Anion-Exchange Membrane “Polikon A” Based on Polyester Fiber Fabric (Functionalized by Low-Temperature High-Frequency Plasma) with Oxidized Metal Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Fabrication
2.2. Materials
2.3. Methods for Studying Properties
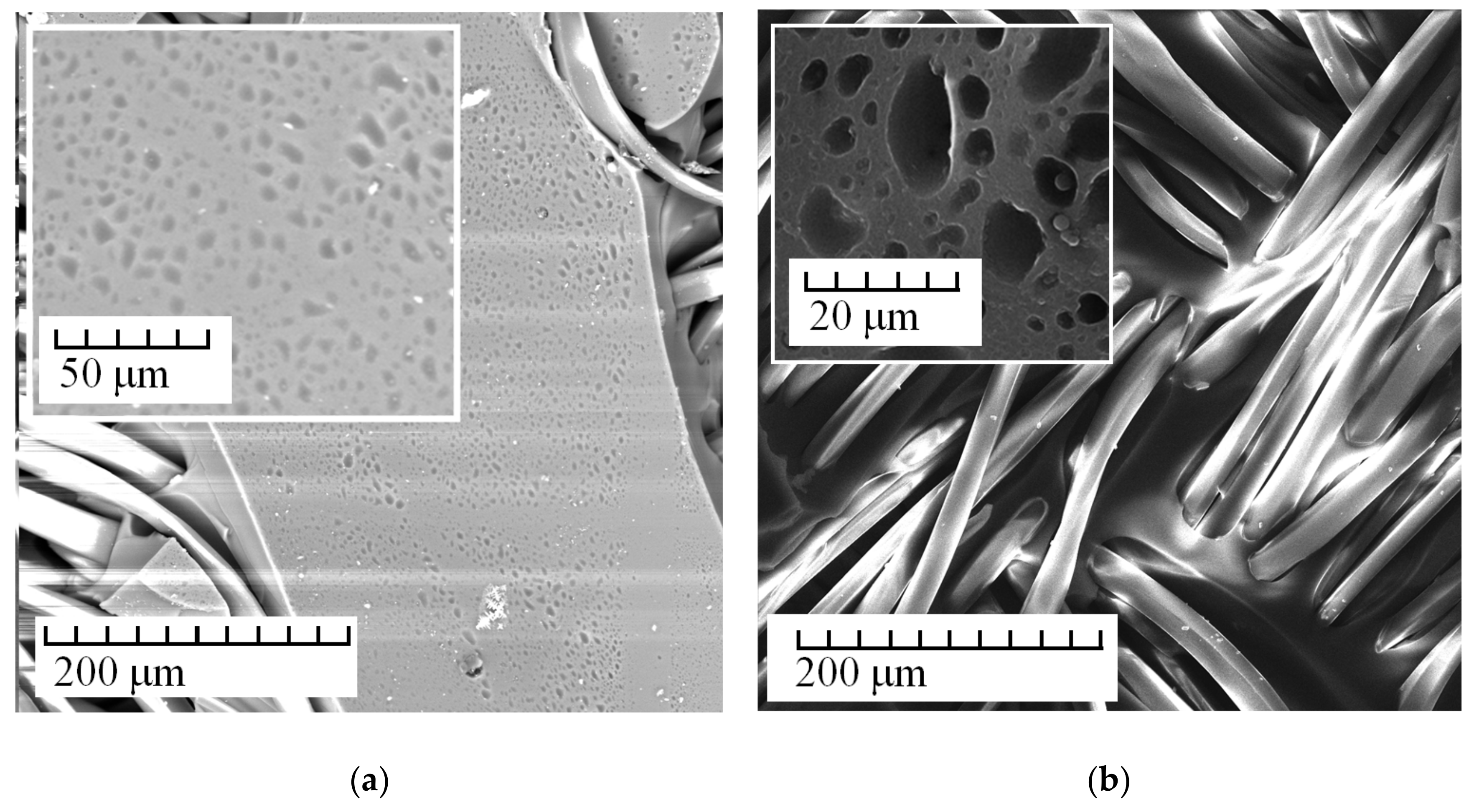
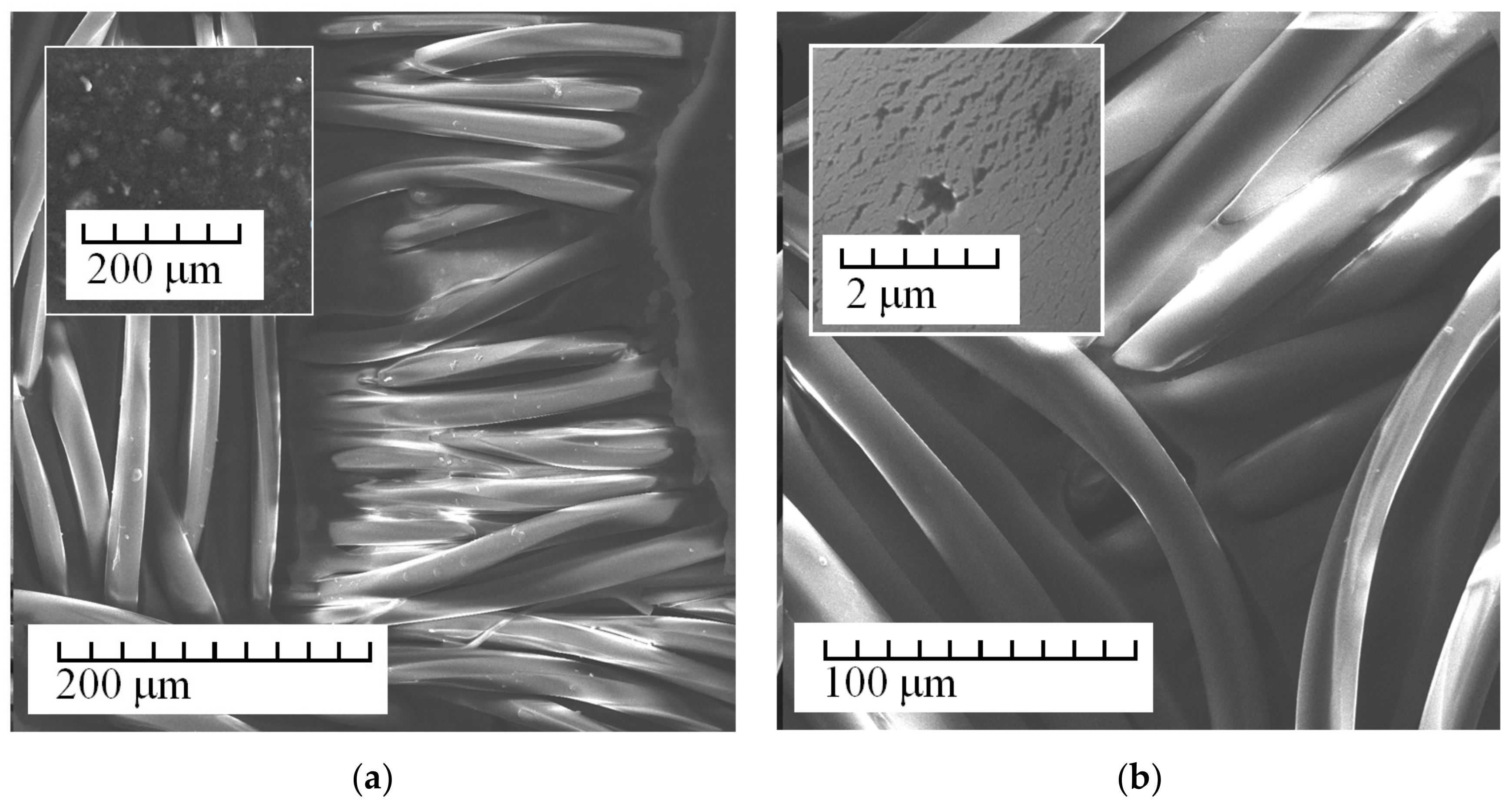
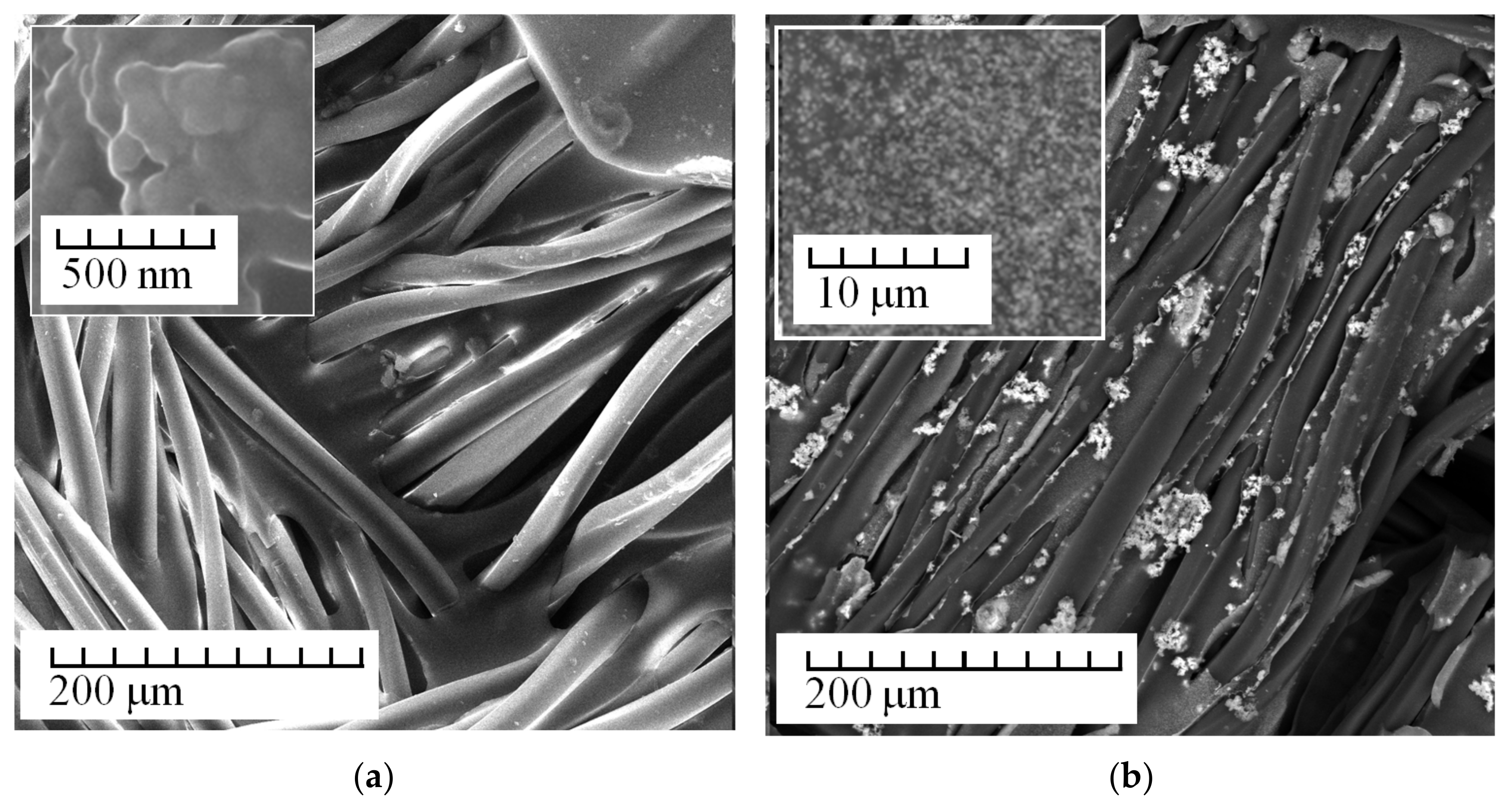
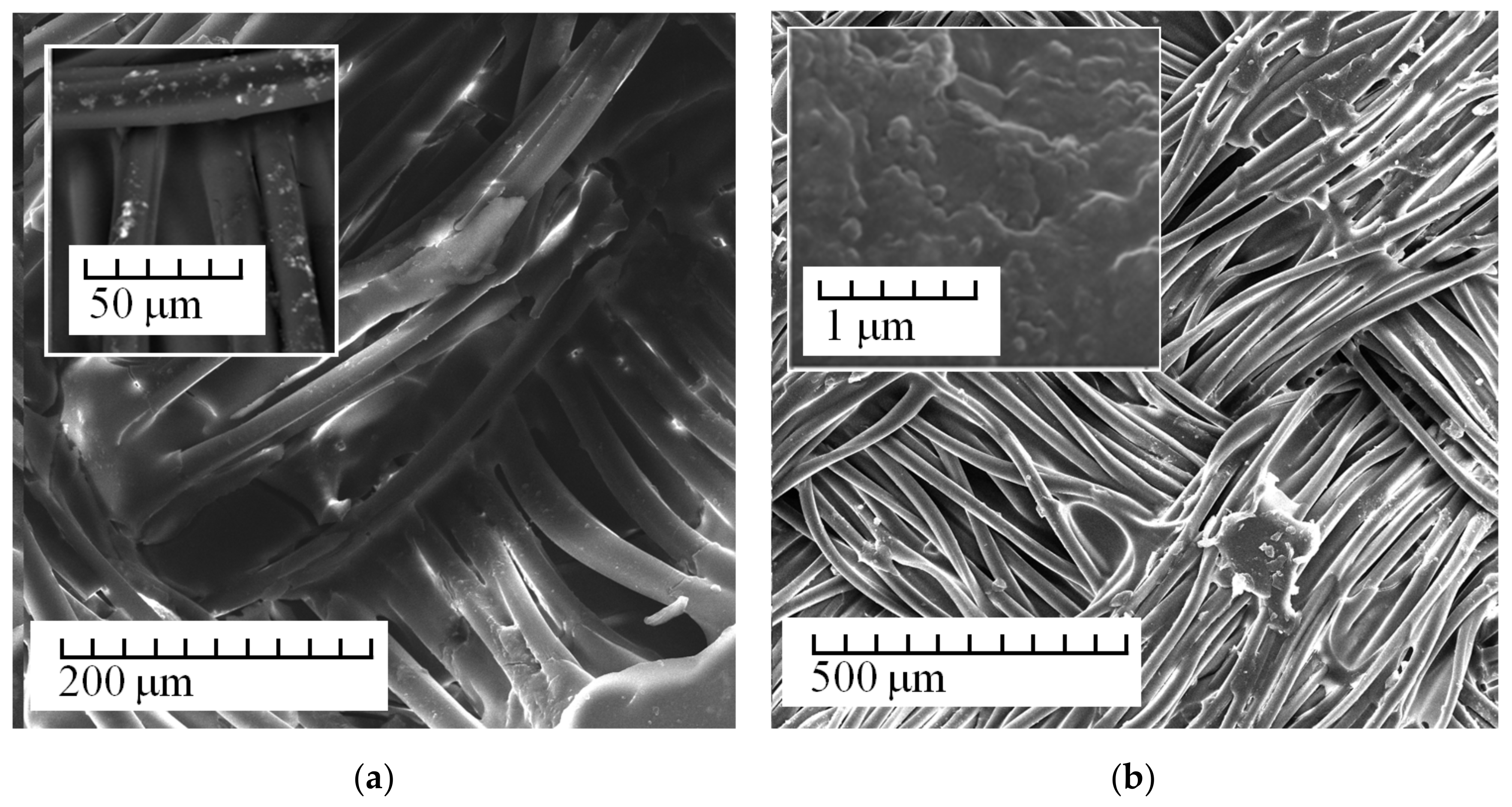
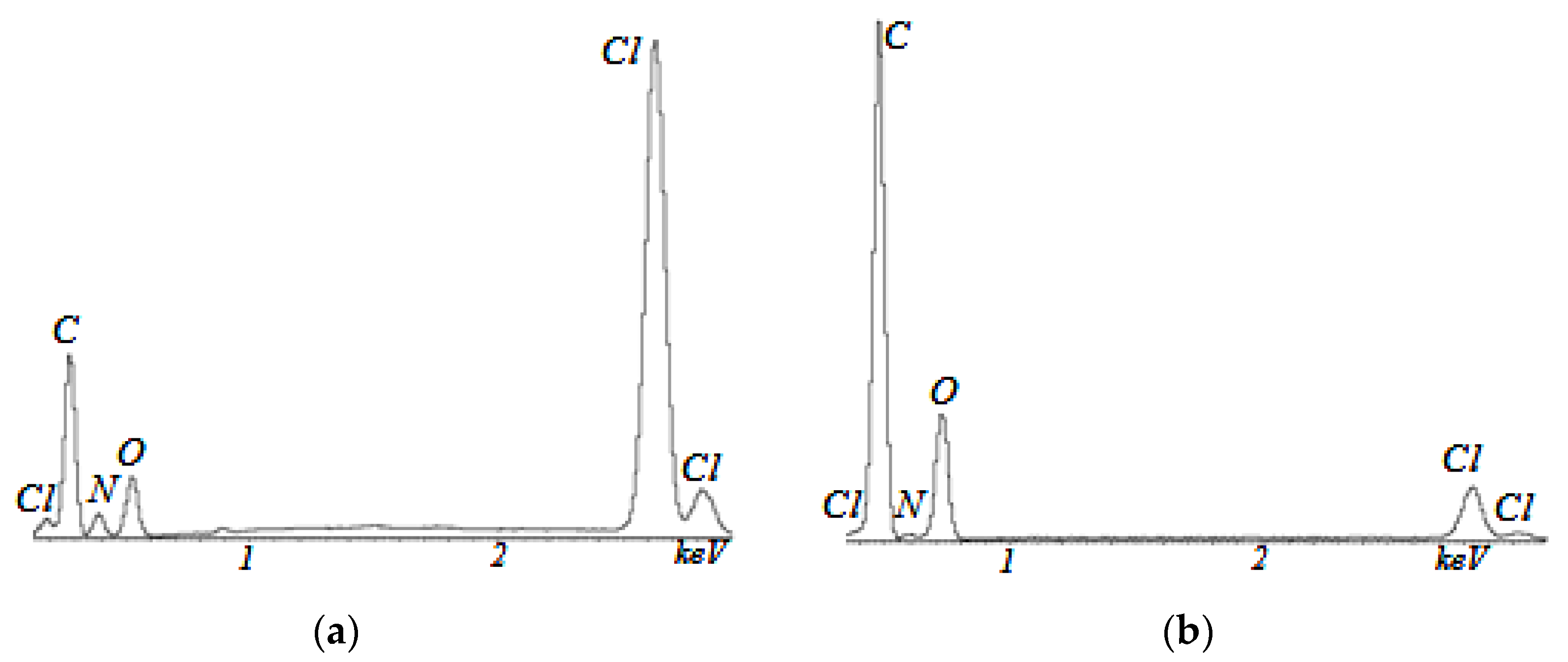
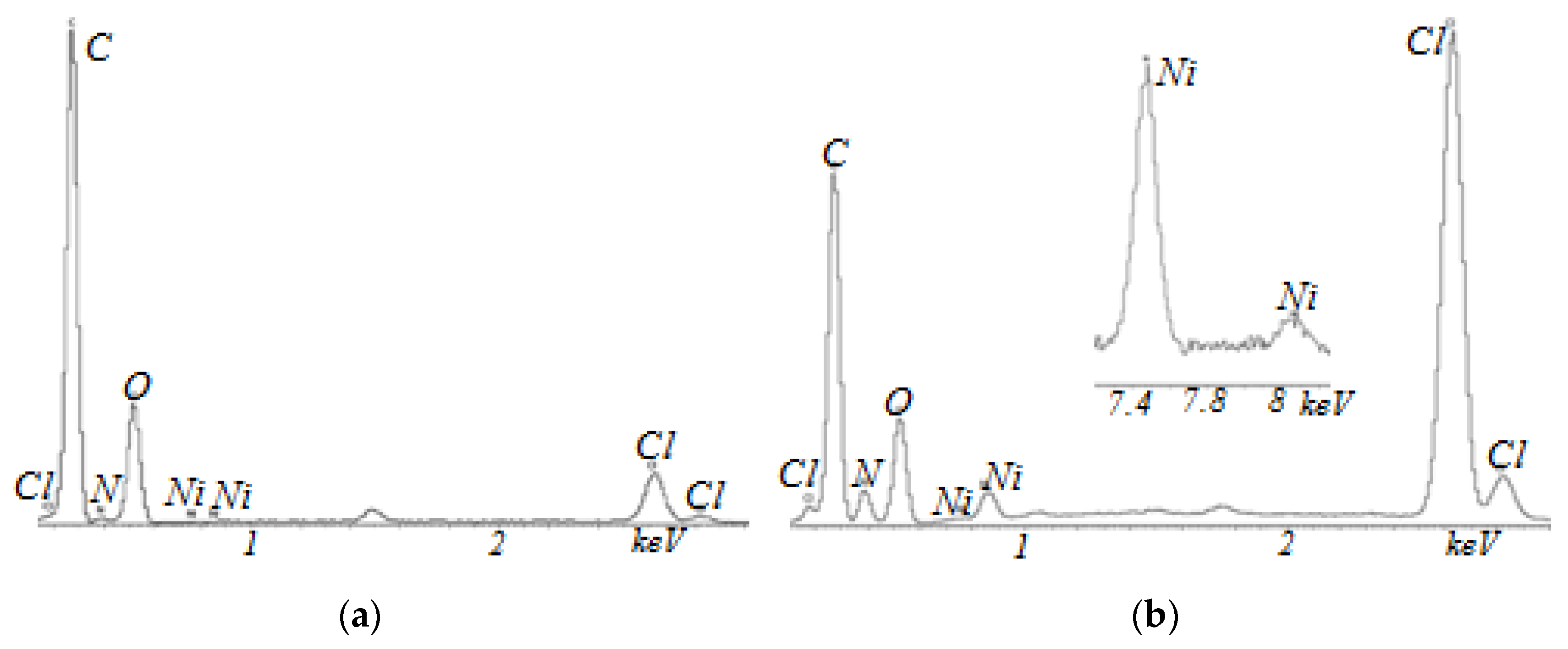
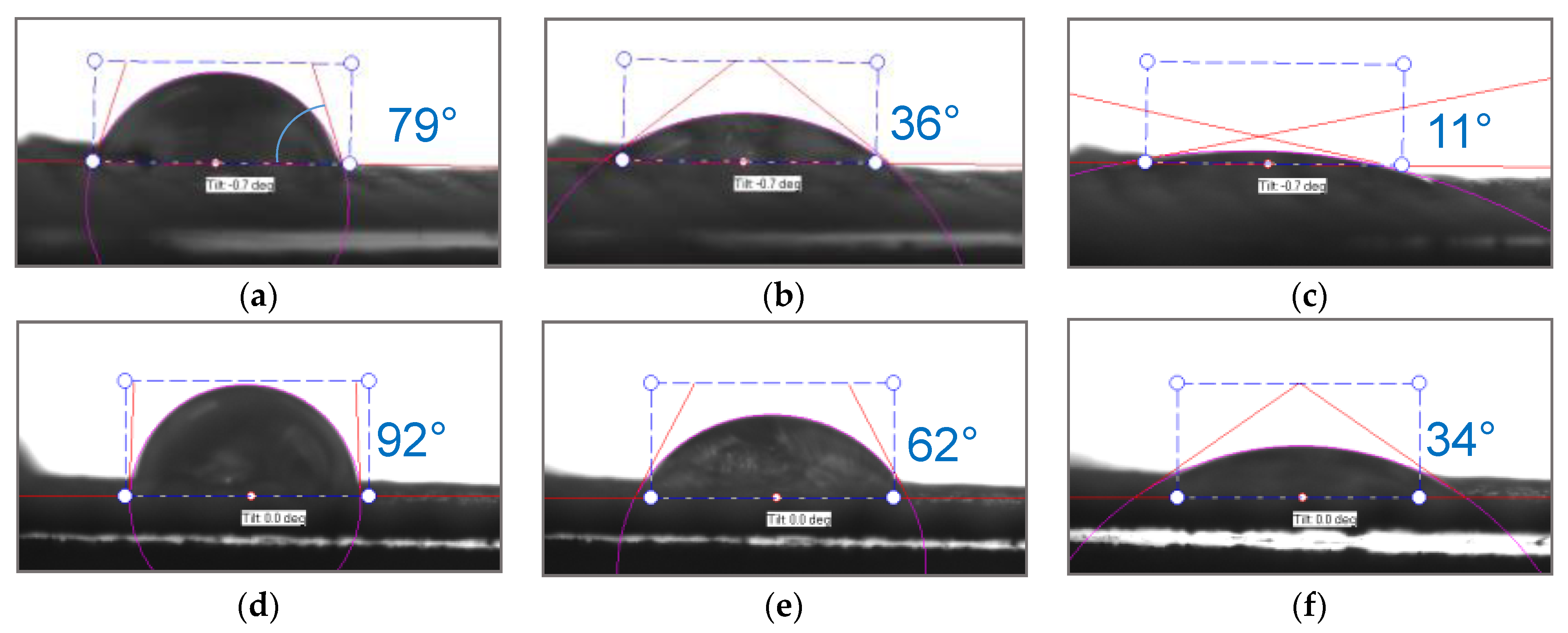
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hickner, M.A.; Herring, A.M.; Coughlin, E.B. Anion exchange membranes: Current status and moving forward. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1727–1735. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef] [PubMed]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the Art Commercial Membranes for Anion Exchange Membrane Water Electrolysis. J. Electrochem. Energy Convers. Storage 2021, 18, 024001. [Google Scholar] [CrossRef]
- Das, G.; Choi, J.-H.; Nguyen, P.K.T.; Kim, D.-J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef] [PubMed]
- Falcão, D.S. Green Hydrogen Production by Anion Exchange Membrane Water Electrolysis: Status and Future Perspectives. Energies 2023, 16, 943. [Google Scholar] [CrossRef]
- Clemens, A.L.; Jayathilake, B.S.; Karnes, J.J.; Schwartz, J.J.; Baker, S.E.; Duoss, E.B.; Oakdale, J.S. Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships. Polymers 2023, 15, 1534. [Google Scholar] [CrossRef] [PubMed]
- Vinodh, R.; Kalanur, S.S.; Natarajan, S.K.; Pollet, B.G. Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review. Polymers 2023, 15, 2144. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, P.; Rana, M.M.; Kang, B.-S.; Sun, H.-J.; Park, G.; Kim, S.-Y.; Lee, H.-K.; Shim, J. Precious Metal-Free CoP Nanorod Electrocatalyst as an Effective Bifunctional Oxygen Electrode for Anion Exchange Membrane-Unitized Regenerative Fuel Cells. Catalysts 2023, 13, 941. [Google Scholar] [CrossRef]
- Pushkarev, A.S.; Pushkareva, I.V.; du Preez, S.P.; Bessarabov, D.G. PGM-Free Electrocatalytic Layer Characterization by Electrochemical Impedance Spectroscopy of an Anion Exchange Membrane Water Electrolyzer with Nafion Ionomer as the Bonding Agent. Catalysts 2023, 13, 554. [Google Scholar] [CrossRef]
- Loh, A.; Li, X.; Sluijter, S.; Shirvanian, P.; Lai, Q.; Liang, Y. Design and Scale-Up of Zero-Gap AEM Water Electrolysers for Hydrogen Production. Hydrogen 2023, 4, 257–271. [Google Scholar] [CrossRef]
- Rakhshani, S.; Araneo, R.; Pucci, A.; Rinaldi, A.; Giuliani, C.; Pozio, A. Synthesis and Characterization of a Composite Anion Exchange Membrane for Water Electrolyzers (AEMWE). Membranes 2023, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ju, P.; Hu, S.; Shi, L.; Yuan, W.; Chen, D.; Wang, Y.; Shi, S. Separation of Hydrochloric Acid and Oxalic Acid from Rare Earth Oxalic Acid Precipitation Mother Liquor by Electrodialysis. Membranes 2023, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Loza, S.; Loza, N.; Kovalchuk, N.; Romanyuk, N.; Loza, J. Comparative Study of Different Ion-Exchange Membrane Types in Diffusion Dialysis for the Separation of Sulfuric Acid and Nickel Sulfate. Membranes 2023, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Pushkareva, I.V.; Solovyev, M.A.; Butrim, S.I.; Kozlova, M.V.; Simkin, D.A.; Pushkarev, A.S. On the Operational Conditions’ Effect on the Performance of an Anion Exchange Membrane Water Electrolyzer: Electrochemical Impedance Spectroscopy Study. Membranes 2023, 13, 192. [Google Scholar] [CrossRef]
- Xu, Z.; Delgado, S.; Atanasov, V.; Morawietz, T.; Gago, A.S.; Friedrich, K.A. Novel Pyrrolidinium-Functionalized Styrene-b-ethylene-b-butylene-b-styrene Copolymer Based Anion Exchange Membrane with Flexible Spacers for Water Electrolysis. Membranes 2023, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Noor Azam, A.M.I.; Ragunathan, T.; Zulkefli, N.N.; Masdar, M.S.; Majlan, E.H.; Mohamad Yunus, R.; Shamsul, N.S.; Husaini, T.; Shaffee, S.N.A. Investigation of Performance of Anion Exchange Membrane (AEM) Electrolysis with Different Operating Conditions. Polymers 2023, 15, 1301. [Google Scholar] [CrossRef]
- Shen, H.; Gong, Y.; Chen, W.; Wei, X.; Li, P.; Cheng, C. Anion Exchange Membrane Based on BPPO/PECH with Net Structure for Acid Recovery via Diffusion Dialysis. Int. J. Mol. Sci. 2023, 24, 8596. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Rybalkina, O.; Solonchenko, K.; Pasechnaya, E.; Sarapulova, V.; Wang, Y.; Jiang, C.; Xu, T.; Nikonenko, V. How Chemical Nature of Fixed Groups of Anion-Exchange Membranes Affects the Performance of Electrodialysis of Phosphate-Containing Solutions? Polymers 2023, 15, 2288. [Google Scholar] [CrossRef]
- Kozmai, A.; Porozhnyy, M.; Ruleva, V.; Gorobchenko, A.; Pismenskaya, N.; Nikonenko, V. Is It Possible to Prepare a “Super” Anion-Exchange Membrane by a Polypyrrole-Based Modification? Membranes 2023, 13, 103. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Q.; Afsar, N.U.; Ge, L.; Xu, T. Poly(alkyl-biphenyl pyridinium)-Based Anion Exchange Membranes with Alkyl Side Chains Enable High Anion Permselectivity and Monovalent Ion Flux. Membranes 2023, 13, 188. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Sahu, S.; Gallo, A.A.; Zhou, X.-D. Efficient Synthesis of High-Performance Anion Exchange Membranes by Applying Clickable Tetrakis(dialkylamino)phosphonium Cations. Polymers 2023, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Min, K.; Mo, Y.-H.; Han, S.-B.; Kim, T.-H. Understanding the Effect of Triazole on Crosslinked PPO–SEBS-Based Anion Exchange Membranes for Water Electrolysis. Polymers 2023, 15, 1736. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Sun, X.; Gao, H.; Pan, L.; Li, N.; Li, Y. Crosslinked Polynorbornene-Based Anion Exchange Membranes with Perfluorinated Branch Chains. Polymers 2023, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Park, J.-S. Fouling and Mitigation Behavior of Foulants on Ion Exchange Membranes with Surface Property in Reverse Electrodialysis. Membranes 2023, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, A.; Mostovoy, A.; Bekeshev, A.; Burmistrov, I.; Arzamastsev, S.; Lopukhova, M. Effect of Microwave Irradiation at Different Stages of Manufacturing Unsaturated Polyester Nanocomposite. Polymers 2022, 14, 4594. [Google Scholar] [CrossRef] [PubMed]
- Mostovoy, A.S.; Yakovlev, A.V.; Lopukhova, M.I. Directional control of physico-chemical and mechanical properties of epoxide composites by the addition of graphite-graphene structures. Polym.-Plast. Technol. Mater. 2020, 59, 874–883. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Zhumabekova, A.; Serikbayeva, G.; Vikulova, M.; Svitkina, V. Effect of Phosphorus and Chlorine Containing Plasticizers on the Physicochemical and Mechanical Properties of Epoxy Composites. J. Compos. Sci. 2023, 7, 178. [Google Scholar] [CrossRef]
- Mostovoi, A.S.; Plakunova, E.V.; Panova, L.G. New epoxy composites based on potassium polytitanates. Int. Polym. Sci. Technol. 2013, 40, 49–51. [Google Scholar] [CrossRef]
- Mostovoi, A.S.; Kurbatova, E.A. Controlling the properties of epoxy composites filled with brick dust. Russ. J. Appl. Chem. 2017, 90, 267–276. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Kadykova, Y.A.; Bekeshev, A.Z.; Tastanova, L.K. Epoxy composites modified with microfibers of potassium polytitanates. J. Appl. Polym. Sci. 2018, 135, 46651. [Google Scholar] [CrossRef]
- Kutepov, A.M.; Zakharov, A.G.; Maksimov, A.I.; Titov, V.A. Plasma modifying of textile materials: Prospects and problems. Ross. Him. Zhurnal 2002, 46, 103–115. (In Russian) [Google Scholar]
- Friedrich, J. Mechanisms of Plasma Polymerization—Reviewed from a Chemical Point of View. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Jelil, R.A. A review of low-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Strilets, I.D.; Kardash, M.M.; Terin, D.V.; Tsyplyayev, S.V.; Druzhinina, T.V. Features of Synthesis of Anion Exchange Matrix “Polikon A” with Oxidated Ultrafine Additives on Lavsan Textile Bases. Membr. Membr. Technol. 2020, 2, 325–331. [Google Scholar] [CrossRef]
- Terin, D.V.; Tsylyaev, S.V.; Cherkasov, V.V.; Kardash, M.M. Influence of the Process Parameters of Obtaining Polikon Mosaic Membranes on Their Structure and Properties. Fibre Chem. 2022, 53, 434–436. [Google Scholar] [CrossRef]
- Kardash, M.M.; Terin, D.V. Application of Viscose Nonwoven Fabrics as a Fibrous Frame of Polykon Mosaic Membranes. Membr. Membr. Technol. 2020, 2, 63–69. [Google Scholar] [CrossRef]
- Terin, D.V.; Kardash, M.M.; Druzhinina, T.V.; Tsyplyaev, S.V. Effect of Structural Heterogeneity of Polikon Mosaic Materials on Their Properties. Membr. Membr. Technol. 2019, 1, 323–330. [Google Scholar] [CrossRef]
- Terin, D.; Kardash, M.; Korchagin, S.; Tsyplyayev, S.; Cherkasov, V.; Druzhinina, T. Features of Thermomechanical Stability of Anionic–Cation Exchange Matrix “Polikon AC” on Viscose Non-Woven Materials. Membranes 2021, 11, 734. [Google Scholar] [CrossRef]
- Kardash, M.M.; Kononenko, N.A.; Fomenko, M.A.; Tyurin, I.A.; Ainetdinov, D.V. Effect of nature of fibrous substrate of composite membranes on their structure, conductive properties, and selectivity. Pet. Chem. 2016, 56, 315–320. [Google Scholar] [CrossRef]
- Bilenko, D.I.; Terin, D.V.; Tozkoparan, O.; Yildirim, O.; Galushka, V.V.; Dincer, I.; Dobrinsky, E.K.; Ellerman, Y.; Venig, S.B. The influence of Morphology, Conditions of Production and External Effects on Nanoparticles’ (in Terms of Iron) Dielectric Properties. Izv. Saratov. Univ. Novaya Ser. Ser. Fiz. 2015, 15, 21. [Google Scholar] [CrossRef]
- Optical Tensiometer. Available online: https://www.biolinscientific.com/attension/optical-tensiometers/theta-lite (accessed on 21 June 2023).
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef]
- Zimon, A.D. Fluid Adhesion and Wetting; Chemistry Publishers: Moscow, Russia, 1974; 416p. (In Russian) [Google Scholar]
- Volfkovich, Y.M.; Bograchev, D.A.; Mikhalin, A.A.; Rychagova, A.Y.; Sosenkin, V.E.; Park, D. Capacitive deionization of aqueous solutions. Modeling and experiments. Desalination Water Treat. 2017, 69, 130–141. [Google Scholar] [CrossRef]


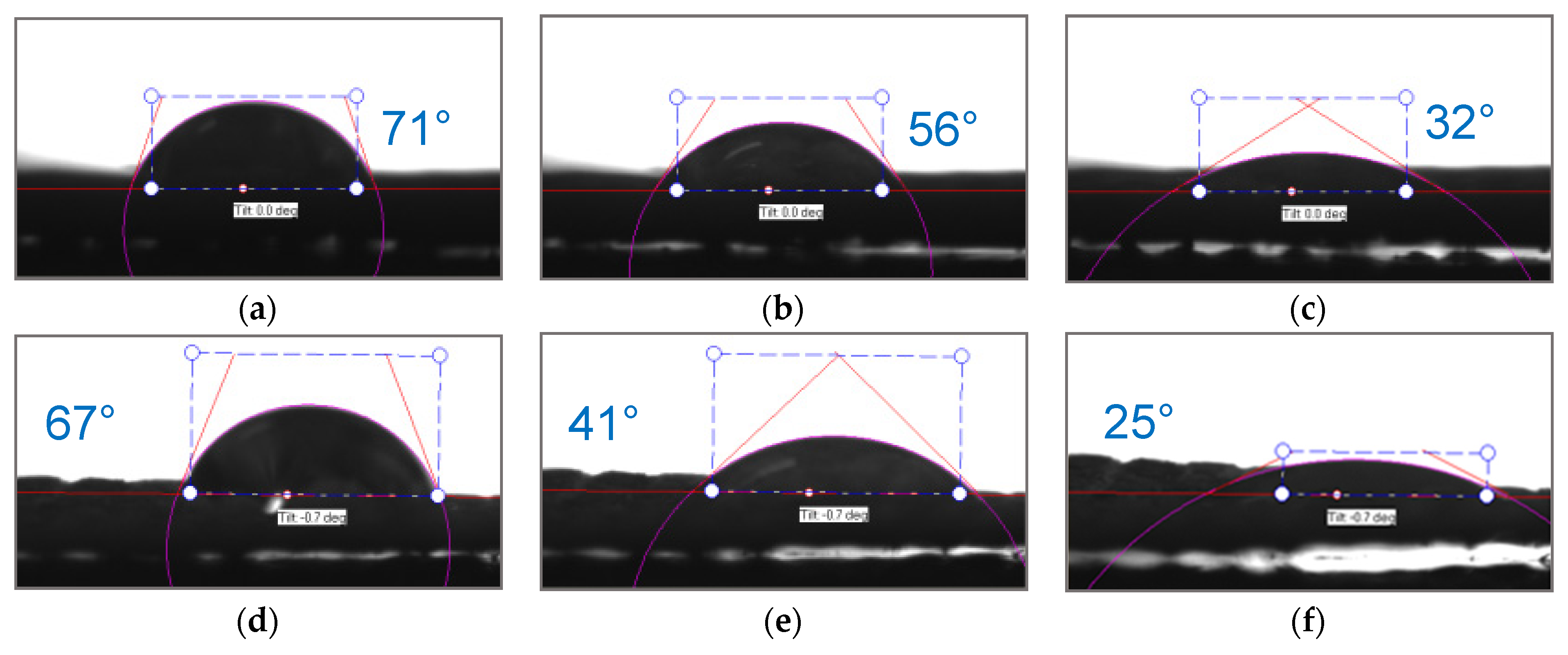

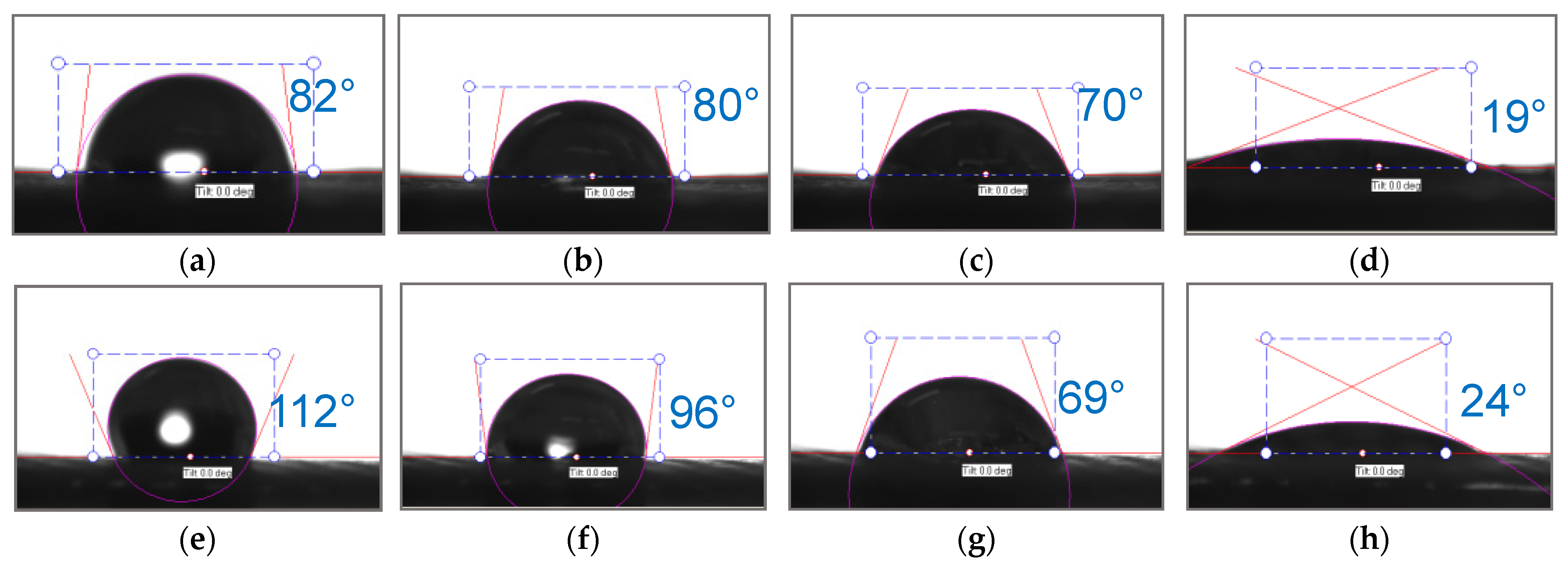

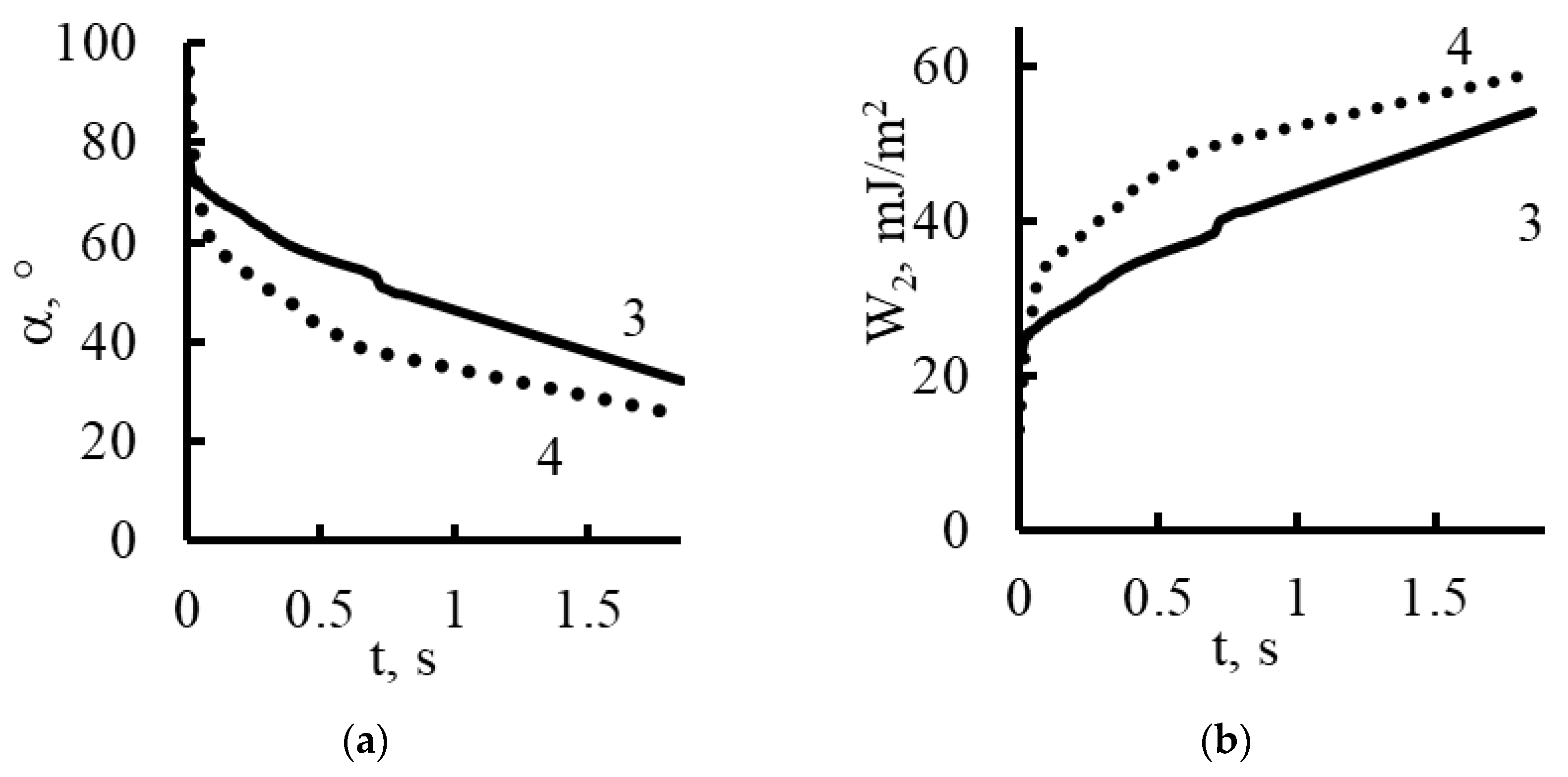

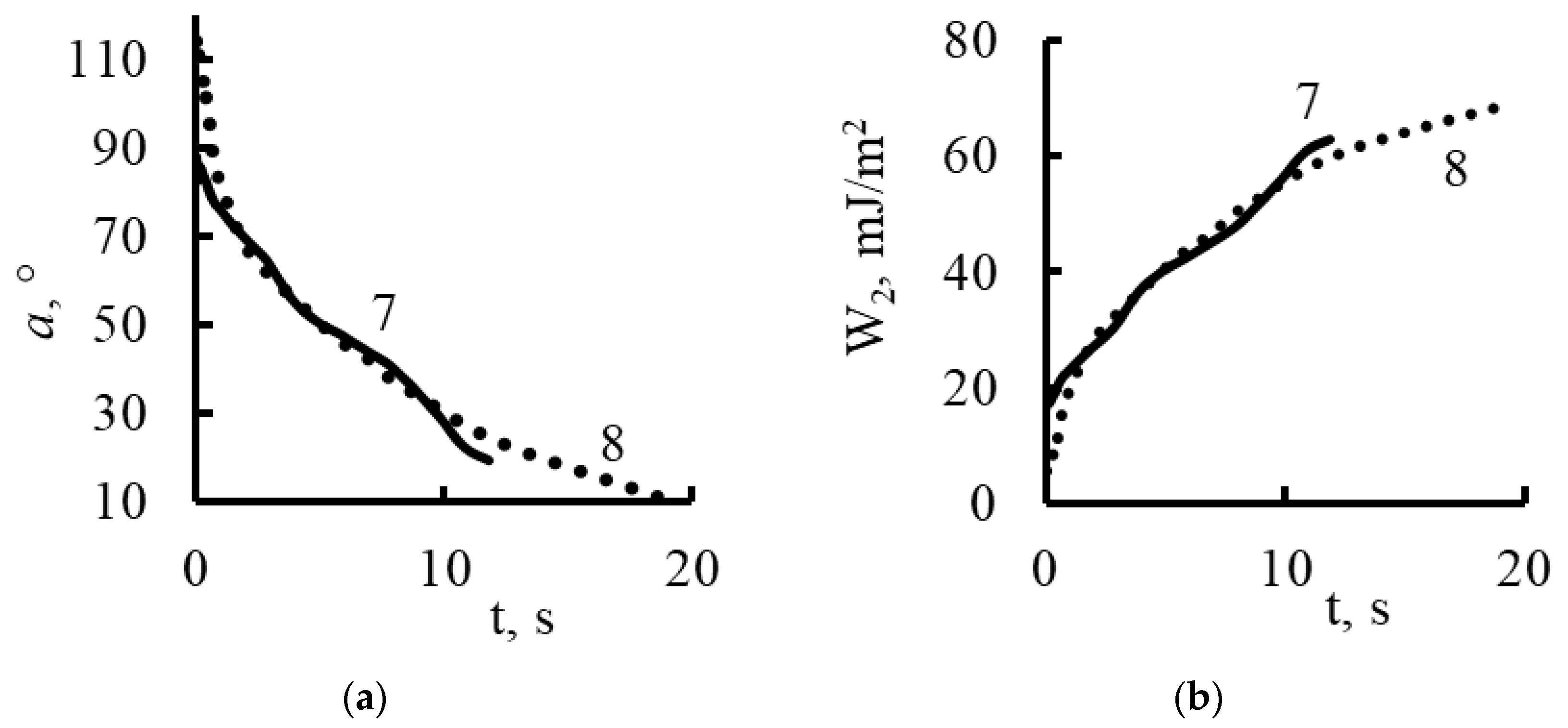
| Polikon A | Ion-Exchange Capacity, mg-eq/g | |
|---|---|---|
| without Plasma Treatment | with Plasma Treatment | |
| without nanoparticles | 2.60 ± 0.05 | 3.12 ± 0.05 |
| Si oxidized nanoparticles | 2.76 ± 0.05 | 4.09 ± 0.05 |
| Fe oxidized nanoparticles | 3.05 ± 0.05 | 3.53 ± 0.05 |
| Ni oxidized nanoparticles | 4.07 ± 0.05 | 4.73 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terin, D.; Kardash, M.; Ainetdinov, D.; Turaev, T.; Sinev, I. Anion-Exchange Membrane “Polikon A” Based on Polyester Fiber Fabric (Functionalized by Low-Temperature High-Frequency Plasma) with Oxidized Metal Nanoparticles. Membranes 2023, 13, 742. https://doi.org/10.3390/membranes13080742
Terin D, Kardash M, Ainetdinov D, Turaev T, Sinev I. Anion-Exchange Membrane “Polikon A” Based on Polyester Fiber Fabric (Functionalized by Low-Temperature High-Frequency Plasma) with Oxidized Metal Nanoparticles. Membranes. 2023; 13(8):742. https://doi.org/10.3390/membranes13080742
Chicago/Turabian StyleTerin, Denis, Marina Kardash, Denis Ainetdinov, Timur Turaev, and Ilya Sinev. 2023. "Anion-Exchange Membrane “Polikon A” Based on Polyester Fiber Fabric (Functionalized by Low-Temperature High-Frequency Plasma) with Oxidized Metal Nanoparticles" Membranes 13, no. 8: 742. https://doi.org/10.3390/membranes13080742
APA StyleTerin, D., Kardash, M., Ainetdinov, D., Turaev, T., & Sinev, I. (2023). Anion-Exchange Membrane “Polikon A” Based on Polyester Fiber Fabric (Functionalized by Low-Temperature High-Frequency Plasma) with Oxidized Metal Nanoparticles. Membranes, 13(8), 742. https://doi.org/10.3390/membranes13080742








