Asymmetric Distribution of Plasmalogens and Their Roles—A Mini Review
Abstract
1. Introduction
2. Plasmalogens
3. Asymmetric Distribution of Ethanolamine Plasmalogen
4. ATP8B2-Mediated Asymmetric Distribution of PlsEtn
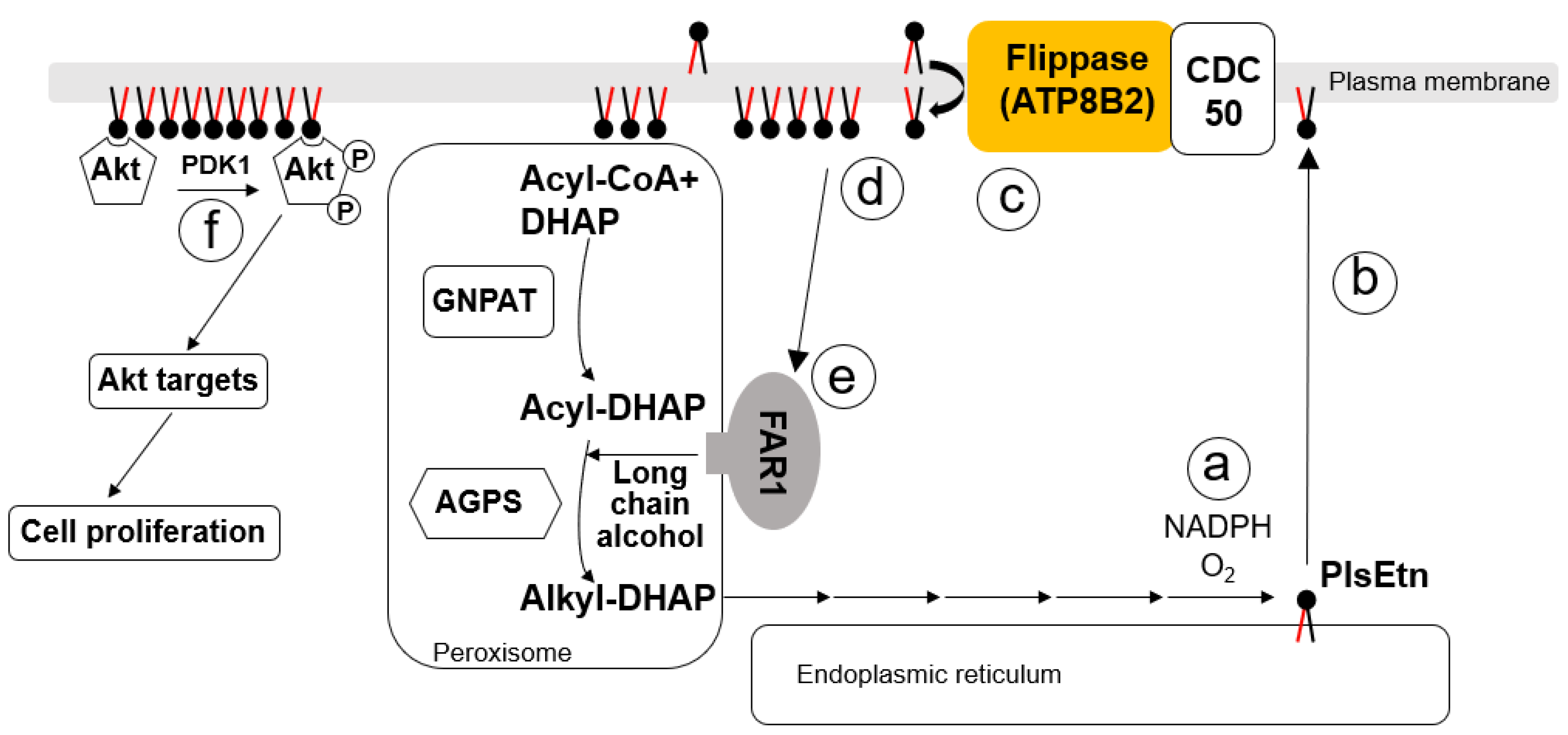
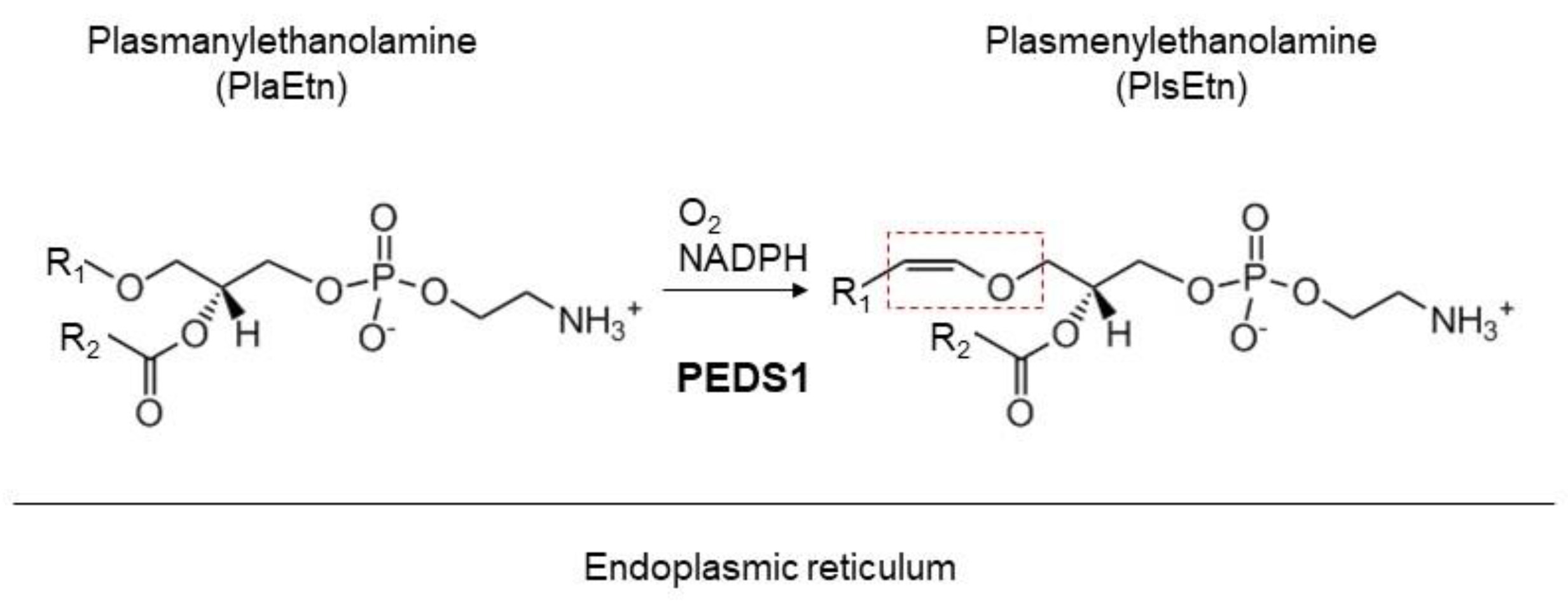
5. Roles of Plasmalogens Located in the Cytoplasmic Leaflet
6. Future Perspective
Funding
Conflicts of Interest
References
- Zachowski, A. Phospholipids in animal eukaryotic membranes: Transverse asymmetry and movement. Biochem. J. 1993, 294, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, G.; Williamson, P.; Holthuis, J.C. On the origin of lipid asymmetry: The flip side of ion transport. Curr. Opin. Chem. Biol. 2007, 11, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.P.; Vestergaard, A.L.; Mikkelsen, S.A.; Mogensen, L.S.; Chalat, M.; Molday, R.S. P4-ATPases as phospholipid flippases—Structure, function, and enigmas. Front. Physol. 2016, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, M.; Fairn, G.D. Phospholipid subcellular localization and dynamics. J. Biol. Chem. 2018, 293, 6230–6240. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Hossain, K.R.; Cao, K. Physiological roles of transverse lipid asymmetry of animal membranes. Biochim. Biophys. Acta 2020, 1862, 183382. [Google Scholar] [CrossRef]
- Shukla, S.; Baumgart, T. Enzymatic trans-bilayer lipid transport: Mechanisms, efficiencies, slippage, and membrane curvature. Biochim. Biophys. Acta 2021, 1863, 183534. [Google Scholar] [CrossRef]
- Auland, M.E.; Roufogalis, B.D.; Devaux, P.F.; Zachowski, A. Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc. Natl. Acad. Sci. USA 1994, 91, 10938–10942. [Google Scholar] [CrossRef]
- Roelants, F.M.; Su, B.M.; von Wulffen, J.; Ramachandran, S.; Sartorel, E.; Trott, A.E.; Thorner, L. Protein kinase Gin4 negatively regulates flippase function and controls plasma membrane asymmetry. J. Cell Biol. 2015, 208, 299–311. [Google Scholar] [CrossRef]
- Yabas, M.; Jing, W.; Shafik, S.; Broer, S.; Enders, A. ATP11C facilitates phospholipid translocation across the plasma membrane of all leukocytes. PLoS ONE 2016, 11, e0146774. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Hara, Y.; Okuda, M.; Itoh, K.; Nishioka, R.; Shiomi, A.T.; Nagata, Y.; Matsuda, R.; Takayama, Y.; Tominaga, M.; et al. Cell surface flip-flop of phosphatidylserine is critical for PIEZO1-mediated myotube formation. Nat. Commun. 2018, 9, 2049. [Google Scholar] [CrossRef]
- Sartorel, E.; Barrey, E.; Lau, R.K.; Thorner, J. Plasma membrane aminoglycerolipid flippase function is required for signaling competence in the yeast mating pheromone response pathway. Mol. Biol. Cell 2015, 26, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Hachiro, T.; Yamamoto, T.; Nakano, K.; Tanaka, K. Phospholipid flippases Lem3p-Dnf1p and Lem3p-Dnf2p are involved in the sorting of the tryptophan permease Tat2p in yeast. J. Biol. Chem. 2013, 288, 3594–3608. [Google Scholar] [CrossRef]
- Muthusamy, B.-P.; Yamamoto, T.; Nakano, K.; Tanaka, K. Control of protein and sterol trafficking by antagonistic activities of a type IV P-type ATPase and oxysterol binding protein homologue. Mol. Biol. Cell 2009, 20, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, P.M.; van der Velden, L.M.; Oorschot, V.; van Faassen, E.E.; Klumperman, J.; Houwen, R.H.J.; Pomorski, T.G.; Holthuis, J.C.M.; Klomp, L.W.J. A flippase-independent function of ATP8B1, the protein affected in familial intrahepatic cholestasis type 1, is required for apical protein expression and microvillus formation in polarized epithelial cells. Hepatology 2010, 51, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Slaughter, B.D.; Unruh, J.R.; Bradford, W.D.; Alexander, R.; Rubinstein, B.; Li, R. Flippase-mediated phospholipid asymmetry promotes fast Cdc42 recycling in dynamic maintenance of cell polarity. Nat. Cell Biol. 2012, 14, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Yabas, M.; Teh, C.E.; Frankenreiter, S.; Lal, D.; Roots, C.M.; Whittle, B.; Andrews, D.T.; Zhang, Y.; Teoh, N.C.; Sprent, J.; et al. ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat. Immunol. 2011, 12, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; Werner, E.R.; Berger, J.; Watshinger, K. Regulation of plasmalogen metabolism and traffic in mammals: The fog begins to lift. Front. Cell Dev. Biol. 2022, 10, 946393. [Google Scholar] [CrossRef]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Honsho, M.; Fujiki, Y. Plasmalogen homeostasis: Regulation of plasmalogen biosynthesis and its physiological consequence in mammals. FEBS Lett. 2017, 591, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Purdue, P.E.; Zhang, J.W.; Skoneczny, M.; Lazarow, P.B. Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat. Genet. 1997, 15, 381–384. [Google Scholar] [CrossRef]
- Motley, A.M.; Hettema, E.H.; Hogenhout, E.M.; Brites, P.; ten Asbroek, A.L.M.A.; Wijburg, F.A.; Baas, F.; Heijmans, H.S.; Tabak, H.F.; Wanders, R.J.A.; et al. Rhizomelic chondrodysplasia punctata is a peroxisomal protein targeting disease caused by a non-functional PTS2 receptor. Nat. Genet. 1997, 15, 377–380. [Google Scholar] [CrossRef]
- Braverman, N.; Steel, G.; Obie, C.; Moser, A.; Moser, H.; Gould, S.J.; Valle, D. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat. Genet. 1997, 15, 369–376. [Google Scholar] [CrossRef]
- Motley, A.M.; Brites, P.; Gerez, L.; Hogenhout, E.; Haasjes, J.; Benne, R.; Tabak, H.F.; Wanders, R.J.A.; Waterham, H.R. Mutational spectrum in the PEX7 gene and functional analysis of mutant alleles in 78 patients with rhizomelic chondrodysplasia punctata type 1. Am. J. Hum. Genet. 2002, 70, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Braverman, N.; Chen, L.; Lin, P.; Obie, C.; Steel, G.; Douglas, P.; Chakraborty, P.K.; Clarke, J.T.R.; Boneh, A.; Moser, A.; et al. Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum. Mutat. 2002, 20, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.A.; Schumacher, H.; Heikoop, J.; Schutgens, R.B.H.; Tager, J.M. Human dihydroxyacetonephosphate acyltransferase deficiency: A new peroxisomal disorder. J. Inherit. Metab. Dis. 1992, 15, 389–391. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Dekker, C.; Hovarth, V.A.; Schutgens, R.B.; Tager, J.M.; van Laer, P.; Lecoutere, D. Human alkyldihydroxyacetonephosphate synthase deficiency: A new peroxisomal disorder. J. Inherit. Metab. Dis. 1994, 17, 315–318. [Google Scholar] [CrossRef]
- Buchert, R.; Tawamie, H.; Smith, C.; Uebe, S.; Innes, A.M.; Al Hallak, B.; Ekici, A.B.; Sticht, H.; Schwarze, B.; Lamont, R.E.; et al. A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am. J. Hum. Genet. 2014, 95, 602–610. [Google Scholar] [CrossRef]
- Barøy, T.; Koster, J.; Strømme, P.; Ebberink, M.S.; Misceo, D.; Ferdinandusse, S.; Holmgren, A.; Hughes, T.; Merckoll, E.; Westvik, J.; et al. A novel type of rhizomelic chondrodysplasia punctata, RCDP5, is caused by loss of the PEX5 long isoform. Hum. Mol. Genet. 2015, 24, 5845–5854. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; McWalter, K.; te Brinke, H.; IJlst, L.; Mooijer, P.M.; Ruiter, J.P.N.; van Lint, A.E.M.; Pras-Raves, M.; Wever, E.; Millan, F.; et al. An autosomal dominant neurological disorder caused by de novo variants in FAR1 resulting in uncontrolled synthesis of ether lipids. Genet. Med. 2021, 23, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Hulshagen, L.; Krysko, O.; Bottelbergs, A.; Huyghe, S.; Klein, R.; Van Veldhoven, P.P.; De Deyn, P.P.; D’Hooge, R.; Hartmann, D.; Baes, M. Absence of functional peroxisomes from mouse CNS causes dysmyelination and axon degeneration. J. Neurosci. 2008, 28, 4015–4027. [Google Scholar] [CrossRef]
- Shindea, A.B.; Baboota, R.K.; Denisb, S.; Loizides-Mangold, U.; Peeters, A.; Espeeld, M.; Malheiroe, A.R.; Riezmanc, H.; Vinckierf, S.; Vazb, F.M.; et al. Mitochondrial disruption in peroxisome deficient cells is hepatocyte selective but is not mediated by common hepatic peroxisomal metabolites. Mitochondrion 2018, 39, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; He, A.; Tan, M.; Johnson, J.M.; Dean, J.M.; Pietka, T.A.; Chen, Y.; Zhang, X.; Hsu, F.F.; Razani, B.; et al. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J. Clin. Investig. 2019, 129, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, L.; Rafique, S.; Xuereb, J.H.; Rapoport, S.I.; Gershfeld, N.L. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995, 698, 223–226. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology 2009, 72, S1–S136. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: Molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef]
- Mawatari, S.; Ohara, S.; Taniwaki, Y.; Tsuboi, Y.; Maruyama, T.; Fujino, T. Improvement of blood plasmalogens and clinical symptoms in Parkinson’s disease by oral administration of ether phospholipids: A preliminary report. Parkinson’s Dis. 2020, 2020, 2671070. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kimura, A.K.; Ren, M.; Berno, B.; Xu, Y.; Schlame, M.; Epand, R.M. Substantial decrease in plasmalogen in the heart associated with tafazzin deficiency. Biochemistry 2018, 57, 2162–2175. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kimura, A.K.; Ren, M.; Monteiro, V.; Xu, Y.; Berno, B.; Schlame, M.; Epand, R.M. Plasmalogen loss caused by remodeling deficiency in mitochondria. Life Sci. Alliance 2019, 2, e201900348. [Google Scholar] [CrossRef]
- Meikle, P.J.; GWong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef]
- Sutter, I.; Klingenberg, R.; Othman, A.; Rohrer, L.; Landmesser, U.; Heg, D.; Rodondi, N.; Mach, F.; Windecker, S.; Matter, C.M.; et al. Decreased phosphati-dylcholine plasmalogens-A putative novel lipid signature in patients with stable coro-nary artery disease and acute myocardial infarction. Atherosclerosis 2016, 246, 130–140. [Google Scholar] [CrossRef]
- Heymans, H.S.A.; Schutgens, R.B.H.; Tan, R.; van den Bosch, H.; Borst, P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature 1983, 306, 69–70. [Google Scholar] [CrossRef]
- Fellmann, P.; Herve, P.; Devaux, P.F. Transmembrane distribution and translocation of spin-labeled plasmalogens in human red blood cells. Chem. Phys. Lipids 1993, 66, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, D.A.; Ganser, A.L. Myelin labeled with mercuric chloride. Asymmetric localization of phosphatidylethanolamine plasmalogen. J. Mol. Biol. 1982, 157, 635–658. [Google Scholar] [CrossRef]
- Honsho, M.; Abe, Y.; Fujiki, Y. Plasmalogen biosynthesis is spatiotemporally regulated by sensing plasmalogens in the inner leaflet of plasma membranes. Sci. Rep. 2017, 7, 43936. [Google Scholar] [CrossRef]
- Emoto, K.; Kobayashi, T.; Yamaji, A.; Aizawa, H.; Yahara, I.; Inoue, K.; Umeda, M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. USA 1996, 93, 12867–12872. [Google Scholar] [CrossRef] [PubMed]
- Bryde, S.; Hennrich, H.; Verhulst, P.M.; Devaux, P.F.; Lenoir, G.; Holthuis, J.C.M. CDC50 proteins are critical components of the human class-1 P4-ATPase transport machinery. J. Biol. Chem. 2010, 285, 40562–40572. [Google Scholar] [CrossRef]
- Coleman, J.A.; Molday, R.S. Critical role of the β-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-ATPase ATP8A2. J. Biol. Chem. 2011, 286, 17205–17216. [Google Scholar] [CrossRef] [PubMed]
- Segawa, K.; Kurata, S.; Nagata, S. The CDC50A extracellular domain is required for forming a functional complex with and chaperoning phospholipid flippases to the plasma membrane. J. Biol. Chem. 2018, 293, 2172–2182. [Google Scholar] [CrossRef]
- Grifell-Junyent, M.; Baum, J.; Valimets, S.; Hermann, A.; Paulusma, C.C.; Lopez-Marques, R.L.; Pomorski, T.G. CDC50A is required for aminophospholipid transport and cell fusion in mouse C2C12 myoblasts. J. Cell Sci. 2022, 135, cs258649. [Google Scholar] [CrossRef] [PubMed]
- Paulusma, C.C.; Elferink, R.P. P4 ATPases--the physiological relevance of lipid flipping transporters. FEBS Lett. 2010, 584, 2708–2716. [Google Scholar] [CrossRef]
- Takatsu, H.; Tanaka, G.; Segawa, K.; Suzuki, J.; Nagata, S.; Nakayama, K.; Shin, H.-W. Phospholipid flippase activities and substrate specificities of human type IV P-type ATPases localized to the plasma membrane. J. Biol. Chem. 2014, 289, 33543–33556. [Google Scholar] [CrossRef]
- Lopez-Marques, R.L.; Gourdon, P.; Pomorski, T.G.; Palmgren, M. The transport mechanism of P4 ATPase lipid flippases. Biochem. J. 2020, 471, 3769–3790. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-W.; Takatsu, H. Substrates of P4-ATPases: Beyond aminophospholipids (phosphatidylserine and phosphatidylethanolamine). FASEB J. 2019, 33, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Honsho, M.; Mawatari, S.; Fujiki, Y. ATP8B2-mediated asymmetric distribution of plasmalogens regulates plasmalogen homeostasis and plays a role in intracellular signaling. Front. Mol. Biosci. 2022, 9, 915457. [Google Scholar] [CrossRef]
- Naito, T.; Takatsu, H.; Miyano, R.; Takada, N.; Nakayama, K.; Shin, H.-W. Phospholipid flippase ATP10A translocates phosphatidylcholine and is Involved in plasma membrane dynamics. J. Biol. Chem. 2015, 290, 15004–15017. [Google Scholar] [CrossRef]
- Segawa, K.; Kurata, S.; Nagata, S. Human Type IV P-type ATPases That Work as Plasma Membrane Phospholipid Flippases and Their Regulation by Caspase and Calcium. J. Biol. Chem. 2016, 291, 762–772. [Google Scholar] [CrossRef]
- Takatsu, H.; Baba, K.; Shima, T.; Umino, H.; Kato, U.; Umeda, M.; Nakayama, K.; Shin, H.-W. ATP9B, a P4-ATPase (a putative aminophospholipid translocase), localizes to the trans-Golgi network in a CDC50 protein-independent manner. J. Biol. Chem. 2011, 286, 38159–38167. [Google Scholar] [CrossRef] [PubMed]
- Rog, T.; Koivuniemi, A. The biophysical properties of ethanolamine plasmalogens revealed by atomistic molecular dynamics simulations. Biochim. Biophys. Acta 2016, 1858, 97–103. [Google Scholar] [CrossRef]
- Honsho, M.; Yagita, Y.; Kinoshita, N.; Fujiki, Y. Isolation and characterization of mutant animal cell line defective in alkyl-dihydroxyacetonephosphate synthase: Localization and transport of plasmalogens to post-Golgi compartments. Biochim. Biophys. Acta 2008, 1783, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Russell, D.W. Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. J. Biol. Chem. 2004, 279, 37789–37797. [Google Scholar] [CrossRef] [PubMed]
- Honsho, M.; Asaoku, S.; Fujiki, Y. Posttranslational regulation of fatty acyl-CoA reductase 1, Far1, controls ether glycerophospholipid synthesis. J. Biol. Chem. 2010, 285, 8537–8542. [Google Scholar] [CrossRef] [PubMed]
- Honsho, M.; Fujiki, Y. Regulation of plasmalogen biosynthesis in mammalian cells and tissues. Brain Res. Bull. 2023, 194, 118–123. [Google Scholar] [CrossRef]
- Otsuka, K.; Sawai-Ogawa, M.; Kihara, A. Formation of fatty alcohols—Components of meibum lipids—By the fatty acyl-CoA reductase FAR2 is essential for dry eye prevention. FASEB J. 2022, 36, e22216. [Google Scholar] [CrossRef] [PubMed]
- Honsho, M.; Asaoku, S.; Fukumoto, K.; Fujiki, Y. Topogenesis and homeostasis of fatty acyl-CoA reductase 1. J. Biol. Chem. 2013, 288, 34588–34598. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.F.; Eira, J.; Lopes, A.T.; Malheiro, A.R.; Sousa, V.; Luoma, A.; Avila, R.L.; Wanders, R.J.A.; Just, W.W.; Kirschner, D.A.; et al. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J. Clin. Investig. 2014, 124, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.F.; Granadeiro, L.S.; Bessa-Neto, D.; Luz, L.L.; Safronov, B.V.; Brites, P. Plasmalogens regulate the AKT-ULK1 signaling pathway to control the position of the axon initial segment. Prog. Neurobiol. 2021, 205, 102123. [Google Scholar] [CrossRef]
- Koivuniemi, A. The biophysical properties of plasmalogens originating from their unique molecular architecture. FEBS Lett. 2017, 591, 2700–2713. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, J. Akt signaling dynamics in plasma membrane microdomains visualized by FRET-based reporters. Commun. Integr. Biol. 2009, 2, 32–34. [Google Scholar] [CrossRef][Green Version]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J.; Han, X.; Chung, K.N.; Gross, R.W. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 2002, 41, 2075–2088. [Google Scholar] [CrossRef]
- Rodemer, C.; Thai, T.P.; Brugger, B.; Kaercher, T.; Werner, H.; Nave, K.A.; Wieland, F.; Gorgas, K.; Just, W.W. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 2003, 12, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Brugger, B.; Glass, B.; Haberkant, P.; Leibrecht, I.; Wieland, F.T.; Krausslich, H.G. The HIV lipidome: A raft with an unusual composition. Proc. Natl. Acad. Sci. USA 2006, 103, 2641–2646. [Google Scholar] [CrossRef]
- Oh, S.; Jo, S.; Bajzikova, M.; Kim, H.S.; Dao, T.T.P.; Rohlena, J.; Kim, J.-M.; Neuzil, J.; Park, S. Non-bioenergetic roles of mitochondrial GPD2 promote tumor progression. Theranostics 2023, 13, 438–457. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.M.; Astudillo, A.M.; Casas, J.; Balboa, M.A.; Balsinde, J. Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens. Front. Immunol. 2018, 9, 1723. [Google Scholar] [CrossRef]
- Gaposchkin, D.P.; W Farber, H.W.; Zoeller, R.A. On the importance of plasmalogen status in stimulated arachidonic acid release in the macrophage cell line RAW 264.7. Biophys. Biochim. Acta 2008, 1781, 213–219. [Google Scholar] [CrossRef]
- Gil-de-Gómez, L.; Astudillo, A.M.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Essential role for ethanolamine plasmalogen hydrolysis in bacterial lipopolysaccharide priming of macrophages for enhanced arachidonic acid release. Front. Immunol. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Lebrero, P.; Astudillo, A.M.; Rubio, J.M.; Fernández-Caballero, L.; Kokotos, G.; Balboa, M.A.; Balsinde, J. Cellular plasmalogen content does not influence arachidonic acid kevels or distribution in macrophages: A role for cytosolic phospholipase A2γ in phospholipid remodeling. Cells 2019, 8, 799. [Google Scholar] [CrossRef]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J.; et al. RAS-RAFMEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Henry, W.S.; Ricq, E.L.; Graham, E.T.; Phadnis, V.V.; Maretich, P.; Paradkar, S.; Boehnke, N.; Deik, A.A.; Reinhardt, F.; et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020, 585, 603–608. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Cmpartmentalized regulation of lipid signaling in oxidative stress and inflammation: Plasmalogens, oxidized lipids and ferroptosis as newq paradigms of bioactive lipid research. Prog. Lipid Res. 2023, 89, 101207. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C., Jr.; Epand, R.M. Plasmalogen replacement therapy. Membranes 2021, 11, 838. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, D.; Gu, W.; Chu, B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021, 28, 2536–2551. [Google Scholar] [CrossRef]
- Albert, D.H.; Anderson, C.E. Ether-linked glycerolipids in human brain tumors. Lipids 1977, 12, 188–192. [Google Scholar] [CrossRef]
- Roos, D.S.; Choppin, P.W. Tumorigenicity of cell lines with altered lipid composition. Proc. Natl. Acad. Sci. USA 1984, 81, 7622–7626. [Google Scholar] [CrossRef]
- Snyder, F.; Wood, R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969, 29, 251–257. [Google Scholar] [PubMed]
- Lackner, K.; Sailer, S.; van Klinken, J.-B.; Wever, E.; Pras-Raves, M.L.; Dane, A.D.; Honsho, M.; Abe, Y.; Keller, M.A.; Golderer, G.; et al. Alterations in ether lipid metabolism and the consequences for the mouse lipidome. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2023, 1868, 159285. [Google Scholar] [CrossRef] [PubMed]
- Dieudonnéa, T.; Kümmererb, F.; Laursena, M.J.; Stocka, C.; Flygaarda, R.K.; Khalidc, S.; Lenoird, G.; Lyonse, J.A.; Lindorff-Larsenb, K.; Nissena, P.a. Activation and substrate specificity of the human P4-ATPase ATP8B1. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mineno, K.; Katafuchi, T. Neuronal orphan G-protein coupled receptor proteins mediate plasmalogens-induced activation of ERK and Akt signaling. PLoS ONE 2016, 11, e0150846. [Google Scholar] [CrossRef] [PubMed]
- Honsho, M.; Mawatari, S.; Fujino, T. Transient Ca2+ entry by plasmalogen-mediated activation of receptor potential cation channel promotes AMPK activity. Front. Mol. Biosci. 2022, 9, 1008626. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Plasmalogens, the vinyl ether-linked glycerophospholipids, enhance learning and memory by regulating brain-derived neurotrophic factor. Front. Cell Dev. Biol. 2022, 10, 828282. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Hashimoto, M.; Haque, A.M.; Nakagawa, K.; Kinoshita, M.; Shido, O.; Miyazawa, T. Oral administration of ethanolamine glycerophospholipid containing a high level of plasmalogen improves memory impairment in amyloid β-infused rats. Lipids 2017, 52, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, L.; Sun, R.; Wang, J.-L.; Wang, J.; Lin, Y.; Lei, S.; Zhang, Y.; Lv, D.; Jiang, F.; et al. Plasmalogens eliminate aging-associated synaptic defects and microglia-mediated neuroin-flammation in mice. J. Front. Cell Dev. Biol. 2022, 9, 815320. [Google Scholar]
- Yamamoto, K.; Miki, Y.; Sato, M.; Taketomi, Y.; Nishito, Y.; Taya, C.; Muramatsu, K.; Ikeda, K.; Nakanishi, H.; Taguchi, R.; et al. The role of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. J. Exp. Med. 2015, 212, 1901–1919. [Google Scholar] [CrossRef]
- Lydic, T.A.; Townsend, S.; Adda, C.A.; Collins, C.; Mathivanan, S.; Reid, G.E. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods 2015, 87, 83–89. [Google Scholar] [CrossRef]
- Su, H.; Rustam, Y.H.; Masters, C.L.; Makalic, E.; McLean, C.A.; Hill, A.F.; Barnham, K.J.; Reid, G.E.; Vella, L.J. Characterization of brain-derived extracellular vesicle lipids in Alzheimer’s diseas. J. Extracell. Vesicles 2021, 10, e12089. [Google Scholar] [CrossRef]
- Blank, M.L.; Snyder, F. Plasmanylethanolamine Δ1-desaturase. Meth. Enzymol. 1992, 209, 390–396. [Google Scholar]
- Padmanabhan, S.; Monera-Girona, A.J.; Pajares-Martinez, E.; Bastida-Martinez, E.; Navalon, I.D.R.; Perez-Castano, R.; Galbis-Martinez, L.M.; Fontes, M.; Elias-Arnanz, M. Plasmalogens and photooxidative stress signaling in myxobacteria, and how it unmasked CarF/TMEM189 as the Δ1’-desaturase PEDS1 for human plasmalogen biosynthesis. Front. Cell Dev. Biol. 2022, 10, 884689. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.H.; Calvo, S.E.; Markhard, A.L.; Skinner, O.S.; To, T.-L.; Ast, T.; Mootha, V.K. Genetic screen for cell fitness in high or low oxygen highlights mitochondrial and lipid metabolism. Cell 2020, 181, 716–727.e11. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.A.; Raetz, C.R. Isolation of animal cell mutants deficient in plasmalogen biosynthesis and peroxisome assembly. Proc. Natl. Acad. Sci. USA 1986, 83, 5170–5174. [Google Scholar] [CrossRef] [PubMed]
- Shimozawa, N.; Tsukamoto, T.; Suzuki, Y.; Orii, T.; Fujiki, Y. Animal cell mutants represent two complementation groups of peroxisome-defective Zellweger syndrome. J. Clin. Investig. 1992, 90, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Morand, O.H.; Allen, L.-A.H.; Zoeller, R.A.; Raetz, C.R.H. A rapid selection for animal cell mutants with defective peroxisomes. Biochim. Biophys. Acta 1990, 1034, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, Y.; Okumoto, K.; Kinoshita, N.; Ghaedi, K. Lessons from peroxisome-deficient Chinese hamster ovary (CHO) cell mutants. Biochim. Biophys. Acta—Mol. Cell Res. 2006, 1763, 1374–1381. [Google Scholar] [CrossRef]
- Brites, P.; Ferreira, A.S.; da Silva, T.F.; Sousa, V.F.; Malheiro, A.R.; Duran, M.; Waterham, H.R.; Baes, M.; Wanders, R.J. Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. PLoS ONE 2011, 6, e28539. [Google Scholar] [CrossRef]
- Fallatah, W.; Smith, T.; Cui, W.; Jayasinghe, D.; Di Pietro, E.; Ritchie, S.A.; Braverman, N. Oral administration of a synthetic vinyl-ether plasmalogen normalizes open field activity in a mouse model of rhizomelic chondrodysplasia punctata. Dis. Model. Mech. 2020, 13, dmm042499. [Google Scholar] [CrossRef]
- Malheiro, A.R.; Correia, B.; Ferreira da Silva, T.; Bessa-Neto, D.; Van Veldhoven, P.P.; Brites, P. Leukodystrophy caused by plasmalogen deficiency rescued by glyceryl 1-myristyl ether treatment. Brain Pathol. 2019, 29, 622–639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honsho, M.; Fujiki, Y. Asymmetric Distribution of Plasmalogens and Their Roles—A Mini Review. Membranes 2023, 13, 764. https://doi.org/10.3390/membranes13090764
Honsho M, Fujiki Y. Asymmetric Distribution of Plasmalogens and Their Roles—A Mini Review. Membranes. 2023; 13(9):764. https://doi.org/10.3390/membranes13090764
Chicago/Turabian StyleHonsho, Masanori, and Yukio Fujiki. 2023. "Asymmetric Distribution of Plasmalogens and Their Roles—A Mini Review" Membranes 13, no. 9: 764. https://doi.org/10.3390/membranes13090764
APA StyleHonsho, M., & Fujiki, Y. (2023). Asymmetric Distribution of Plasmalogens and Their Roles—A Mini Review. Membranes, 13(9), 764. https://doi.org/10.3390/membranes13090764





