Effect of Heat Treatment on Yellow Field Pea (Pisum sativum) Protein Concentrate Coupled with Membrane Ultrafiltration on Emulsification Properties of the Isolated >50 kDa Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
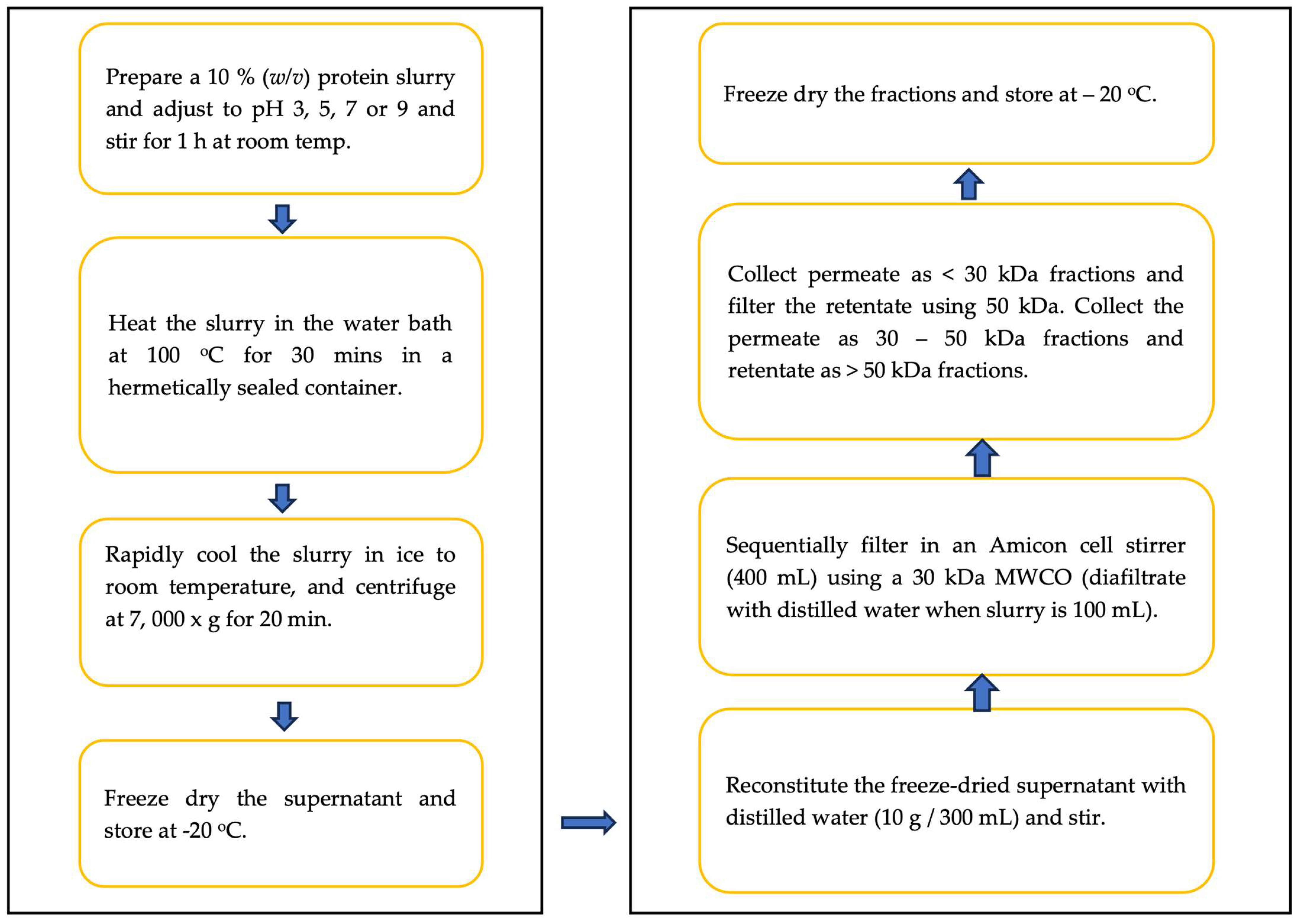
2.2. Methods
2.2.1. Membrane Ultrafiltration of Heat-Treated Pea Protein Concentrate
2.2.2. Circular Dichroism Spectra Analysis
2.2.3. Microstructure of Emulsion
2.2.4. Droplet Size Distribution
2.2.5. Flocculation (FI) and Coalescence Indices (CI)
2.2.6. Interfacial Protein Concentration (Г)
2.2.7. Statistical Analysis
3. Results and Discussion
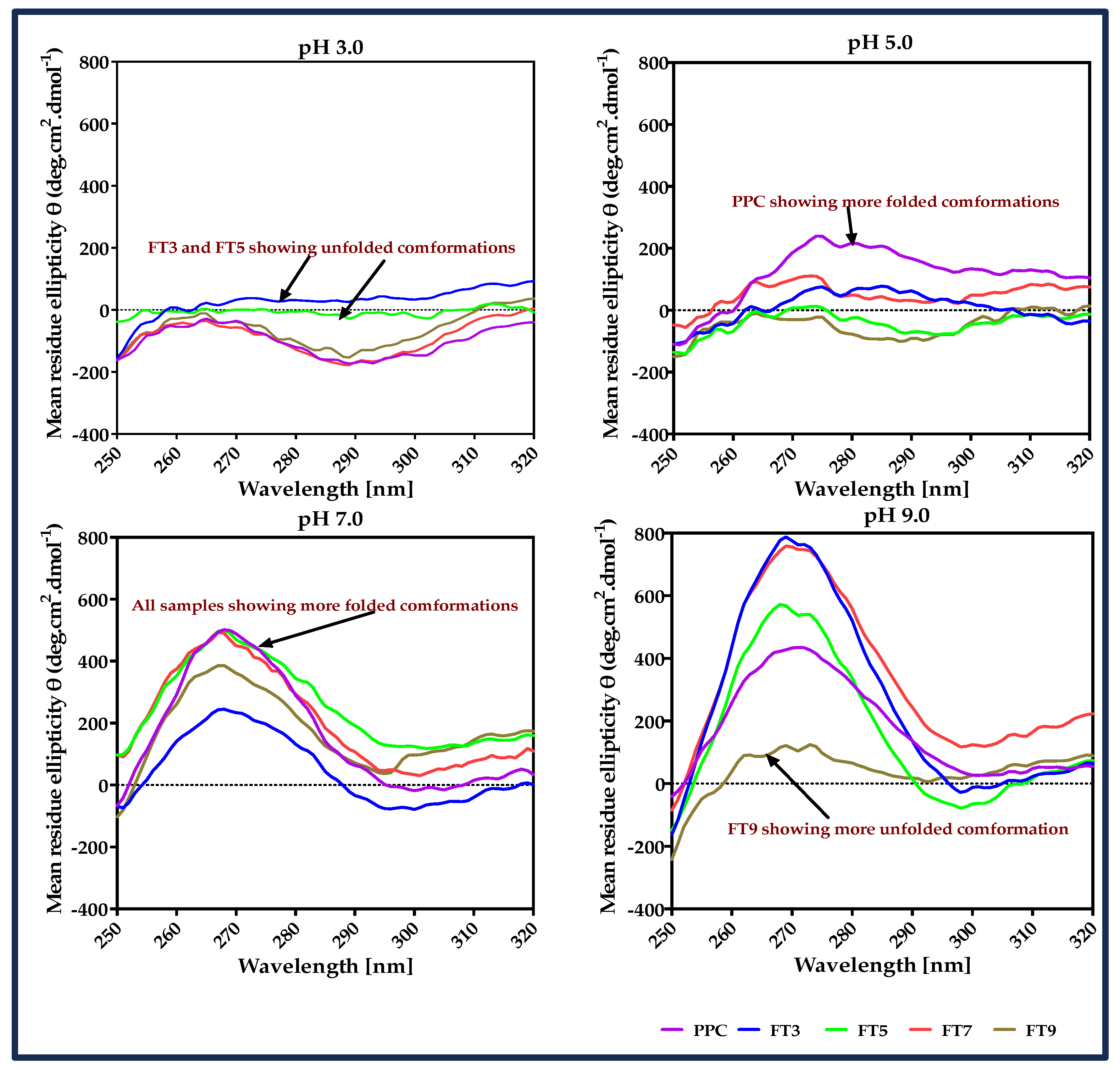
3.1. Circular Dichroism (CD) Spectra
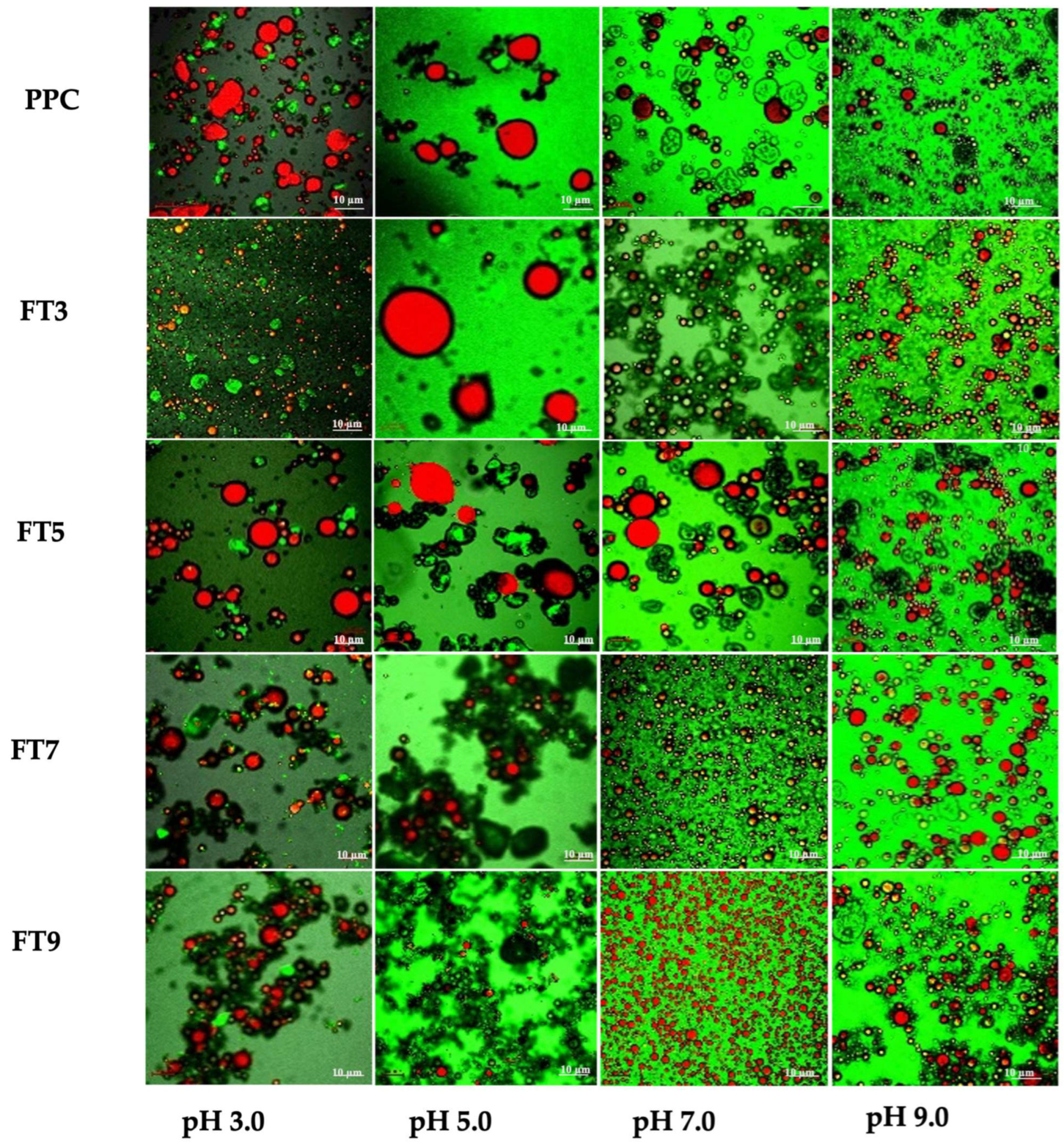
3.2. Microstructure of the Emulsions
3.3. Macrostructure of the Oil-in-Water Emulsions at Different pH and 10 mg/mL Protein Concentration
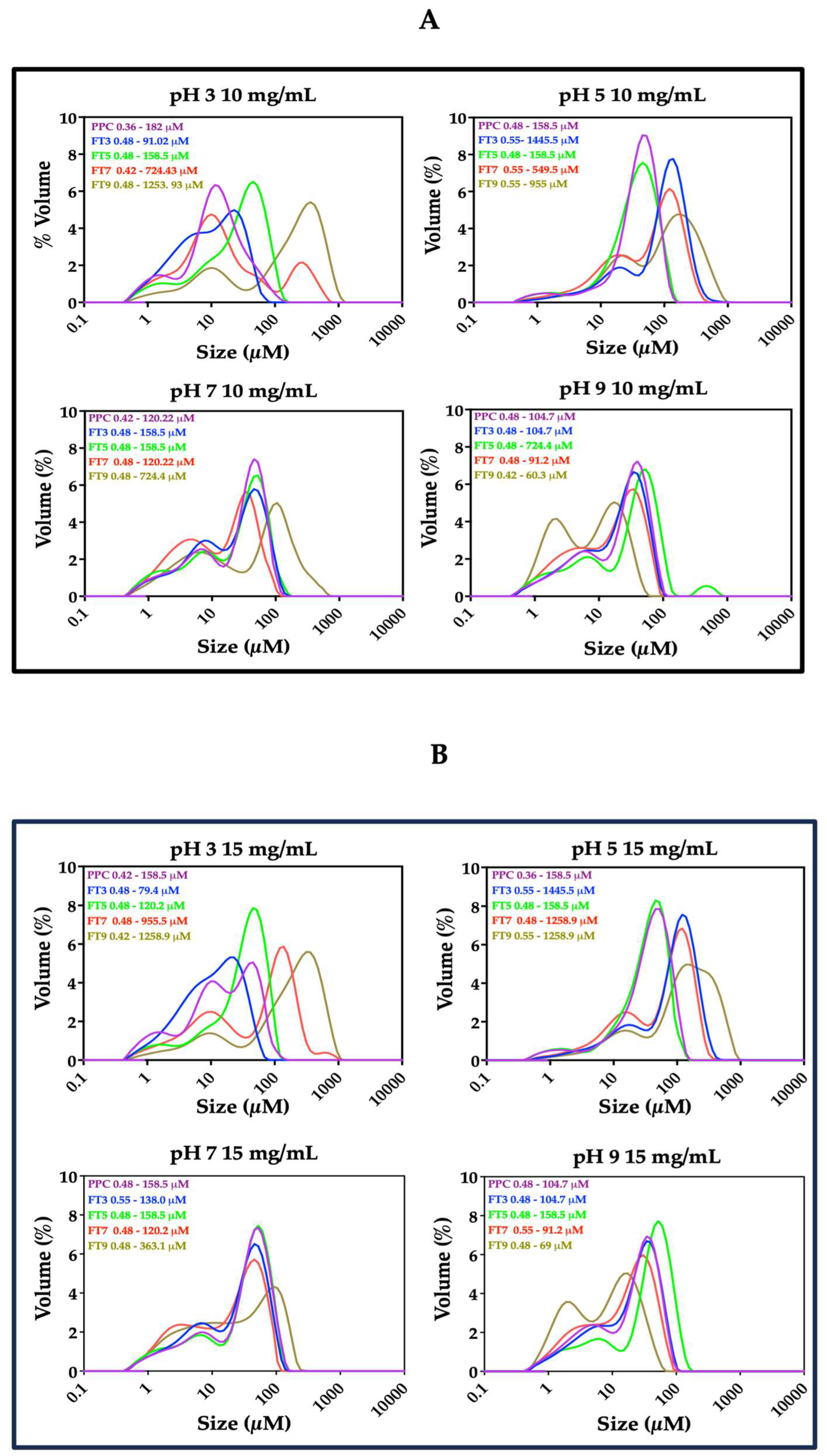
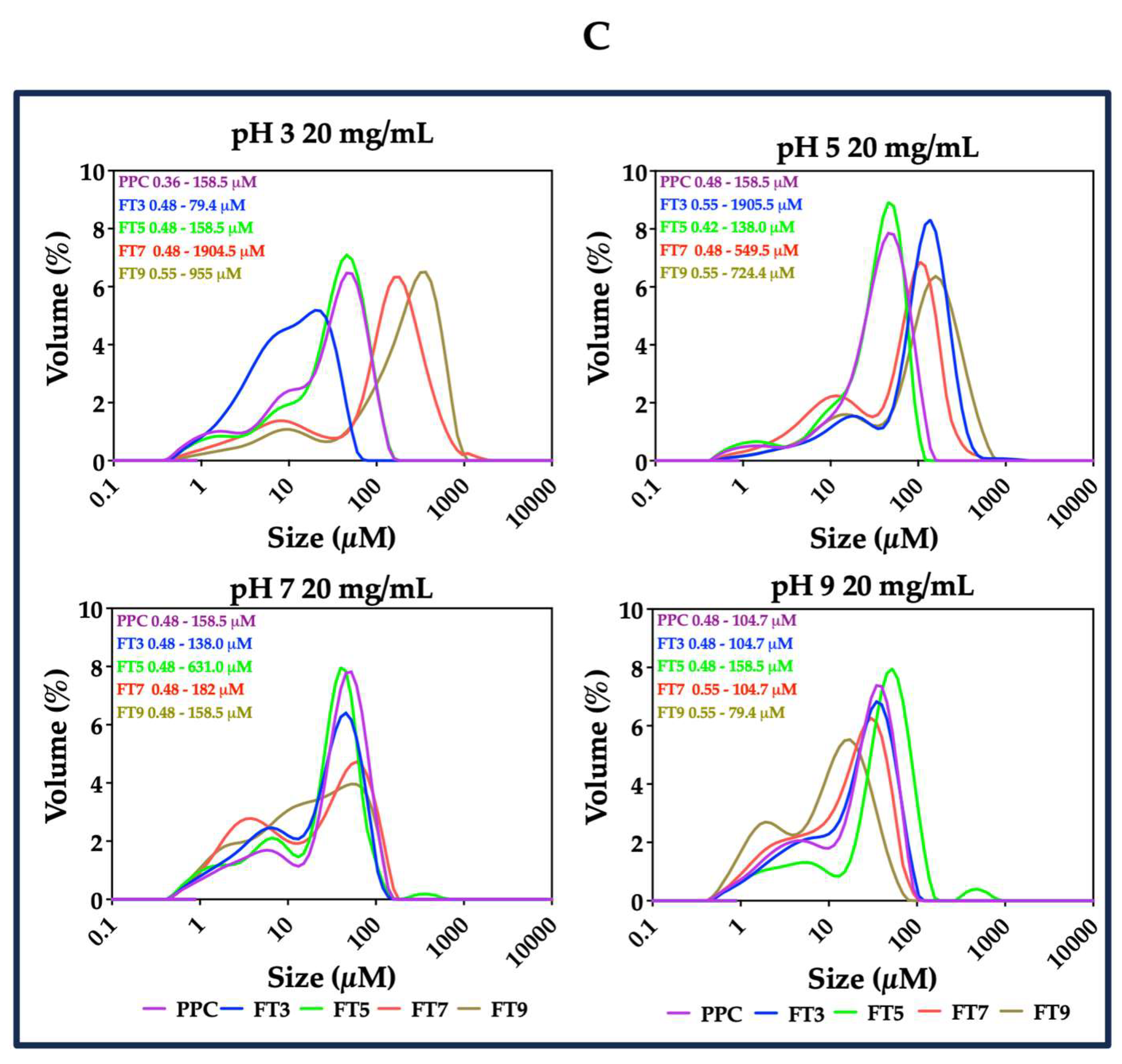
3.4. Particle Size Distribution
3.5. Interfacial Protein Concentration (Г)
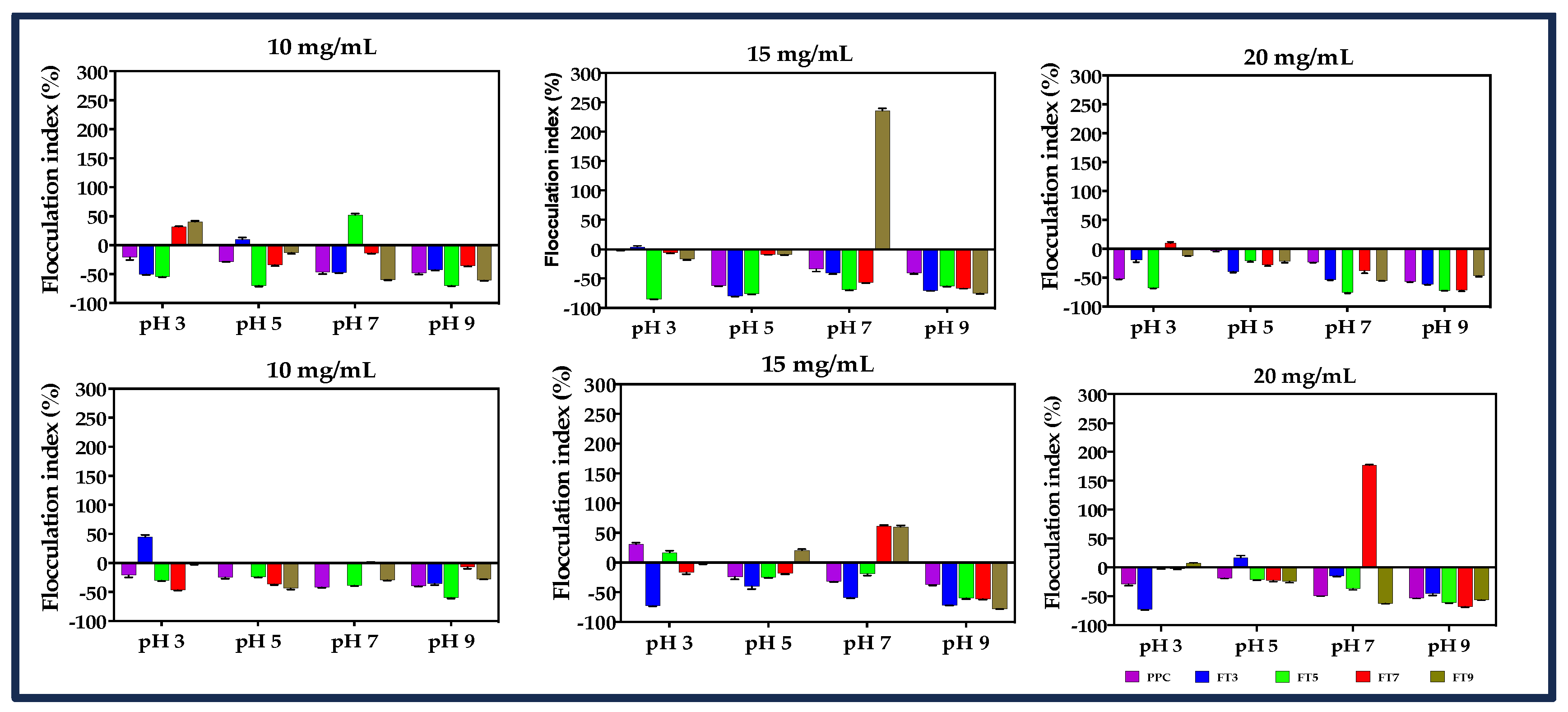
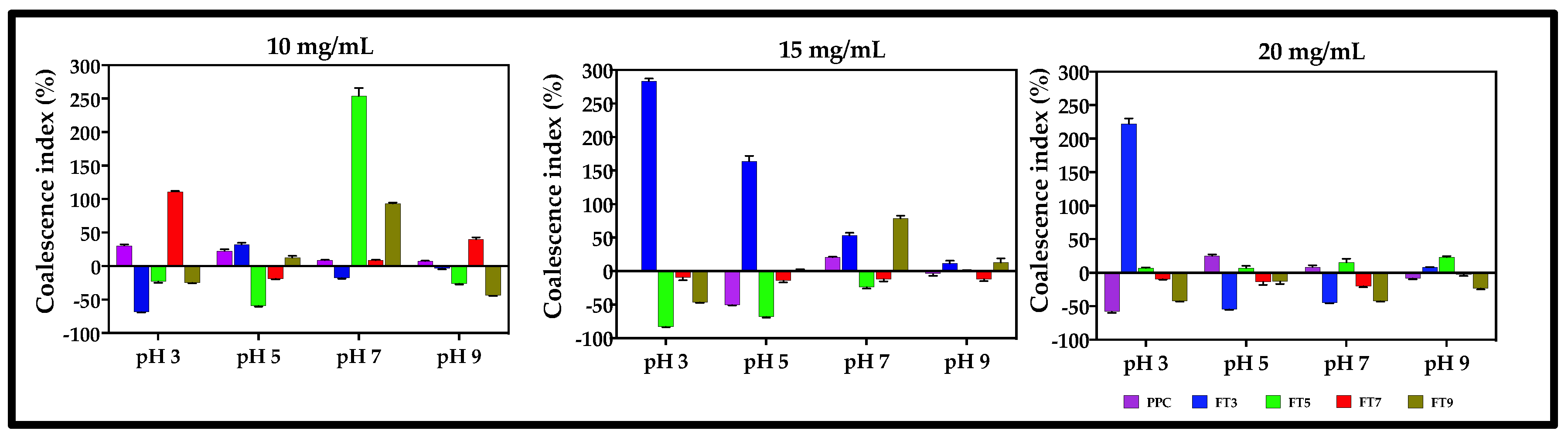
3.6. Flocculation and Coalescence Index
4. Conclusions
5. Limitations and Future Prospects of Study
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J. Protein-Stabilized Emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Hadidi, M.; Motamedzadegan, A.; Jelyani, A.Z.; Khashadeh, S. Nanoencapsulation of Hyssop Essential Oil in Chitosan-Pea Protein Isolate Nano-Complex. Food Sci. Technol. 2021, 144, 111254. [Google Scholar] [CrossRef]
- Oliete, B.; Potin, F.; Cases, E.; Saurel, R. Microfluidization as Homogenization Technique in Pea Globulin-Based Emulsions. Food Bioprocess Technol. 2019, 12, 877–882. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.J.; Bitter, J.H.; Nikiforidis, C.V. Pea Flour as Stabilizer of Oil-in-Water Emulsions: Protein Purification Unnecessary. Food Hydrocoll. 2020, 101, 105533. [Google Scholar] [CrossRef]
- Peng, W.; Kong, X.; Chen, Y.; Zhang, C.; Yang, Y.; Hua, Y. Effects of Heat Treatment on the Emulsifying Properties of Pea Proteins. Food Hydrocoll. 2016, 52, 301–310. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, C.H. Characteristics and Oxidative Stability of Soy Protein-Stabilized Oil-in-Water Emulsions: Influence of Ionic Strength and Heat Pretreatment. Food Hydrocoll. 2014, 37, 149–158. [Google Scholar] [CrossRef]
- Krstonošić, V.S.; Kalić, M.D.; Dapčević-Hadnađev, T.R.; Lončarević, I.S.; Hadnađev, M.S. Physico-Chemical Characterization of Protein Stabilized Oil-in-Water Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125045. [Google Scholar] [CrossRef]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M.T. Effect of PH on the Inter-Relationships between the Physicochemical, Interfacial and Emulsifying Properties for Pea, Soy, Lentil and Canola Protein Isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. Pea Protein Exhibits a Novel Pickering Stabilization for Oil-in-Water Emulsions at PH 3.0. LWT-Food Sci. Technol. 2014, 58, 463–469. [Google Scholar] [CrossRef]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Formation and Stability of ω-3 Oil Emulsion-Based Delivery Systems Using Plant Proteins as Emulsifiers: Lentil, Pea, and Faba Bean Proteins. Food Biophys. 2017, 12, 186–197. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use in Food Products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P. Improved Solubility and Emulsification of Wet-Milled Corn Germ Protein Recovered by Ultrafiltration–Diafiltration. J. Am. Oil Chem. Soc. 2014, 91, 1623–1631. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicrob. Proteins 2019, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, Y.; Acevedo, N.C. Effects of Pre-Heating Soybean Protein Isolate and Transglutaminase Treatments on the Properties of Egg-Soybean Protein Isolate Composite Gels. Food Chem. 2020, 318, 126421. [Google Scholar] [CrossRef]
- Jiang, S.; Altaf Hussain, M.; Cheng, J.; Jiang, Z.; Geng, H.; Sun, Y.; Sun, C.; Hou, J. Effect of Heat Treatment on Physicochemical and Emulsifying Properties of Polymerized Whey Protein Concentrate and Polymerized Whey Protein Isolate. LWT 2018, 98, 134–140. [Google Scholar] [CrossRef]
- Dapčević-Hadnađev, T.; Dizdar, M.; Pojić, M.; Krstonošić, V.; Zychowski, L.M.; Hadnađev, M. Emulsifying Properties of Hemp Proteins: Effect of Isolation Technique. Food Hydrocoll. 2019, 89, 912–920. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Physicochemical and Functional Properties of Membrane-Fractionated Heat-Induced Pea Protein Aggregates. Front. Nutr. 2022, 9, 852225. [Google Scholar] [CrossRef] [PubMed]
- Baldasso, C.; Silvestre, W.P.; Silveira, N.; Vanin, A.P.; Cardozo, N.S.M.; Tessaro, I.C. Ultrafiltration and Diafiltration Modeling for Improved Whey Protein Purification. Sep. Sci. Technol. 2022, 57, 1926–1935. [Google Scholar] [CrossRef]
- Das, R. Multienzyme Modification of Hemp Protein for Functional Peptides Synthesis. J. Food Process. 2015, 2015, 738984. [Google Scholar] [CrossRef]
- Alvarez, P.A.; Emond, C.; Gomaa, A.; Remondetto, G.E.; Subirade, M. Predictive Response Surface Model for Heat-Induced Rheological Changes and Aggregation of Whey Protein Concentrate. J. Food Sci. 2015, 80, E326–E333. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of Low Moisture Extrusion on a Pea Protein Isolate’s Expansion, Solubility, Molecular Weight Distribution and Secondary Structure as Determined by Fourier Transform Infrared Spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
- Guo, F.; Xiong, Y.L.; Qin, F.; Jian, H.; Huang, X.; Chen, J. Surface Properties of Heat-Induced Soluble Soy Protein Aggregates of Different Molecular Masses. J. Food Sci. 2015, 80, C279–C287. [Google Scholar] [CrossRef]
- Schratter, P. Purification and Concentration by Ultrafiltration. In Protein Purification Protocols; Humana Press: Totowa, NJ, USA, 2004; pp. 115–134. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Enujiugha, V.N.; Aluko, R.E. In Vitro Digestibility, Structural and Functional Properties of Moringa Oleifera Seed Proteins. Food Hydrocoll. 2020, 101, 105574. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Z.; Zhang, P.; Taha, A.; Hu, H.; Pan, S. The Role of Conformational State of PH-Shifted β-Conglycinin on the Oil/Water Interfacial Properties and Emulsifying Capacities. Food Hydrocoll. 2020, 108, 105990. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Aluko, R.E. Functional Properties of Protein Fractions Obtained from Commercial Yellow Field Pea (Pisum sativum L.) Seed Protein Isolate. Food Chem. 2011, 128, 902–908. [Google Scholar] [CrossRef]
- Castellani, O.; Belhomme, C.; David-Briand, E.; Guérin-Dubiard, C.; Anton, M. Oil-in-Water Emulsion Properties and Interfacial Characteristics of Hen Egg Yolk Phosvitin. Food Hydrocoll. 2006, 20, 35–43. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. PH-Dependent Emulsifying Properties of Pea [Pisum sativum (L.)] Proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Palazolo, G.; Sorgentini, D.; Wagner, J. Coalescence and Flocculation in o/w Emulsions of Native and Denatured Whey Soy Proteins in Comparison with Soy Protein Isolates. Food Hydrocoll. 2005, 19, 595–604. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, C.F.; Malomo, S.A.; Fagbemi, T.N.; Aluko, R.E. Polypeptide Composition and Functional Properties of African Yam Bean Seed (Sphenostylis stenocarpa) Albumin, Globulin and Protein Concentrate. Food Hydrocoll. 2016, 56, 189–200. [Google Scholar] [CrossRef]
- Schmid, F.X. Spectral Methods of Characterizing Protein Conformation and Conformational Changes in Protein Structure: A Practical Approach; Creighton, T.E., Ed.; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Ijarotimi, O.S.; Malomo, S.A.; Fagbemi, T.N.; Osundahunsi, O.F.; Aluko, R.E. Structural and Functional Properties of Buchholzia Coriacea Seed Flour and Protein Concentrate at Different PH and Protein Concentrations. Food Hydrocoll. 2018, 74, 275–288. [Google Scholar] [CrossRef]
- Qin, X.; Yu, J.; Wang, Q.; Zhang, H.; Chen, H.; Hu, Z.; Lv, Q.; Liu, G. Preparation of Camellia Oil Pickering Emulsion Stabilized by Glycated Whey Protein Isolate and Chitooligosaccharide: Effect on Interfacial Behavior and Emulsion Stability. LWT 2022, 153, 112515. [Google Scholar] [CrossRef]
- Sha, L.; Koosis, A.O.; Wang, Q.; True, A.D.; Xiong, Y.L. Interfacial Dilatational and Emulsifying Properties of Ultrasound-Treated Pea Protein. Food Chem. 2021, 350, 129271. [Google Scholar] [CrossRef]
- Keivaninahr, F.; Gadkari, P.; Zoroufchi Benis, K.; Tulbek, M.; Ghosh, S. Prediction of Emulsification Behaviour of Pea and Faba Bean Protein Concentrates and Isolates from Structure–Functionality Analysis. RSC Adv. 2021, 11, 12117–12135. [Google Scholar] [CrossRef]
- Mcclements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, J.A.; Aluko, R.E. Influence of Enzymatic Hydrolysis, PH and Storage Temperature on the Emulsifying Properties of Canola Protein Isolate and Hydrolysates. Int. J. Food Sci. Technol. 2018, 53, 2316–2324. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in Natural Emulsifiers for Utilization in Food Emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Destribats, M.; Rouvet, M.; Gehin-Delval, C.; Schmitt, C.; Binks, B.P. Emulsions Stabilised by Whey Protein Microgel Particles: Towards Food-Grade Pickering Emulsions. Soft Matter 2014, 10, 6941–6954. [Google Scholar] [CrossRef]
- Aluko, R.E.; Mine, Y. Competitive Adsorption of Hen’s Egg Yolk Granule Lipoproteins and Phosvitin in Oil-in-Water Emulsions. J. Agric. Food Chem. 1997, 45, 4564–4570. [Google Scholar] [CrossRef]
- Hebishy, E.; Zamora, A.; Buffa, M.; Blasco-Moreno, A.; Trujillo, A.-J. Characterization of Whey Protein Oil-In-Water Emulsions with Different Oil Concentrations Stabilized by Ultra-High Pressure Homogenization. Processes 2017, 5, 6. [Google Scholar] [CrossRef]
- Protte, K.; Weiss, J.; Hinrichs, J. Insignificance of Lactose Impurities on Generation and Structural Characteristics of Thermally Stabilised Whey Protein-Pectin Complexes. Int. Dairy J. 2018, 80, 46–51. [Google Scholar] [CrossRef]
- Sağlam, D.; Venema, P.; de Vries, R.; Shi, J.; van der Linden, E. Concentrated Whey Protein Particle Dispersions: Heat Stability and Rheological Properties. Food Hydrocoll. 2013, 30, 100–109. [Google Scholar] [CrossRef]
- Bos, M.A.; van Vliet, T. Interfacial Rheological Properties of Adsorbed Protein Layers and Surfactants: A Review. Adv. Colloid Interface Sci. 2001, 91, 437–471. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P. Physical Chemistry of Foods; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Qamar, S.; Bhandari, B.; Prakash, S. Effect of Different Homogenisation Methods and UHT Processing on the Stability of Pea Protein Emulsion. Food Res. Int. 2019, 116, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2008. [Google Scholar]

| † Adsorbed Protein (%) | |||||
|---|---|---|---|---|---|
| * Sample | Protein Conc. (mg/mL) | pH 3 | pH 5 | pH 7 | pH 9 |
| PPC | 10 | 98.30 ± 3.50 a | 97.30 ± 2.00 a | 97.94 ± 1.00 a | 97.60 ± 0.80 a |
| 15 | 96.50 ± 3.50 a | 99.73 ± 1.68 a | 65.43 ± 1.42 e | 94.08 ± 2.02 b | |
| 20 | 98.20 ± 3.50 a | 96.80 ± 2.40 a | 76.35 ± 1.26 d | 81.75 ± 1.80 c | |
| FT3 | 10 | 86.85 ± 3.30 c | 97.60 ± 2.31 a | 92.04 ± 1.63 b | 82.83 ± 2.02 c |
| 15 | 49.27 ± 0.48 e | 94.74 ± 2.19 b | 69.72 ± 2.29 e | 78.72 ± 3.61 d | |
| 20 | 66.09 ± 4.15 d | 82.39 ± 1.84 c | 60.15 ± 1.68 f | 99.46 ± 4.68 a | |
| FT5 | 10 | 98.20 ± 3.92 a | 98.60 ± 2.57 a | 98.10 ± 2.01 a | 96.30 ± 1.49 a |
| 15 | 99.64 ± 4.20 a | 98.20 ± 3.10 a | 63.95 ± 2.31 e | 89.52 ± 2.59 c | |
| 20 | 91.48 ± 4.65 b | 96.90 ± 2.34 a | 81.97 ± 1.91 c | 79.22 ± 1.69 d | |
| FT7 | 10 | 97.50 ± 4.70 a | 97.30 ± 2.63 a | 93.91 ± 1.21 b | 99.97 ± 1.30 a |
| 15 | 96.80 ± 4.55 a | 98.20 ± 2.28 a | 46.19 ± 2.33 h | 80.95 ± 4.52 c | |
| 20 | 97.90 ± 4.26 a | 86.64 ± 1.85 c | 55.62 ± 4.51 g | 59.39 ± 2.13 f | |
| FT9 | 10 | 98.30 ± 5.08 a | 98.70 ± 1.66 a | 77.18 ± 2.00 d | 69.16 ± 0.84 e |
| 15 | 99.46 ± 4.68 a | 98.30 ± 2.90 a | 37.36 ± 2.48 i | 71.70 ± 4.70 d | |
| 20 | 97.60 ± 4.51 a | 98.10 ± 2.90 a | 68.40 ± 1.51 e | 80.82 ± 4.02 c |
| † Surface Protein Concentration (mg/m2) | |||||
|---|---|---|---|---|---|
| * Sample | ** Protein Conc. (mg/mL) | pH 3 | pH 5 | pH 7 | pH 9 |
| PPC | 10 | 1.53 ± 0.00 f | 3.65 ± 0.00 g | 1.89 ± 0.02 c | 1.87 ± 0.01 d |
| 15 | 2.38 ± 0.00 e | 4.82 ± 0.05 f | 3.61 ± 0.06 a | 2.64 ± 0.06 c | |
| 20 | 4.23 ± 0.00 c | 6.74 ± 0.02 d | 3.71 ± 0.06 a | 3.37 ± 0.07 b | |
| FT3 | 10 | 1.14 ± 0.03 f | 6.35 ± 0.01 d | 1.79 ± 0.03 c | 1.69 ± 0.04 d |
| 15 | 1.17 ± 0.01 f | 7.94 ± 0.18 c | 2.23 ± 0.07 b | 2.24 ± 0.06 c | |
| 20 | 2.01 ± 0.04 e | 10.68 ± 0.24 b | 2.41 ± 0.07 b | 4.26 ± 0.09 a | |
| FT5 | 10 | 2.23 ± 0.00 e | 3.54 ± 0.00 g | 1.89 ± 0.09 c | 2.03 ± 0.00 c |
| 15 | 3.59 ± 0.09 d | 4.84 ± 0.34 f | 3.13 ± 0.07 a | 3.10 ± 0.03 b | |
| 20 | 4.48 ± 0.14 c | 5.90 ± 0.00 e | 3.33 ± 0.08 a | 4.17 ± 0.08 a | |
| FT7 | 10 | 1.70 ± 0.00 f | 4.40 ± 0.00 f | 1.44 ± 0.02 c | 1.63 ± 0.00 d |
| 15 | 3.47 ± 0.00 d | 6.60 ± 0.06 d | 1.20 ± 0.06 c | 1.99 ± 0.05 d | |
| 20 | 7.85 ± 0.09 b | 6.61 ± 0.14 d | 1.86 ± 0.15 c | 2.12 ± 0.08 c | |
| FT9 | 10 | 3.99 ± 0.00 d | 6.10 ± 0.00 d | 1.75 ± 0.04 c | 0.74 ± 0.01 e |
| 15 | 7.50 ± 0.15 b | 11.11 ± 0.00 b | 1.04 ± 0.07 c | 1.11 ± 0.02 d | |
| 20 | 12.69 ± 0.00 a | 12.53 ± 0.00 a | 2.29 ± 0.05 b | 1.90 ± 0.04 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asen, N.D.; Aluko, R.E. Effect of Heat Treatment on Yellow Field Pea (Pisum sativum) Protein Concentrate Coupled with Membrane Ultrafiltration on Emulsification Properties of the Isolated >50 kDa Proteins. Membranes 2023, 13, 767. https://doi.org/10.3390/membranes13090767
Asen ND, Aluko RE. Effect of Heat Treatment on Yellow Field Pea (Pisum sativum) Protein Concentrate Coupled with Membrane Ultrafiltration on Emulsification Properties of the Isolated >50 kDa Proteins. Membranes. 2023; 13(9):767. https://doi.org/10.3390/membranes13090767
Chicago/Turabian StyleAsen, Nancy D., and Rotimi E. Aluko. 2023. "Effect of Heat Treatment on Yellow Field Pea (Pisum sativum) Protein Concentrate Coupled with Membrane Ultrafiltration on Emulsification Properties of the Isolated >50 kDa Proteins" Membranes 13, no. 9: 767. https://doi.org/10.3390/membranes13090767






