Breathable Films with Self-Cleaning and Antibacterial Surfaces Based on TiO2-Functionalized PET Membranes
Abstract
:1. Introduction
2. Experimental
2.1. “PET TM + TiO2” Systems Formation
2.2. Morphology Control
2.3. Structural Analysis and Measurement of Water and Gas Permeability
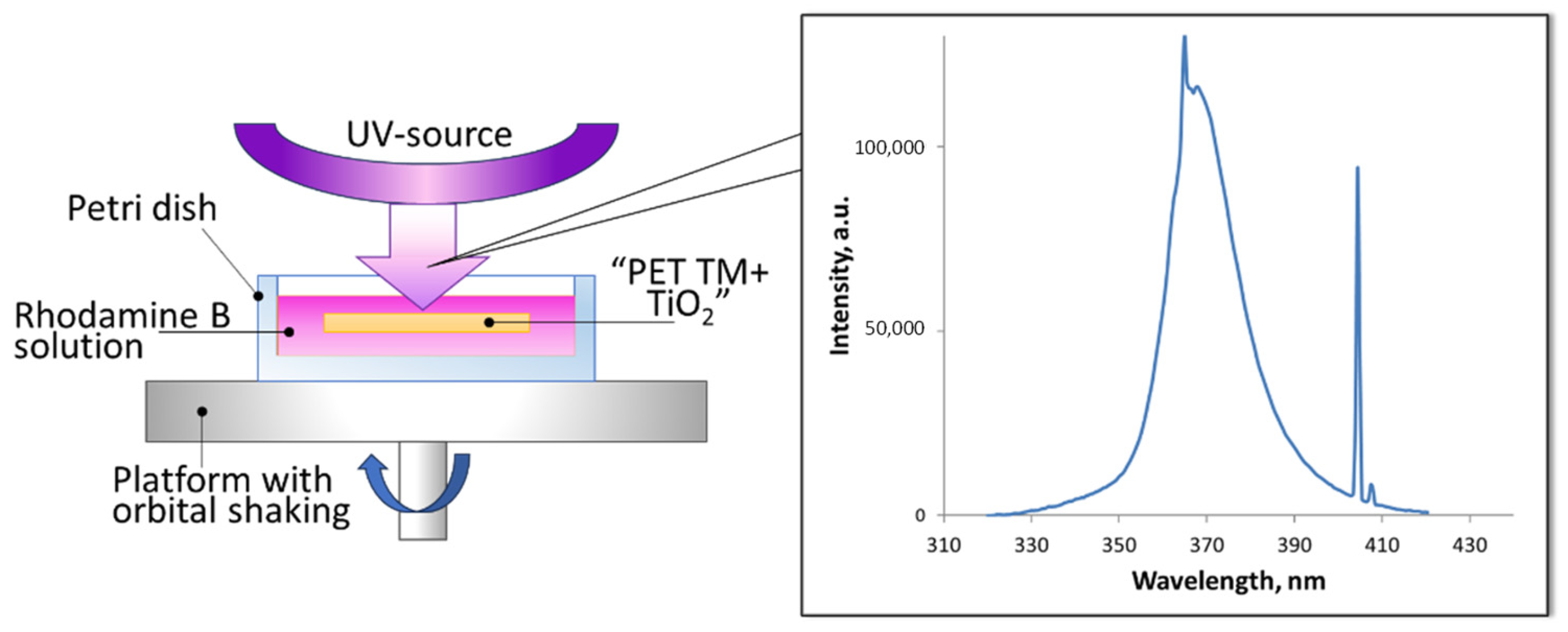
2.4. Surface Adsorption Properties Control
2.5. Analysis of Photocatalytic Activity
2.6. Antibacterial Properties Control
3. Results and Discussion
3.1. Morphology Control
3.2. Structural Analysis
3.3. Evaluation of Applicability
3.3.1. Surface Adsorption Properties Control
3.3.2. Analysis of Photocatalytic Activity
3.3.3. Antibacterial Properties Control
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maury, A.; de Belie, N. State of the art of TiO2 containing cementitious materials: Self-cleaning properties. Mater. Constr. 2010, 60, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Ünal, S.; Canbaz, M. Effect of industrial wastes on self-cleaning properties of concrete containing anatase-TiO2. Rev. Construcción 2022, 21, 493–505. [Google Scholar] [CrossRef]
- Abbas, M.; Iftikhar, H.; Malik, M.H.; Nazir, A. Surface coatings of TiO2 nanoparticles onto the designed fabrics for enhanced self-cleaning properties. Coatings 2018, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Sallehudin, M.E.; Affandi, N.D.N.; Harun, A.M.; Alam, M.K.; Indrie, L. Morphological Structures and Self-Cleaning Properties of Nano-TiO2 Coated Cotton Yarn at Different Washing Cycles. Nanomaterials 2023, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, L.; Iglesias, A.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial surfaces with self-cleaning properties functionalized by photocatalytic ZnO electrosprayed coatings. J. Hazard. Mater. 2019, 369, 665–673. [Google Scholar] [CrossRef]
- Chi, L.; Qian, Y.; Zhang, B.; Zhang, Z.; Jiang, Z. Surface engineering and self-cleaning properties of the novel TiO2/PAA/PTFE ultrafiltration membranes. Appl. Petrochem. Res. 2016, 6, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Linnik, O.; Khoroshko, L. Nonporous Nitrogen and Ruthenium Co-Doped Titania Films for Photocatalysis. Int. J. Nanosci. 2019, 18, 1940043. [Google Scholar] [CrossRef]
- Khoroshko, L.S. Two-dimensional porous anodic alumina for optoelectronics and photocatalytic application. J. Phys. Conf. Ser. 2015, 643, 012110. [Google Scholar] [CrossRef]
- Kutuzau, M.; Shumskaya, A.; Kaniukov, E.; Alisienok, O.; Shidlouskaya, V.; Melnikova, G.; Shemukhin, A.; Nazarov, A.; Kozlovskiy, A.; Zdorovets, M. Photocatalytically active filtration systems based on modified with titanium dioxide PET-membranes. Nucl. Instrum. Methods Phys. Res. B 2019, 460, 212–215. [Google Scholar] [CrossRef]
- Kumar, S.; Bhawna; Sharma, R.; Gupta, A.; Dubey, K.K.; Khan, A.; Singhal, R.; Kumar, R.; Bharti, A.; Singh, P.; et al. TiO2 based Photocatalysis membranes: An efficient strategy for pharmaceutical mineralization. Sci. Total. Environ. 2022, 845, 157221. [Google Scholar] [CrossRef]
- Azani, A.; Che Halin, D.S.; Razak, K.A.; Abdullah, M.M.A.B.; Salleh, M.A.A.M.; Mahmed, N.; Ramli, M.M.; Sepeai, S.; Sharin, M.F.; Chobpattana, V. Self-cleaning property of graphene oxide/TiO2 thin film. AIP Conf. Proc. 2019, 2129, 020062. [Google Scholar] [CrossRef]
- Yasin, S.A.; Abbas, J.A.; Ali, M.M.; Saeed, I.A.; Ahmed, I.H. Methylene blue photocatalytic degradation by TiO2 nanoparticles supported on PET nanofibers. Mater. Today Proc. 2020, 20, 482–487. [Google Scholar] [CrossRef]
- Joomjarearn, P.; Achiwawanich, S.; Setthayanond, J.; Suwanruji, P. Self-cleaning Property of Polyester Fabrics Finished with 3DOM TiO2. Fibers Polym. 2020, 21, 1975–1982. [Google Scholar] [CrossRef]
- Homocianu, M.; Pascariu, P. High-performance photocatalytic membranes for water purification in relation to environmental and operational parameters. J. Environ. Manag. 2022, 311, 114817. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Park, Y.C. Super-hydrophilic anatase TiO2 thin film in-situ deposited by DC magnetron sputtering. Thin Solid Films 2017, 638, 9–16. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, X.; Liu, Z.; Shen, R.; Rogachev, A.V. Preparation and characterization of TiO2 thin film by thermal oxidation of sputtered Ti film. Mater. Sci. Semicond. Process. 2013, 16, 513–519. [Google Scholar] [CrossRef]
- Miyauchi, M.; Tokudome, H. Super-hydrophilic and transparent thin films of TiO2 nanotube arrays by a hydrothermal reaction. J. Mater. Chem. 2007, 17, 2095–2100. [Google Scholar] [CrossRef]
- Sun, X.; Chen, P.; Mujahid, M.; Zhou, L. Effect of withdrawal speed on the microstructure, optical, and self-cleaning properties of TiO2 thin films. J. Sol-Gel Sci. Technol. 2020, 93, 62–69. [Google Scholar] [CrossRef]
- Khoroshko, L.; Borisenko, V.; Baltrukovich, P.; Nurmonov, S.; Ruzimuradov, O. One-step sol-gel fabrication of TiO2/(CuO + Cu2O) photocatalysts. J. Sol-Gel Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Salvaggio, M.G.; Passalacqua, R.; Abate, S.; Perathoner, S.; Centi, G.; Lanza, M.; Stassi, A. Functional nano-textured titania-coatings with self-cleaning and antireflective properties for photovoltaic surfaces. Sol. Energy 2016, 125, 227–242. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties. Adv. Powder Technol. 2018, 29, 211–219. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Z.; Shi, W.; Liu, Y.; Gao, H.; Mao, Y. The optimum titanium precursor of fabricating TiO2 compact layer for perovskite solar cells. Nanoscale Res. Lett. 2017, 12, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasikhudin; Ismaya, E.P.; Diantoro, M.; Kusumaatmaja, A.; Triyana, K. Preparation of PVA/TiO2 Composites Nanofibers by using Electrospinning Method for Photocatalytic Degradation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012011. [Google Scholar] [CrossRef]
- Che Halin, D.S.; Razak, K.A.; Mohamad Sukeri, N.S.; Azani, A.; Abdullah, M.M.A.B.; Salleh, M.A.A.M.; Mahmed, N.; Ramli, M.M.; Azhari, A.W.; Chobpattana, V. The Effect of Polyethylene Glycol (PEG) on TiO2 Thin Films via Sol-Gel Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 743, 012007. [Google Scholar] [CrossRef]
- Bu, S.; Jin, Z.; Liu, X.; Yang, L.; Cheng, Z. Fabrication of TiO2 porous thin films using peg templates and chemistry of the process. Mater. Chem. Phys. 2004, 88, 273–279. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Borgekov, D.; Kenzhina, I.; Zdorovets, M.; Korolkov, I.; Kaniukov, E.; Kutuzau, M.; Shumskaya, A. PET ion-track membranes: Formation features and basic applications. In NANO 2018: Nanocomposites, Nanostructures, and Their Applications; Fesenko, O., Yatsenko, L., Eds.; Springer Proceedings in Physics; Springer: Cham, Switzerland, 2019; Volume 221, pp. 461–479. [Google Scholar] [CrossRef]
- Kaniukov, E.; Shumskaya, A.; Yakimchuk, D.; Kozlovskiy, A.; Ibrayeva, A.; Zdorovets, M. Characterization of pet track membrane parameters. In NANO 2016: Nanophysics, Nanomaterials, Interface Studies, and Applications; Fesenko, O., Yatsenko, L., Eds.; Springer Proceedings in Physics; Springer: Cham, Switzerland, 2016; Volume 195, pp. 79–91. [Google Scholar] [CrossRef]
- Murashkevich, A.N.; Alisienok, O.A.; Zharskiy, I.M.; Novitskaya, M.S.; Fedorova, O.V.; Maximovskikh, A.I. Titania sols as precursors in sol-gel technologies of composite materials for photocatalysis, electrorheology, sorption. J. Sol-Gel Sci. Technol. 2019, 92, 254–263. [Google Scholar] [CrossRef]
- Alisienok, O.A.; Korol’kov, I.V.; Kozlovskij, A.L.; Kutuzov, M.D.; Shumskaya, E.E.; Kanyukov, E.Y.; Balakshin, Y.V.; Kozhemyako, A.A.; Shemukhin, A.A. Effect of ionizing radiation on structure and optical properties of «PETF+TiO2» system. In Proceedings of the Summaries of Reports of XLIX International Tulinov Conference on Physics of Interactions of Charged Particles with Crystals, Moscow, Russia, 29–31 May 2019; Volume 37. [Google Scholar]
- Kuriechen, S.K.; Murugesan, S.; Raj, S.P. Mineralization of Azo Dye Using Combined Photo-Fenton and Photocatalytic Processes under Visible Light. J. Catal. 2013, 2013, 104019. [Google Scholar] [CrossRef] [Green Version]
- Korolkov, I.V.; Narmukhamedova, A.R.; Melnikova, G.B.; Muslimova, I.B.; Yeszhanov, A.B.; Zhatkanbayeva, Z.K.; Chizhik, S.A.; Zdorovets, M.V. Preparation of hydrophobic PET track-etched membranes for separation of oil–water emulsion. Membranes 2021, 11, 637. [Google Scholar] [CrossRef]







| Overpressure, kPa | Performance, mL/min*m2 |
|---|---|
| 0 | 0 |
| 12 | 3.057 |
| 20 | 15.286 |
| 40 | 152 |
| 60 | 713 |
| 80 | 815 |
| 120 | 917 |
| Spectra (Figure 7) | Normalized Absorption, a.u. | Concentrarion of Rhodamine B, mg/L | Decrease in Concentration, mg/L | Decrease in Concentration, % |
|---|---|---|---|---|
| 1 | 1 | 2500 | -- | -- |
| 2 | 0.971 | 2431 | 0.069 | 2.76 |
| 3 | 0.959 | 2399 | 0.101 | 4.04 |
| 4 | 0.658 | 1654 | 0.846 | 33.84 |
| Test Bacteria | Concentration of Bacterial Cells, CFU/mL | Lg Red | |||
|---|---|---|---|---|---|
| C0 | Experiment CTiO2+UV | Control CUV | “PET TM + TiO2” + UV | UV | |
| E. coli | 5.0 × 105 | 2.3 × 102 | 2.2 × 105 | 3.34 | 0.36 |
| St. aureus | 8.6 × 105 | 5.0 × 101 | 4.7 × 104 | 4.24 | 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alisiyonak, O.; Lavitskaya, A.; Khoroshko, L.; Kozlovskiy, A.L.; Zdorovets, M.; Korolkov, I.; Yauseichuk, M.; Kaniukov, E.; Shumskaya, A. Breathable Films with Self-Cleaning and Antibacterial Surfaces Based on TiO2-Functionalized PET Membranes. Membranes 2023, 13, 733. https://doi.org/10.3390/membranes13080733
Alisiyonak O, Lavitskaya A, Khoroshko L, Kozlovskiy AL, Zdorovets M, Korolkov I, Yauseichuk M, Kaniukov E, Shumskaya A. Breathable Films with Self-Cleaning and Antibacterial Surfaces Based on TiO2-Functionalized PET Membranes. Membranes. 2023; 13(8):733. https://doi.org/10.3390/membranes13080733
Chicago/Turabian StyleAlisiyonak, Olga, Anna Lavitskaya, Liudmila Khoroshko, Artem L. Kozlovskiy, Maxim Zdorovets, Ilya Korolkov, Maryia Yauseichuk, Egor Kaniukov, and Alena Shumskaya. 2023. "Breathable Films with Self-Cleaning and Antibacterial Surfaces Based on TiO2-Functionalized PET Membranes" Membranes 13, no. 8: 733. https://doi.org/10.3390/membranes13080733
APA StyleAlisiyonak, O., Lavitskaya, A., Khoroshko, L., Kozlovskiy, A. L., Zdorovets, M., Korolkov, I., Yauseichuk, M., Kaniukov, E., & Shumskaya, A. (2023). Breathable Films with Self-Cleaning and Antibacterial Surfaces Based on TiO2-Functionalized PET Membranes. Membranes, 13(8), 733. https://doi.org/10.3390/membranes13080733










