google Scholar] [CrossRef] [Green Version]
- Kind, M.; Wöll, C. Organic surfaces exposed by self-assembled organothiol monolayers: Preparation, characterization, and application. Prog. Surf. Sci. 2009, 84, 230–278. [Google Scholar] [CrossRef]
- Smith, R.K.; Lewis, P.A.; Weiss, P.S. Patterning self-assembled monolayers. Prog. Surf. Sci. 2004, 75, 1–68. [Google Scholar] [CrossRef]
- Krämer, S.; Fuierer, R.R.; Gorman, C.B. Scanning probe lithography using self-assembled monolayers. Chem. Rev. 2003, 103, 4367–4418. [Google Scholar] [CrossRef]
- Gölzhäuser, A.; Wöll, C. Interfacial Systems Chemistry: Out of the Vacuum—Through the Liquid—Into the Cell. ChemPhysChem 2010, 11, 3201–3213. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef]
- Wilbur, J.L.; Kumar, A.; Kim, E.; Whitesides, G.M. Microfabrication by microcontact printing of self-assembled monolayers. Adv. Mater. 1994, 6, 600–604. [Google Scholar] [CrossRef]
- Rogers, J.A.; Jackman, R.J.; Whitesides, G.M. Microcontact printing and electroplating on curved substrates: Production of free-standing three-dimensional metallic microstructures. Adv. Mater. 1997, 9, 475–477. [Google Scholar] [CrossRef]
- Wilbur, J.L.; Kumar, A.; Biebuyck, H.A.; Kim, E.; Whitesides, G.M. Microcontact printing of self-assembled monolayers: Applications in microfabrication. Nanotechnology 1996, 7, 452. [Google Scholar] [CrossRef]
- Xia, Y.; Rogers, J.A.; Paul, K.E.; Whitesides, G.M. Unconventional methods for fabricating and patterning nanostructures. Chem. Rev. 1999, 99, 1823–1848. [Google Scholar] [CrossRef]
- Kane, R.S.; Takayama, S.; Ostuni, E.; Ingber, D.E.; Whitesides, G.M. Patterning proteins and cells using soft lithography. Biomaterials 1999, 20, 2363–2376. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Schlenoff, J.B.; Decher, G. (Eds.) Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kissel, P.; Erni, R.; Schweizer, W.B.; Rossell, M.D.; King, B.T.; Bauer, T.; Götzinger, S.; Schlüter, A.D.; Sakamoto, J. A two-dimensional polymer prepared by organic synthesis. Nat. Chem. 2012, 4, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Kory, M.J.; Bergeler, M.; Reiher, M.; Schlüter, A.D. Facile Synthesis and Theoretical Conformation Analysis of a Triazine-Based Double-Decker Rotor Molecule with Three Anthracene Blades. Chem.–A Eur. J. 2014, 20, 6934–6938. [Google Scholar] [CrossRef]
- Kambe, T.; Sakamoto, R.; Kusamoto, T.; Pal, T.; Fukui, N.; Hoshiko, K.; Shimojima, T.; Wang, Z.; Hirahara, T.; Ishizaka, K.; et al. Redox control and high conductivity of nickel bis (dithiolene) complex π-nanosheet: A potential organic two-dimensional topological insulator. J. Am. Chem. Soc. 2014, 136, 14357–14360. [Google Scholar] [CrossRef]
- Eck, W.; Küller, A.; Grunze, M.; Völkel, B.; Gölzhäuser, A. Freestanding nanosheets from crosslinked biphenyl self-assembled monolayers. Adv. Mater. 2005, 17, 2583–2587. [Google Scholar] [CrossRef]
- Gebeshuber, I.C. Biomimetic Nanotechnology Vol. 2. Biomimetics 2022, 7, 16. [Google Scholar] [CrossRef]
- Jakšić, Z.; Jakšić, O. Biomimetic nanomembranes: An overview. Biomimetics 2020, 5, 24. [Google Scholar] [CrossRef]
- Albisa, A.; Espanol, L.; Prieto, M.; Sebastian, V. Polymeric nanomaterials as nanomembrane entities for biomolecule and drug delivery. Curr. Pharm. Des. 2017, 23, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, R.; Viefhues, M.; Goett-Zink, L.; Gilzer, D.; Hellweg, T.; Gölzhäuser, A.; Kottke, T.; Anselmetti, D. Characterization of Robust and Free-Standing 2D-Nanomembranes of UV-Polymerized Diacetylene Lipids. Langmuir 2018, 34, 3256–3263. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.; Rodler, A.; Tscheliessnig, R.; Jungbauer, A. Freely suspended perforated polymer nanomembranes for protein separations. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Arif, S.; Umar, M.; Kim, S. Interacting metal–insulator–metal resonator by nanoporous silver and silk protein nanomembranes and its water-sensing application. Acs Omega 2019, 4, 9010–9016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Campolongo, M.J.; Cha, J.J.; Tan, S.J.; Umbach, C.C.; Muller, D.A.; Luo, D. Free-standing nanoparticle superlattice sheets controlled by DNA. Nat. Mater. 2009, 8, 519–525. [Google Scholar] [CrossRef]
- Han, D.; Park, Y.; Kim, H.; Lee, J.B. Self-assembly of free-standing RNA membranes. Nat. Commun. 2014, 5, 4367. [Google Scholar] [CrossRef] [Green Version]
- Bechhold, H. Kolloidstudien mit der Filtrationsmethode. Z. Für Phys. Chem. 1907, 60, 257–318. [Google Scholar] [CrossRef] [Green Version]
- Nosheen, S. Nanomembrane applications in environmental engineering. In Nanotechnology Applications in Environmental Engineering; IGI Global: Hershey, PA, USA, 2019; pp. 103–120. [Google Scholar]
- Afreen, S.; Omar, R.A.; Talreja, N.; Chauhan, D.; Ashfaq, M. Carbon-based nanostructured materials for energy and environmental remediation applications. In Approaches in Bioremediation: The New Era of Environmental Microbiology and Nanobiotechnology; Springer International Publishing: Cham, Switzerland, 2018; pp. 369–392. [Google Scholar]
- Turchanin, A.; Gölzhäuser, A. Carbon nanomembranes. Adv. Mater. 2016, 28, 6075–6103. [Google Scholar] [CrossRef]
- Ai, M.; Shishatskiy, S.; Wind, J.; Zhang, X.; Nottbohm, C.T.; Mellech, N.; Winter, A.; Vieker, H.; Qiu, J.; Dietz, K.J.; et al. Carbon nanomembranes (CNMs) supported by polymer: Mechanics and gas permeation. Adv. Mater. 2014, 26, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kunitake, T. A large, freestanding, 20 nm thick nanomembrane based on an epoxy resin. Adv. Mater. 2007, 19, 909–912. [Google Scholar] [CrossRef]
- Ahmadipouya, S.; Mousavi, S.A.; Shokrgozar, A.; Mousavi, D.V. Improving dye removal and antifouling performance of polysulfone nanofiltration membranes by incorporation of UiO-66 metal-organic framework. J. Environ. Chem. Eng. 2022, 10, 107535. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; El-Sayed, A.A.; Khalil, A.M. Polysulfone nanofiltration membranes enriched with functionalized graphene oxide for dye removal from wastewater. J. Polym. Eng. 2020, 40, 833–841. [Google Scholar] [CrossRef]
- Amini, M.; Arami, M.; Mahmoodi, N.M.; Akbari, A. Dye removal from colored textile wastewater using acrylic grafted nanomembrane. Desalination 2011, 267, 107–113. [Google Scholar] [CrossRef]
- Rzepka, S.; Neidhart, B. Transport processes through track-etch membrane filters in a reagent delivery cell. Fresenius J. Anal. Chem. 2000, 366, 336–340. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamaki, T.; Koshikawa, H.; Asano, M.; Voss, K.-O.; Neumann, R.; Yoshida, M. Preparation of ion-track membranes of poly(p-phenylene terephthalamide): Control of pore shape by irradiation with different ion beams. Nucl. Instrum. Methods Phys. Res. B 2007, 260, 693–695. [Google Scholar] [CrossRef]
- Varobiev, E.D.; Ovchinnikov, V.V.; Shestakov, V.D. Some peculiarities of use of the polymeric nuclear track membranes in clean rooms. JINR Rep. 1989, 18, 89–529. [Google Scholar]
- Vater, P. Production and applications of nuclear track microfilters. Radiat. Meas. 1988, 15, 743–749. [Google Scholar] [CrossRef]
- Yamazaki, I.M.; Paterson, R.; Geraldo, L.P. A new generation of track etched membranes for microfiltration and ultrafiltration. Part I. Preparation and characterisation. J. Membr. Sci. 1996, 118, 239–245. [Google Scholar] [CrossRef]
- Ziaie, F.; Shadman, M.; Yeganegi, S.; Mollaie, A.; Majdabadi, A. Investigation on polycarbonate nanomembrane production based on alpha particles irradiation. Nukleonika 2009, 54, 157–161. [Google Scholar]
- Wang, X.Y.; Zhu, K.D.; Zhu, J.; Ding, S.N. Photonic crystal of polystyrene nanomembrane: Signal amplification and low triggered potential electrochemiluminescence for tetracycline detection. Anal. Chem. 2021, 93, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Rahman, M.M.; Asmatulu, E. Sustainable freshwater harvesting from atmosphere through nanocomposite fibers of recycled polystyrene foams. In Behavior and Mechanics of Multifunctional Materials IX; SPIE: Bellingham, WA, USA, 2020; Volume 11377, pp. 183–191. [Google Scholar]
- Yang, Z.Y.; Ning, X.N.; Wang, H.; Liu, J.Y. Preparation of electrospinning polystyrene nanofibrous membrame and nanofiltration for simulated dyeing wastewater. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; Volume 55, pp. 1554–1559. [Google Scholar]
- Rana, A.K.; Gupta, V.K.; Saini, A.K.; Voicu, S.I.; Abdellattifaand, M.H.; Thakur, V.K. Water desalination using nanocelluloses/cellulose derivatives-based membranes for sustainable future. Desalination 2021, 520, 115359. [Google Scholar] [CrossRef]
- Gao, X.; Xu, L.-P.; Xue, Z.; Feng, L.; Peng, J.; Wen, Y.; Wang, S.; Zhang, X. Dual-scaled porous nitrocellulose membranes with underwater superoleophobicity for highly efficient oil/water separation. Adv. Mater. 2014, 26, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Tang, J.; Luo, J.; Wang, P.; Ding, B.; Tam, K.C. Applications of nanotechnology in oil and gas industry: Progress and perspective. Can. J. Chem. Eng. 2018, 96, 91–100. [Google Scholar] [CrossRef]
- Qi, P.; Jia, H.; Wang, Q.; Su, G.; Xu, S.; Zhang, M.; Qu, Y.; Pei, F. Ionic liquid-modified polyimide membranes with in-situ-grown polydopamine for separation of oil–water emulsions. High Perform. Polym. 2022, 34, 717–727. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Pant, H.R.; Kim, H.J.; Joshi, M.K.; Pant, B.; Park, C.H.; Kim, J.I.; Hui, K.; Kim, C.S. One-step fabrication of multifunctional composite polyurethane spider-web-like nanofibrous membrane for water purification. J. Hazard. Mater. 2014, 264, 25–33. [Google Scholar] [CrossRef]
- Asman, G. Use of Poly (methyl methacrylate-co-methacrylic acid) Membranes in the Ultrafiltration of Aqueous Fe3+ Solutions by Complexing with Poly (vinyl pyrrolidone) and Dextran. Sep. Sci. Technol. 2009, 44, 1164–1180. [Google Scholar] [CrossRef]
- Dutta, D.; Dubey, R.; Borah, J.P.; Puzari, A. Smart pH-responsive polyaniline-coated hollow polymethylmethacrylate microspheres: A potential pH neutralizer for water purification systems. ACS Omega 2021, 6, 10095–10105. [Google Scholar] [CrossRef]
- Abuin, G.C.; Fuertes, M.C.; Corti, H.R. Substrate effect on the swelling and water sorption of Nafion nanomembranes. J. Membr. Sci. 2013, 428, 507–515. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Costa, C.; Calvo, J.I.; Saelee, R. Novel polytetrafluoroethylene tubular membranes for membrane distillation. Desalination Water Treat. 2015, 53, 1559–1564. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A review on porous polymeric membrane preparation. Part II: Production techniques with polyethylene, polydimethylsiloxane, polypropylene, polyimide, and polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Albargi, H.; Ali, M.; Batool, M.; Nazir, A.; Qadir, M.B.; Khaliq, Z.; Arshad, S.N.; Jalalah, M.; Harraz, F.A. Differential carbonization-shrinkage induced hierarchically rough PAN/PDMS nanofiber composite membrane for robust multimodal superlipophilic applications. J. Sci. Adv. Mater. Devices 2023, 8, 100536. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic contaminants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Pan, J.; Yao, H.; Xu, L.; Ou, H.; Huo, P.; Li, X.; Yan, Y. Selective recognition of 2, 4, 6-trichlorophenol by molecularly imprinted polymers based on magnetic halloysite nanotubes composites. J. Phys. Chem. C 2011, 115, 5440–5449. [Google Scholar] [CrossRef]
- García-Torres, J.; Lázaro, C.; Sylla, D.; Lanzalaco, S.; Ginebra, M.P.; Alemán, C. Combining 2D organic and 1D inorganic nanoblocks to develop free-standing hybrid nanomembranes for conformable biosensors. J. Nanostruct. Chem. 2022, 1–11. [Google Scholar] [CrossRef]
- Watanabe, H.; Muto, E.; Ohzono, T.; Nakao, A.; Kunitake, T. Giant nanomembrane of covalently-hybridized epoxy resin and silica. J. Mater. Chem. 2009, 19, 2425–2431. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, F.; Cui, J.; Xu, B.; Mei, Y. Diverse Polymer Nanomembranes Toward Task-Specific Applications. Nanomembr. Mater. Prop. Appl. 2022, 57–83. [Google Scholar] [CrossRef]
- Shayesteh, M.; Samimi, A.; Shafiee Afarani, M.; Khorram, M. Synthesis of titania–γ-alumina multilayer nanomembranes on performance-improved alumina supports for wastewater treatment. Desalination Water Treat. 2016, 57, 9115–9122. [Google Scholar] [CrossRef]
- Cavallo, F.; Lagally, M.G. Semiconductors turn soft: Inorganic nanomembranes. Soft Matter 2010, 6, 439–455. [Google Scholar] [CrossRef]
- Singh, E.; Osmani, R.A.M.; Banerjee, R. Nanomembranes for ultrapurification and water treatment. In Membrane-Based Hybrid Processes for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 657–691. [Google Scholar]
- Tai, Y.C.; Yeh, P.L.; An, S.; Cheng, H.H.; Kim, M.; Chang, G.E. Strain-free GeSn nanomembranes enabled by transfer-printing techniques for advanced optoelectronic applications. Nanotechnology 2020, 31, 445301. [Google Scholar] [CrossRef]
- Seo, J.H.; Zhang, K.; Kim, M.; Zhao, D.; Yang, H.; Zhou, W.; Ma, Z. Flexible phototransistors based on single-crystalline silicon nanomembranes. Adv. Opt. Mater. 2016, 4, 120–125. [Google Scholar] [CrossRef]
- Yin, L.; Huang, W.; Xiao, R.; Peng, W.; Zhu, Y.; Zhang, Y.; Pi, X.; Yang, D. Optically stimulated synaptic devices based on the hybrid structure of silicon nanomembrane and perovskite. Nano Lett. 2020, 20, 3378–3387. [Google Scholar] [CrossRef]
- Yin, J.; Kim, E.S.; Yang, J.; Deng, B. Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM-41 silica nanoparticles (NPs) for water purification. J. Membr. Sci. 2012, 423, 238–246. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Sabir, A.; Shafiq, M.; Islam, A.; Jabeen, F.; Shafeeq, A.; Ahmad, A.; Zahid Butt, M.T.; Jacob, K.I.; Jamil, T. Conjugation of silica nanoparticles with cellulose acetate/polyethylene glycol 300 membrane for reverse osmosis using MgSO4 solution. Carbohydr. Polym. 2016, 136, 551–559. [Google Scholar] [CrossRef]
- Ahmad, A.; Waheed, S.; Khan, S.M.; Shafiq, M.; Farooq, M.; Sanaullah, K.; Jamil, T. Effect of silica on the properties of cellulose acetate/polyethylene glycol membranes for reverse osmosis. Desalination 2015, 355, 1–10. [Google Scholar] [CrossRef]
- Pang, R.; Zhang, K. Fabrication of hydrophobic fluorinated silica-polyamide thin film nanocomposite reverse osmosis membranes with dramatically improved salt rejection. J. Colloid Interface Sci. 2018, 510, 127–132. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.-Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wang, Z.; Hou, D.; Lin, S. Coaxially electrospun super-amphiphobic silica-based membrane for anti-surfactant-wetting membrane distillation. J. Membr. Sci. 2017, 531, 122–128. [Google Scholar] [CrossRef]
- Lee, E.J.; An, A.K.; He, T.; Woo, Y.C.; Shon, H.K. Electrospun nanofiber membranes incorporating fluorosilane-coated TiO2 nanocomposite for direct contact membrane distillation. J. Membr. Sci. 2016, 520, 145–154. [Google Scholar] [CrossRef]
- Shafiq, M.; Sabir, A.; Islam, A.; Khan, S.M.; Gull, N.; Hussain, S.N.; Butt, M.T.Z. Cellulaose acetate based thin film nanocomposite reverse osmosis membrane incorporated with TiO2 nanoparticles for improved performance. Carbohydr. Polym. 2018, 186, 367–376. [Google Scholar] [CrossRef]
- Emami, F.; Shekarriz, S.; Shariatinia, Z.; Moridi Mahdieh, Z. Self-cleaning properties of nylon 6 fabrics treated with corona and TiO2 nanoparticles under both ultraviolet and daylight irradiations. Fibers Polym. 2018, 19, 1014–1023. [Google Scholar] [CrossRef]
- Stan, M.S.; Badea, M.A.; Pircalabioru, G.G.; Chifiriuc, M.C.; Diamandescu, L.; Dumitrescu, I.; Trica, B.; Lambert, C.; Dinischiotu, A. Designing cotton fibers impregnated with photocatalytic graphene oxide/Fe, N-doped TiO2 particles as prospective industrial self-cleaning and biocompatible textiles. Mater. Sci. Eng. C 2019, 94, 318–332. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Kim, S.H.; Kim, S.S. Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Membr. Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Ren, L.F.; Xia, F.; Chen, V.; Shao, J.; Chen, R.; He, Y. TiO2-FTCS modified superhydrophobic PVDF electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Desalination 2017, 423, 1–11. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Nasr, M.; Zayed, M.; Ali, S.S.; Alshaikh, H.; Abd El-Salam, H.M.; Shaban, M. Fabrication of PES Modified by TiO2/Na2Ti3O7 Nanocomposite Mixed-Matrix Woven Membrane for Enhanced Performance of Forward Osmosis: Influence of Membrane Orientation and Feed Solutions. Membranes 2023, 13, 654. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Mohammad, A.W.; Ba-Abbad, M.M.; Razzaz, Z. Enhancement of polysulfone membrane with integrated ZnO nanoparticles for the clarification of sweetwater. Int. J. Environ. Sci. Technol. 2018, 15, 561–570. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Aber, S.; Vatanpour, V.; Mahmoodi, N.M. The effect of amine functionalization of CuO and ZnO nanoparticles used as additives on the morphology and the permeation properties of polyethersulfone ultrafiltration nanocomposite membranes. Compos. Part B Eng. 2018, 154, 388–409. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Aber, S.; Mahmoodi, N.M. Preparation and characterization of a novel polyethersulfone (PES) ultrafiltration membrane modified with a CuO/ZnO nanocomposite to improve permeability and antifouling properties. Sep. Purif. Technol. 2018, 192, 369–382. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Reconstitution of cell membrane structure in vitro andits transformation into an excitable system. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef]
- Tabushi, I.; Kuroda, Y.; Yokota, K. A, B, D, F-tetrasubstituted β-cyclodextrin as artificial channelcompound. Tetrahedron Lett. 1982, 23, 4601–4604. [Google Scholar] [CrossRef]
- Anitha, S.; Brabu, B.; Thiruvadigal, D.J.; Gopalakrishnan, C.; Natarajan, T.S. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym. 2012, 87, 1065–1072. [Google Scholar] [CrossRef]
- Hussain, N.; Bilal, M.; Iqbal, H.M. Carbon-based nanomaterials with multipurpose attributes for water treatment: Greening the 21st-century nanostructure materials deployment. Biomater. Polym. Horiz. 2022, 1, 48–58. [Google Scholar] [CrossRef]
- Manakhov, A.; Orlov, M.; Grokhovsky, V.; AlGhunaimi, F.I.; Ayirala, S. Functionalized Nanomembranes and Plasma Technologies for Produced Water Treatment: A Review. Polymers 2022, 14, 1785. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788. [Google Scholar] [CrossRef]
- Gohil, J.M.; Choudhury, R.R. Introduction to nanostructured and nano-enhanced polymeric membranes: Preparation, function, and application for water purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–57. [Google Scholar]
- Huang, Q.L.; Huang, Y.; Xiao, C.F.; You, Y.W.; Zhang, C.X. Electrospun ultrafine fibrous PTFE-supported ZnO porous membrane with self-cleaning function for vacuum membrane distillation. J. Membr. Sci. 2017, 534, 73–82. [Google Scholar] [CrossRef]
- Chen, L.H.; Huang, A.; Chen, Y.R.; Chen, C.H.; Hsu, C.C.; Tsai, F.Y.; Tung, K.L. Omniphobic membranes for direct contact membrane distillation: Effective deposition of zinc oxide nanoparticles. Desalination 2018, 428, 255–263. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Losic, D.; Kurkuri, M.D. Graphene-Based Nanomembranes for Sustainable Water Purification Applications. In Environmental Applications of Carbon Nanomaterials-Based Devices; WILEY-VCH GmbH: Weinheim, Germany, 2021; pp. 1–31. [Google Scholar]
- Franke, V.; McCleaf, P.; Lindegren, K.; Ahrens, L. Efficient removal of per-and polyfluoroalkyl substances (PFASs) in drinking water treatment: Nanofiltration combined with active carbon or anion exchange. Environ. Sci. Water Res. Technol. 2019, 5, 1836–1843. [Google Scholar] [CrossRef] [Green Version]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathy, D.B.; Gupta, A. Nanomembranes-Affiliated Water Remediation: Chronology, Properties, Classification, Challenges and Future Prospects. Membranes 2023, 13, 713. https://doi.org/10.3390/membranes13080713
Tripathy DB, Gupta A. Nanomembranes-Affiliated Water Remediation: Chronology, Properties, Classification, Challenges and Future Prospects. Membranes. 2023; 13(8):713. https://doi.org/10.3390/membranes13080713
Chicago/Turabian StyleTripathy, Divya Bajpai, and Anjali Gupta. 2023. "Nanomembranes-Affiliated Water Remediation: Chronology, Properties, Classification, Challenges and Future Prospects" Membranes 13, no. 8: 713. https://doi.org/10.3390/membranes13080713




