Facile Preparation of Dense Polysulfone UF Membranes with Enhanced Salt Rejection by Post-Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
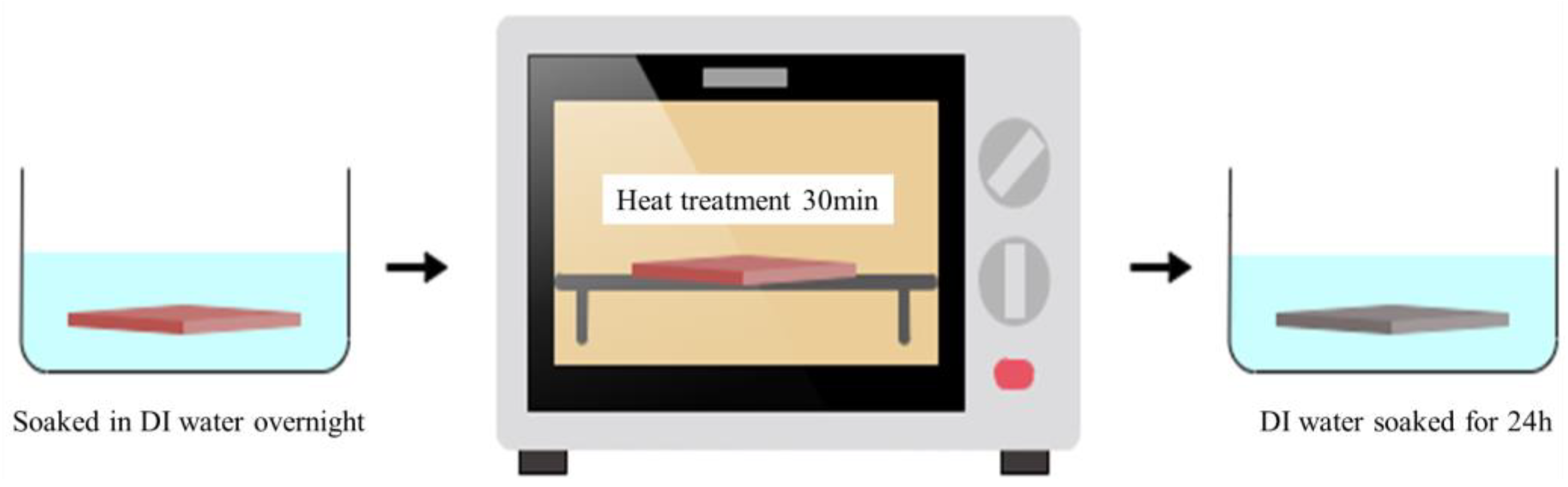
2.2. Heat Treatment of PSf Membranes and Performance Evaluation
2.3. Operational Procedures for Membrane Performance Evaluation
2.4. Characterization and Analytical Methods
3. Results and Discussion
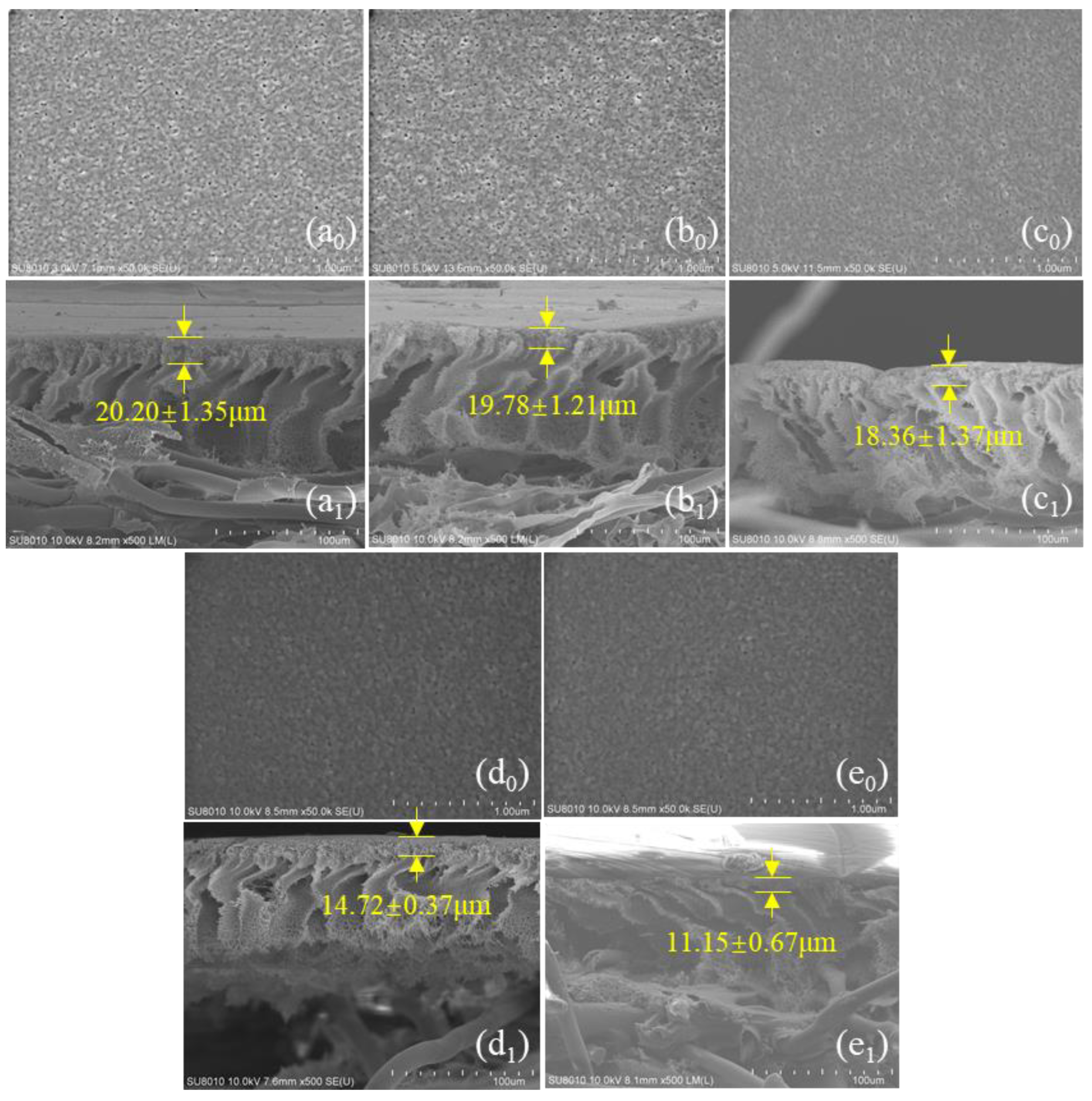
3.1. Characterizations of PSf Membranes
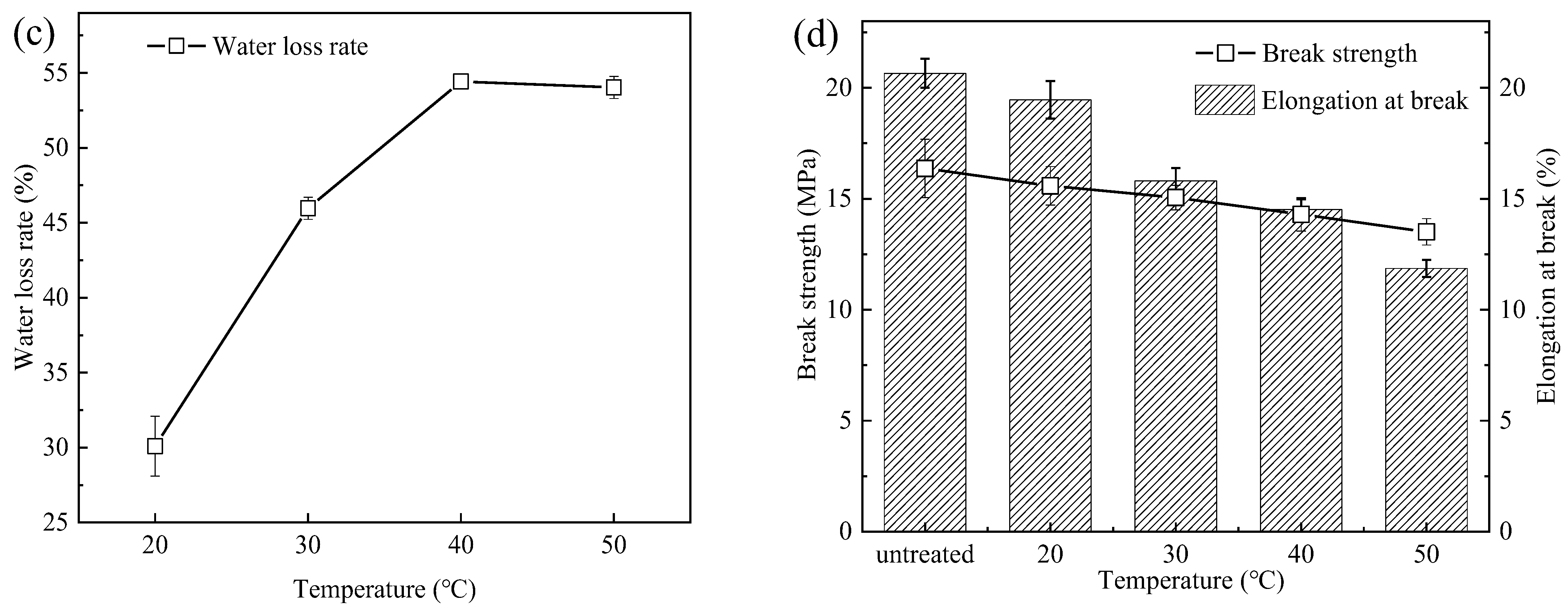
3.2. Performance of PSf Membranes
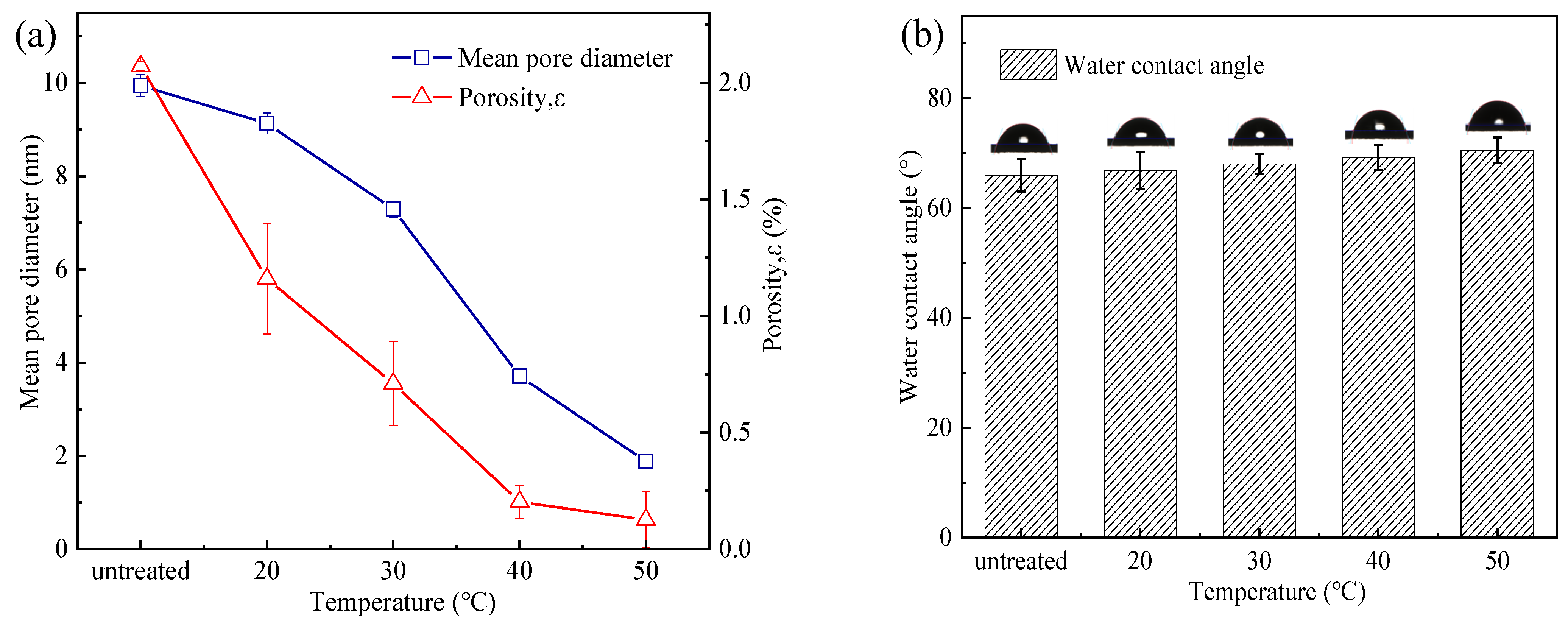
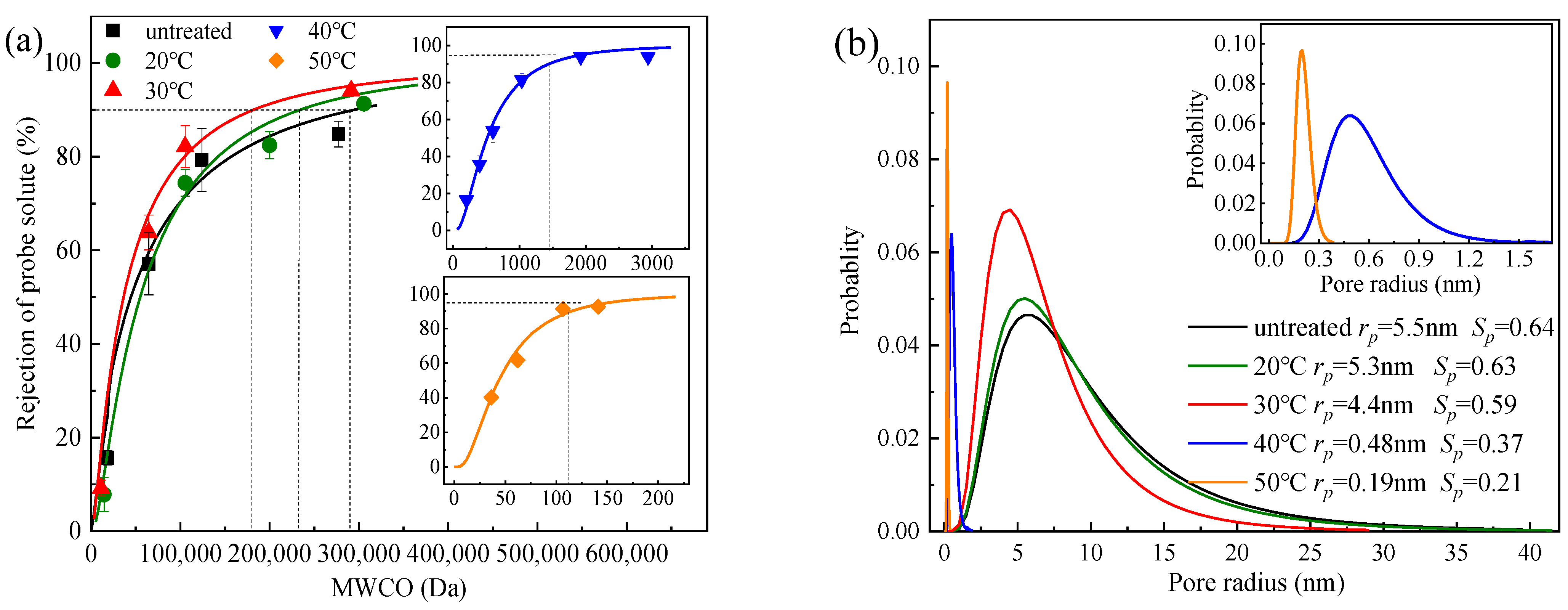
3.2.1. MWCO and Pore Size Distribution
3.2.2. Slat Rejection
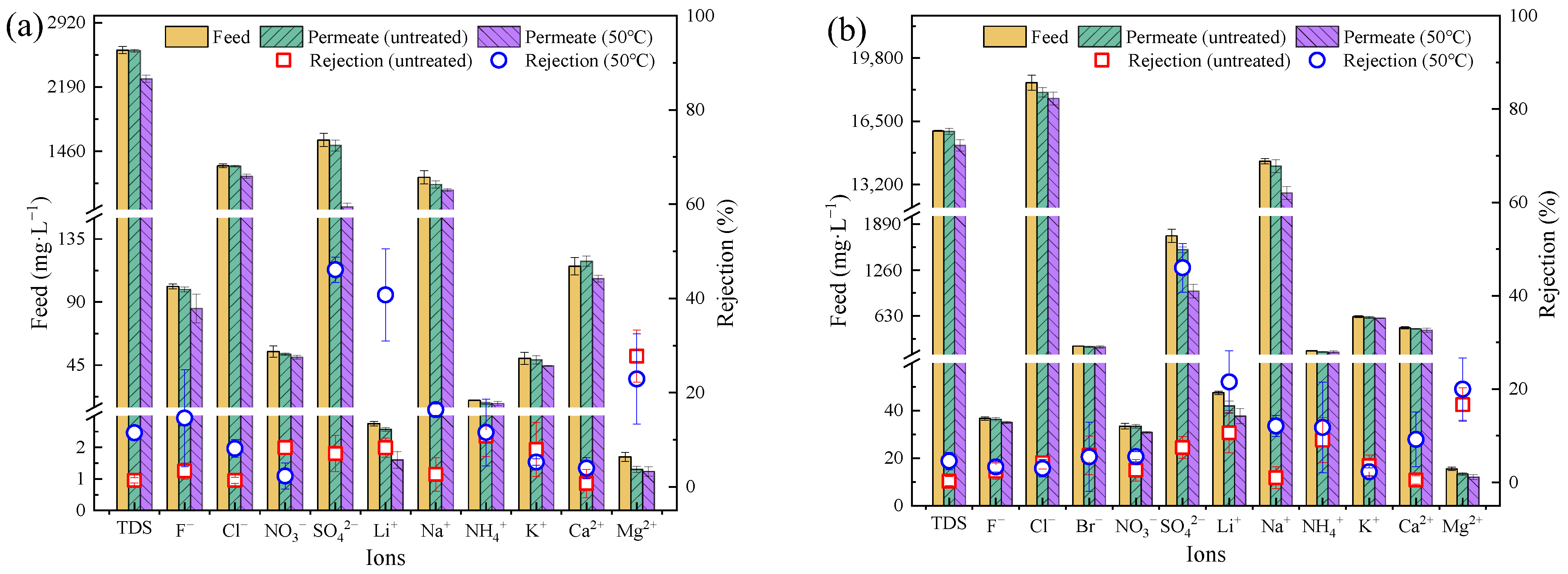
3.3. Desalinization Performance of Industrial Wastewater
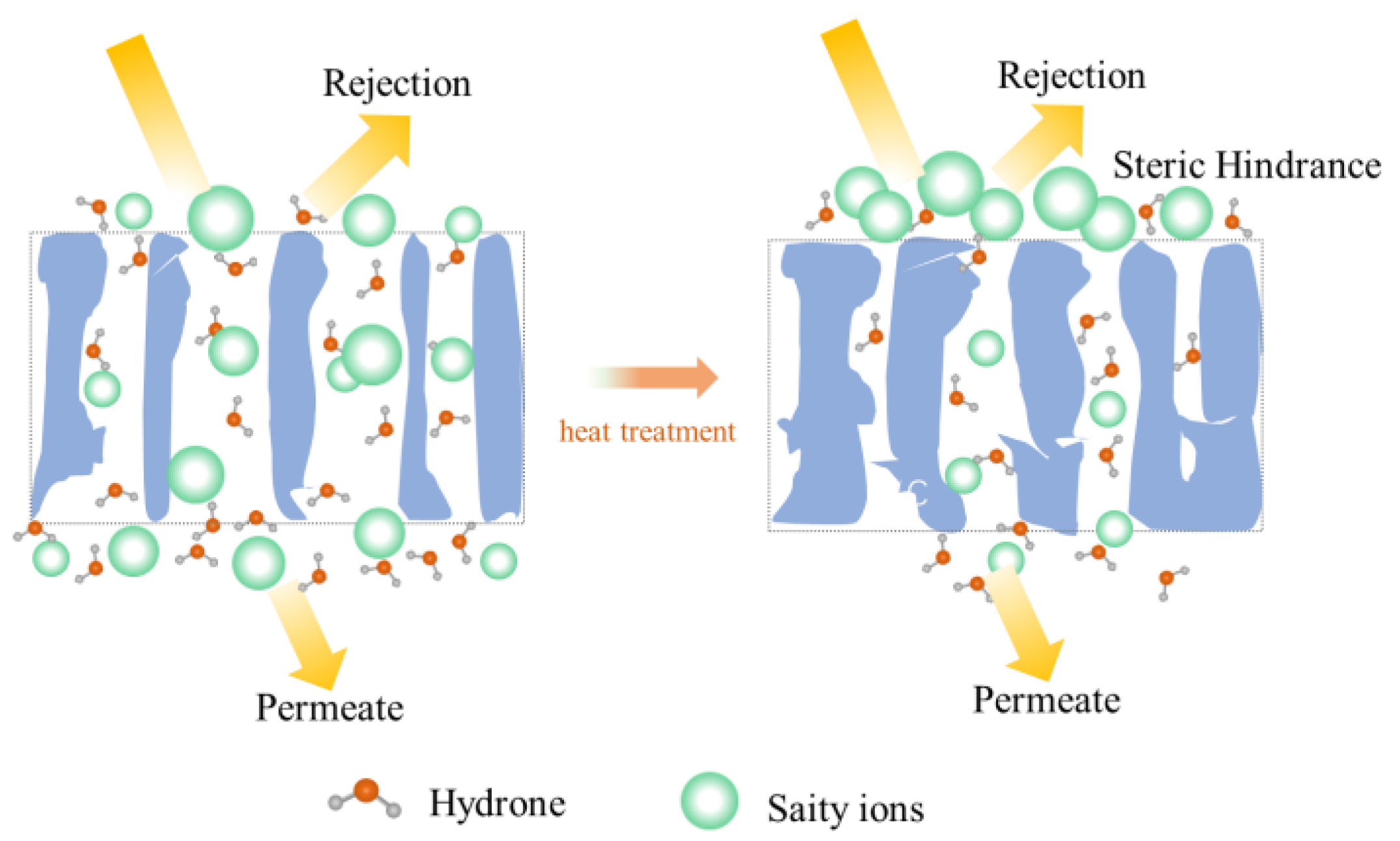
3.4. Implication of Heating on the Structure and Performance of the PSf Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, J.; Ulbricht, M. Poly(ethylene oxide)-block-poly(methyl methacrylate) diblock copolymers as functional additive for poly(vinylidene fluoride) ultrafiltration membranes with tailored separation performance. J. Membr. Sci. 2018, 545, 301–311. [Google Scholar] [CrossRef]
- Vickerman, J.C.; Gilmore, I. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2009. [Google Scholar]
- Labhasetwar, P.K.; Yadav, A. Membrane Based Point-of-Use Drinking Water Treatment Systems; IWA Publishing: London, UK, 2023. [Google Scholar]
- Kheirieh, S.; Asghari, M.; Afsari, M. Application and modification of polysulfone membranes. Rev. Chem. Eng. 2018, 34, 657–693. [Google Scholar] [CrossRef]
- Xu, Z.; Liao, J.; Tang, H.; Li, N. Antifouling polysulfone ultrafiltration membranes with pendent sulfonamide groups. J. Membr. Sci. 2018, 548, 481–489. [Google Scholar] [CrossRef]
- Wang, R.; Lin, S. Pore model for nanofiltration: History, theoretical framework, key predictions, limitations, and prospects. J. Membr. Sci. 2021, 620, 118809. [Google Scholar] [CrossRef]
- Su, J.; Yang, Q.; Teo, J.F.; Chung, T.-S. Cellulose acetate nanofiltration hollow fiber membranes for forward osmosis processes. J. Membr. Sci. 2010, 355, 36–44. [Google Scholar] [CrossRef]
- Hu, P.; Huang, B.; Miao, Q.; Wang, H.; Liu, L.; Tai, W.; Liu, T.; Li, Z.; Chen, S.; Qian, L. Ion Transport Behavior through Thermally Reduced Graphene Oxide Membrane for Precise Ion Separation. Crystals 2019, 9, 214. [Google Scholar] [CrossRef]
- Tong, D.Q.; Wang, X.Z.; Ali, M.; Lan, C.Q.; Wang, Y.; Drioli, E.; Wang, Z.H.; Cui, Z.L. Preparation of hyflon AD60/PVDF composite hollow fiber membranes for vacuum membrane distillation. Sep. Purif. Technol. 2016, 157, 1–8. [Google Scholar] [CrossRef]
- Gong, G.H.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Reverse osmosis performance of layered-hybrid membranes consisting of an organosilica separation layer on polymer supports. J. Membr. Sci. 2015, 494, 104–112. [Google Scholar] [CrossRef]
- Singh, S.; Khulbe, K.; Matsuura, T.; Ramamurthy, P. Membrane characterization by solute transport and atomic force microscopy. J. Membr. Sci. 1998, 142, 111–127. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Modelling of the retention of uncharged molecules with nanofiltration. Water Res. 2002, 36, 1360–1368. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, X.; Chen, Y. Fit-for-purpose design of nanofiltration membranes for simultaneous nutrient recovery and micropollutant removal. Environ. Sci. Technol. 2021, 55, 3352–3361. [Google Scholar] [CrossRef]
- AZydney, A.L.; Aimar, P.; Meireles, M.; Pimbley, J.M.; Belfort, G. Use of the log-normal probability density function to analyze membrane pore size distributions: Functional forms and discrepancies. J. Membr. Sci. 1994, 91, 293–298. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, F.; Chung, T.-S. Substrate modifications and alcohol treatment on thin film composite membranes for osmotic power. Chem. Eng. Sci. 2013, 87, 40–50. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.; Amirinejad, M.; Mansourpanah, Y.; Zereshki, S. The effect of heat treatment of PES and PVDF ultrafiltration membranes on morphology and performance for milk filtration. J. Membr. Sci. 2008, 330, 189–204. [Google Scholar] [CrossRef]
- Li, C.; Jia, M.M.; Zhang, M.L.; Qin, Z.P.; Ma, Y.C.; Liang, Y.C.; Guo, H.X. The interface polymerized nanofiltration membrane with hydroxypropyl-β-cyclodextrin as aqueous monomer. J. Membr. Sci. 2021, 41, 118–125. [Google Scholar]
- Staude, E. Characterization of ultrafiltration membranes by drying. J. Membr. Sci. 1986, 28, 209–223. [Google Scholar] [CrossRef]
- Xu, H.M.; Wei, J.F.; Wang, X.L.; Zhao, K.Y. Study on pore size evolution regularity of hollow fiber ultrafiltration membrane in drying process. J. Tianjin Polytech. Univ. 2014, 33, 7–11. [Google Scholar]
- Yuan, G.L.; Xu, Z.L.; Wei, Y.M. Effect of heat-treatment on morphology and performance of PVDF-PFSA hollow fiber UF blend membranes. J. East China Univ. Ence Technol. (Nat. Ence Ed.) 2009, 35, 501–505. [Google Scholar]
- Mohammad, A.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Yian, C.; Soomin, K.; Anditya, R.; Yoram, C. Tuning the hydraulic permeability and molecular weight cutoff (MWCO) of surface nano-structured ultrafiltration membranes. J. Membr. Sci. 2021, 629, 119180. [Google Scholar]
- Yang, Z.; Guo, H.; Tang, C.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Jie, X.; Cao, Y.; Qin, J.-J.; Liu, J.; Yuan, Q. Influence of drying method on morphology and properties of asymmetric cellulose hollow fiber membrane. J. Membr. Sci. 2004, 246, 157–165. [Google Scholar] [CrossRef]
- Murtiningrum; Suryadarma, P.; Suryani, A.; Manguwidjaja, D. Determination of ultrafiltration resistance using series resistance model in inulin purification from red fruit (Pandanus conoideus L.) pedicel extract. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012086. [Google Scholar] [CrossRef]
- Syahirah, S.N.; Norherdawati, K.; Ebrahim, M.; Juliana, S.I.; Wahab, M.A.; Fathiah, M.Z.; LailiAzua, J.N. Rejection mechanism of ionic solute removal by nanofiltration membranes: An overview. Nanomaterials 2022, 12, 437. [Google Scholar]
- Kong, F.-X.; Yang, H.-W.; Wang, X.-M.; Xie, Y.F. Assessment of the hindered transport model in predicting the rejection of trace organic compounds by nanofiltration. J. Membr. Sci. 2015, 498, 57–66. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Zhang, L.; Gasquet, P.; Blankert, B.; Elimelech, M.; van der Meer, W.G.J. Ion selectivity in brackish water desalination by reverse osmosis: Theory, measurements, and implications. Environ. Sci. Technol. Lett. 2019, 7, 42–47. [Google Scholar] [CrossRef]
- Harrison, C.J.; Le Gouellec, Y.A.; Cheng, R.C.; Childress, A.E. Bench-scale testing of nanofiltration for seawater desalination. J. Environ. Eng. 2007, 133, 1004–1014. [Google Scholar] [CrossRef]
- Sablani, S.; Goosen, M.; Al-Belushi, R.; Wilf, M. Concentration polarization in ultrafiltration and reverse osmosis: A critical review. Desalination 2001, 141, 269–289. [Google Scholar] [CrossRef]
- Kang, G.; Yu, H.; Liu, Z.; Cao, Y. Surface modification of a commercial thin film composite polyamide reverse osmosis membrane by carbodiimide-induced grafting with poly(ethylene glycol) derivatives. Desalination 2011, 275, 252–259. [Google Scholar] [CrossRef]
- Sun, Z.; Dong, F.; Wu, Q.; Tang, Y.; Zhu, Y.; Gao, C.; Xue, L. High water permeating thin film composite polyamide nanofiltration membranes showing thermal responsive gating properties. J. Water Process. Eng. 2020, 36, 101355. [Google Scholar] [CrossRef]
- Chaubey, S.; Mehra, S.; Yadav, A.; Kumar, A.; Shahi, V.K. Investigation of antifouling and antibacterial properties of curcumin-enriched surfactant nanoparticles modified polysulfone nanocomposite membranes. Mater. Today Chem. 2022, 26, 101130. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Aber, S. Improving the permeability and antifouling property of PES ultrafiltration membranes using the drying method and incorporating the CuO-ZnO nanocomposite. J. Water Process Eng. 2019, 31, 100891. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.; You, L.; Zhang, D.; Sun, G.; Chen, J. Facile Preparation of Dense Polysulfone UF Membranes with Enhanced Salt Rejection by Post-Heating. Membranes 2023, 13, 759. https://doi.org/10.3390/membranes13090759
Kong F, You L, Zhang D, Sun G, Chen J. Facile Preparation of Dense Polysulfone UF Membranes with Enhanced Salt Rejection by Post-Heating. Membranes. 2023; 13(9):759. https://doi.org/10.3390/membranes13090759
Chicago/Turabian StyleKong, Fanxin, Lian You, Dingwen Zhang, Guangdong Sun, and Jinfu Chen. 2023. "Facile Preparation of Dense Polysulfone UF Membranes with Enhanced Salt Rejection by Post-Heating" Membranes 13, no. 9: 759. https://doi.org/10.3390/membranes13090759
APA StyleKong, F., You, L., Zhang, D., Sun, G., & Chen, J. (2023). Facile Preparation of Dense Polysulfone UF Membranes with Enhanced Salt Rejection by Post-Heating. Membranes, 13(9), 759. https://doi.org/10.3390/membranes13090759





